Abstract
Data now suggest that current strategies in the treatment of rheumatoid arthritis (RA) should focus on early identification and diagnosis, followed by early initiation of DMARD therapy. Initiation of treatment in early RA—ideally, less than 3–6 months after symptom onset—improves the success of achieving disease remission and reduces joint damage and disability. While the optimal treatment regimen in early RA is unclear, use of initial DMARD mono- or combination therapy with prompt escalation to achieve low disease activity or remission is an appropriate approach. Ultimately, the goal of RA management should be the prevention of inflammatory joint disease and, thereby, prevention of disability. To date, studies have shown that pharmacologic interventions can delay progression from undifferentiated inflammatory arthritis to classifiable RA. However, further investigation is needed to identify asymptomatic individuals at high risk for future RA and to intervene early enough in the pathogenesis of RA to prevent progression to clinical disease.
Keywords: Rheumatoid arthritis, Early treatment, Prevention, Treatment, Strategies, Management, Therapy, DMARD, Joint damage, Autoimmunity
Introduction
In the past 1–2 decades, treatment paradigms in rheumatoid arthritis (RA) have shifted dramatically from initial treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), followed by cautiously progressive addition of disease-modifying antirheumatic drugs (DMARDs), to the current treatment approach of aggressive initiation of DMARD therapy soon after the diagnosis of RA has been made. This change in RA management results from increasing data supporting improved prognosis and outcomes with the initiation of DMARD therapy early in the course of symptomatic disease. Since the goals in RA management include not only disease remission, but also improved functional status, which is strongly associated with radiographic joint damage, an understanding of the impact that the initiation of appropriate treatment during early RA has on these outcomes is essential. In this review, we will discuss the major studies supporting the efficacy and impact of early initiation of DMARDs in the management of RA, as well as discussing issues regarding the definitions and diagnosis of early RA. Furthermore, we will review and discuss potential preventive approaches for RA.
What Is the Definition of “Early” Rheumatoid Arthritis?
Accurate diagnosis of early RA begins with clear definitions of RA, as well as early. There is considerable variability in the literature regarding the time frame defining early RA [1]. Previous intervention studies in early RA have included early RA as disease duration from 3 months to 3 years; however, with the knowledge of improved outcomes with earlier treatment in RA, it becomes clear that a shorter time interval for classification of early RA is clinically significant. Due to the wide range of definitions of early RA presented in the literature, it is difficult to characterize the specific time frame that defines early RA. However, it is now generally accepted that early RA is the onset of symptoms of joint (typically polyarticular) pain, stiffness, or swelling within the past 3 months [2, 3•], although in practical terms it may be difficult for rheumatologists to evaluate patients within that 3-month time frame, due to a variety of factors, including delay in referral of patients with early symptoms of inflammatory arthritis (IA) or delays in patients seeking medical attention for their symptoms [4]. Therefore, guidelines such as the 2012 ACR updates for the treatment of RA (discussed below) that suggest initiation of treatment of RA <6 months after onset of symptoms may be more clinically applicable [3•]. Of note, it is likely that the onset of inflammatory joint symptoms is the best time to begin the clock on the “start” of RA, rather than using the first time IA was identified by a health care provider—in large part, because delays in patient assessments can often delay an “official” diagnosis of IA by months [4]. However, this approach does have the caveat that patient-reported onset of symptoms of RA may be faulty, especially if the time from onset of symptoms to a diagnosis of RA is prolonged or if the onset of symptoms is subtle [5].
Until recently, RA was classified according to the 1987 ACR criteria. This classification scheme was well accepted, although many thought that it was inadequate for identifying patients with early RA [6, 7]. As such, the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria were designed in part to make earlier diagnosis of RA attainable [8]. While still limited, with a sensitivity of 58–91 % in subjects with a symptom duration of less than 2 years and 62–74 % in subjects with symptoms present for less than 3 months [9], these new criteria appear to be successful in establishing a diagnosis of RA earlier in the disease course. For example, retrospective studies from early-arthritis cohorts have confirmed that the 2010 ACR/EULAR criteria detect more patients at baseline who would eventually need DMARD therapy, as compared with the 1987 ACR criteria (68 vs. 42 %) [10••, 11]. However, concerns with the 2010 criteria include the overdiagnosis of RA, since it also appears from these studies that the 2010 criteria diagnosed RA in more patients that would have disease resolution at 18 months without the use of DMARDs (8 vs. 2 %) or were classified with a form of arthritis other than RA after 1 year (18 %). Additional studies will be necessary to understand the specific role the 2010 criteria will play in the early diagnosis and management of RA and how utilization of the 2010 criteria will affect longer-term outcomes in RA.
Benefits of Early DMARD Treatment for Response to Therapy in RA
Multiple studies have evaluated the benefits of early treatment of RA, including several that have evaluated the impact of early DMARD treatment on successful response to therapy (see Table 1). In particular, a meta-analysis of ~1,400 RA patients from 14 randomized controlled trials identified that one of the strongest predictors of response to therapy was a shorter disease duration at the time of treatment initiation [12]. In this meta-analysis, treatment response was defined as achievement of an ACR20 response, and regardless of the specific DMARD used, 53 % of RA patients with disease duration ≤1 year achieved an ACR20 response, as compared with only 35–43 % of those with a disease duration >1 year [12]. Additionally, in a 2007 review article by Cush, subgroup analyses of adalimumab, etanercept, and infliximab trials demonstrated improved ACR20 response rates in patients treated who had a <2–3 year disease duration, as compared with patients with disease durations ≥2–3 years [13].
Table 1.
Summary of studies supporting that early treatment of RA results in improved outcomes
| Anderson et al., 2000 [12] | Meta-analysis demonstrating that RA patients with a shorter disease duration respond better to similar therapies, as compared with patients with longer-term disease. |
| Lard et al., 2001 [17] | In this nonrandomized study, the initiation of treatment of RA at a median of 15 days after diagnosis resulted in improved disease activity at 2 years, as compared with treatment initiated a median of 123 days after diagnosis. |
| Mottonen et al., 2002 [19] | Delay of initiation of RA therapy by 4 months after the onset of symptoms decreased the ability for a single drug to induce remission in early RA. |
| Nell et al., 2004 [14] | A case–control study demonstrating that patients with a median RA duration of 3 months had improved outcomes with similar therapies when compared with patients with a median duration of disease of ~12 months. |
| Finckh et al., 2006 [20] | Meta-analysis demonstrating that early treatment of RA (<1 year) results in reduced long-term radiographic progression rates, as compared with patients treated later (≥1 year). |
| Cush, 2007 [13] | Review article that summarizes data from subanalyses of several trials of biologic therapies in RA. Shows that early treatment (<2–3 years of disease duration) results in improved outcomes, as compared to treatment initiated in disease of ≥2–3 years duration. |
| van der Woude et al., 2009 [15] | Data from two large early arthritis cohorts demonstrated that sustained, DMARD-free remission of RA was significantly associated with shorter duration of symptoms of IA at time of initiation of therapy. |
| van der Linden et al., 2010 [21] | In a study of an early arthritis cohort, only 31 % of patients with RA were assessed within 3 months of symptom onset; assessment and treatment of RA within 3 months of symptom onset was associated with increased chance of DMARD-free remission and less joint destruction. |
In a small prospective study by Nell et al., 40 patients with newly diagnosed RA started on DMARD therapy were compared: 20 subjects had symptoms for <3 months, and 20 matched subjects had symptoms for 9–42 months (median 12 months) prior to treatment [14]. They found that while both groups had similar baseline disease activity as measured by a 28 joint disease activity score (DAS28), the patients treated within 3 months of symptom onset had significantly higher rates of reduction in DAS28 scores. This difference was evident by the first 3-month follow-up (40 % vs. 12 %; p< 0.05) and remained significant at 3 years, demonstrating that a delay in DMARD treatment makes it more challenging to achieve improvements in disease activity.
Finally, van der Woude et al. evaluated factors that predicted remission in RA, using a very rigid definition of remission, and found similar enhanced remission rates in patients treated earlier in the disease course. In this study, remission was defined as no swollen joints off DMARDs for >1 year. Subjects from two large early-RA cohorts, the Leiden Early Arthritis Clinic and the British Early Rheumatoid Arthritis Study with symptom duration <2 years prior to initiation of DMARD therapy, were evaluated, and symptom duration at baseline was found to predict sustained DMARD-free remission [15].
Reduction of Joint Damage and Improved Function With Early Treatment in RA
In the management of RA, an important long-term objective is the reduction of functional decline and disability. Since function in RA is strongly associated with radiographic joint damage, inhibiting progressive joint damage is a key treatment goal in RA [16].
In 2001, Lard et al. retrospectively compared early and delayed initiation of DMARD treatment in an early-RA cohort. On the basis of differences in treatment paradigms at the time of diagnosis, 109 patients received DMARDs only after several months of inadequate NSAID response, and this “delayed” treatment group was compared with 97 patients initiated on DMARD therapy as early as possible [17]. In those with early initiation of DMARDs (within 2 weeks of symptom onset), there was less progression of radiographic joint damage from baseline at 2 years of follow-up, as compared with the delayed DMARD treatment group with median symptom duration of 4 months (p<.05).
In the study discussed earlier by Nell et al. [14], patients initiated on DMARDs earlier (median of 3 months of symptoms) had significant reductions in the progression of joint destruction and improvements in function, as compared with those with a longer duration of symptoms prior to the start of therapy (median 12 months). While it is not surprising that the patients with longer disease duration prior to treatment had worse baseline radiographic joint damage, they also had a greater progression of joint damage over 3 years, as compared with the patients treated earlier. Furthermore, despite similar baseline functional status as assessed by the health assessment questionnaire (HAQ), patients treated earlier had significantly improved functional status within the first 3 months of treatment, and this difference persisted throughout 3 years of follow-up.
One challenge in applying the currently available data in support of earlier initiation of treatment in RA is the lack of long-term follow-up data for joint damage. Most studies are limited to 2 years or less of follow-up, and this time frame may be too short to understand the full impact of early treatment on function and joint damage. To address this concern, Finckh et al. performed a meta-analysis in 2006, evaluating the degree of radiographic joint damage up to 5 years after initiation of therapy (median 3 years) in over 1,000 RA patients treated with DMARDs. They found that radiographic joint damage was reduced by 33 % in RA patients treated within 1 year of symptom onset, as compared with those started on DMARD therapy after >1 year of symptoms. Additionally, a recent study by van der Linden et al. found that clinical assessment by a rheumatologist within 3 months of symptom onset resulted in a 1.34-fold decrease in progression of radiographic joint damage at 6 years (median 4 years), as compared with those assessed >3 months after onset of symptoms [18].
What Treatment Strategy Is Optimal in Early RA?
The benefits of early initiation of DMARDs in achieving remission and reducing joint damage are discussed above, but understanding which specific treatment regimen to choose in early RA is an equally important issue, although one without a clear answer. One study addressing this issue was the Behandel Strategieen (BeST) trial that randomized RA patients with less than a 2-year duration of symptoms (median 6 months) to one of four arms of therapy [22]. While the BeST study demonstrated that combination therapy (methotrexate [MTX], sulfasalazine [SSZ] plus prednisone, or MTX plus infliximab) was, overall, superior to sequential monotherapy or step-up combination DMARD therapy in early RA, a substantial number of subjects in the sequential monotherapy group (53 %) and in the step-up combination therapy group (64 %) achieved low disease activity at 1 year, as defined by a DAS44 [23]. Similar rates of DAS low disease activity were observed in the MTX monotherapy arm of the COMET study, with 47 % of patients achieving low disease activity at 1 year [24]. Regarding radiographic progression, the BeST trial did demonstrate that initial combination therapy resulted in a small but significant reduction in radiographic progression at 1 year, as compared with sequential monotherapy or step-up combination therapy, which was associated with a significant improvement in function as assessed by the Dutch version of the HAQ.
The Finnish Rheumatoid Arthritis Combination Therapy (FIN-RACo) study that compared single versus combination DMARD therapy in early RA (<2 years of symptoms prior to diagnosis) identified increased remission rates at 2 years in RA patients who were started on combination DMARD therapy (SSZ, MTX, hydroxychloroquine [HCQ], and prednisolone), as compared with single DMARD therapy with SSZ (37 % vs. 18 %, respectively; p=.03) [25, 26]. However, similar to other studies, in the FIN-RACo study, up to 35 % of patients responded to single DMARD therapy, and in those receiving SSZ alone, earlier initiation of treatment (<4 months symptom duration) was associated with increased remission rates, as compared with delayed initiation of SSZ monotherapy (4- to 24-month symptom duration; 35 % and 11 %, respectively; p=.021) [19].
While additional studies including the PREMIER trial [27] have demonstrated improved long-term outcomes with combination therapy, as compared with monotherapy, in early RA, a recent comparative effectiveness study of the Treatment of Early Aggressive RA (TEAR) trial concluded that initial use of MTX monotherapy with addition at 6 months of either SSZ plus HCQ or etanercept if needed to achieve disease remission is a reasonable management strategy for early RA [28•]. The TEAR trial randomized RA patients with <3 years of symptoms to (1) initial MTX with step-up therapy if DAS28 scores did not indicate low disease activity at 6 months, (2) initial triple therapy with MTX, SSZ, and HCQ, and (3) initial MTX plus etanercept. At 24 weeks, more patients in the initial combination groups had achieved DAS28 low disease activity (41 %–43 %), as compared with the initial MTX monotherapy group (28 %); however, after step-up algorithms were applied, there was no difference in DAS28 scores between groups at 48 and 102 weeks of follow-up. Additionally, there was no difference between groups in functional assessment by HAQ at 1 and 2 years of follow-up, and at 2 years, there was no clear difference in radiographic erosions between patients treated initially with step-up therapy, triple therapy, or MTX plus etanercept. Furthermore, the SWEFOT study showed that ~34 % of patients with early RA (<1-year duration of symptoms) had “good” responses as measured by the DAS28 in response to MTX monotherapy, although a factor associated with decreased response was a longer duration of symptoms prior to the use of MTX [29, 30•]. However, the SWEFOT investigators also noted that over 2 years, there was some radiographic progression of disease in patients treated with MTX monotherapy, even though these patients had low disease activity (DAS28 score ≤3.2) [31].
Overall, these studies demonstrate that a substantial proportion (28 %–34 %) of patients with early RA respond well in terms of clinical disease activity to single DMARD therapy alone, and this consistent observation becomes clinically relevant in the balance of risks, benefits, and costs in the initial treatment of patients with RA, although the potential for radiographic progression of disease, despite good clinical responses from monotherapy, needs to be evaluated in future studies.
How Should Early RA Be Managed?
It is clear that identification and treatment of patients with early RA is beneficial, but as discussed above, the optimal initial treatment regimen for an individual patient is difficult to establish. While some studies, such as the FIN-RACo, BeST, and PREMIER trials, suggest that early combination DMARD/biologic therapy is superior, other studies, such as the TEAR and SWEFOT trials, show that a certain proportion of patients respond well to initial therapy with MTX or do not appear to have significant long-term adverse events if their disease requires additions to initial MTX monotherapy over time. Therefore, the optimal initial therapy for a patient with early RA remains unclear. This question has been more formally addressed in the ACR’s 2012 updates of treatment for RA, where initiating DMARD monotherapy in any early-RA patient without poor prognostic factors (functional limitations, extra-articular disease, seropositivity, or erosions) is recommended. However, in early-RA patients with moderate to high disease activity and poor prognostic factors, they recommend initial treatment with combination DMARDs or anti-TNF therapy (±MTX) within the first 6 months of disease. They acknowledge that some patients with high disease activity may respond to DMARD monotherapy, but given the presence of poor prognostic factors, more aggressive treatment is recommended to prevent irreversible joint damage and preserve function over the long term.
This approach to the management of early RA as recommended by the ACR is reasonable; however, there are several caveats to it, which include (1) difficulty in practical clinical experience of identifying and treating patients with “early RA” (and especially of disease of <6-month duration since symptom onset; in particular, a 2011 study of European patients found that the median duration from onset of symptoms to assessment by a rheumatologist was ~24 weeks [32]), (2) limited head-to-head randomized trials comparing early and delayed institution of specific treatment regimens in RA, (3) limitations in excluding spontaneous disease remission and avoiding potential overtreatment with early therapy in using current definitions of RA, especially the 2010 criteria, and (4) a limited understanding of the specific prognostic factors and biomarkers that should be used to guide initial therapy of individuals with early RA (e.g., what specific findings would indicate that use of MTX monotherapy is adequate, as compared with MTX plus a biologic agent?). These issues will need to be addressed in future studies that, hopefully, will identify optimal methodologies for evaluating and treating patients with RA soon after onset of symptoms and methods (biomarker or otherwise) for identifying which specific early treatments are optimal for individual patients.
How Does Early Treatment of RA Result in Improved Outcomes?
We have yet to understand fully the pathogenesis of RA, especially in its earliest phases of development. However, it is of interest that there is an enhanced treatment effect and higher likelihood of remission when immunosuppression is started earlier. While the mechanisms of this are unclear, it may be that the immune system can be altered within a certain window after the symptoms of joint inflammation appear, during which reversal of disease is possible [13, 33]. Early initiation of therapy may also prevent subtle joint damage that, if allowed to develop, may result over time in more significant disability due to secondary degenerative effects. Also, past a certain point, the pathogenesis and mechanisms of sustained inflammation may be altered to such an extent that the opportunity for reversal of disease and cure is lost. For example, in longstanding RA, synovial fibroblasts may be largely autonomous in their production of destructive factors and poorly responsive to treatment [34]. Future studies are needed to better understand the etiology for this enhanced impact on disease with early initiation of treatment.
Prevention of RA
While early treatment of RA leads to improved outcomes, ultimately, the goal of RA management should be the prevention of disease. What makes the concept of prevention of RA more enticing, as well as possible to achieve, is the growing number of studies demonstrating that RA-specific biomarkers precede the development of clinically apparent RA [35–38]. These findings have led to a model of development of RA outlined in Fig. 1. In this model, initially genetic and environmental risk factors for RA (phase 1) “trigger” a period of asymptomatic RA-related autoimmunity (phase 2) during which abnormalities of biomarkers, such as rheumatoid factor and antibodies to citrullinated protein antigens (ACPAs), are present. This phase is followed by a period in which a subject develops symptoms of inflammatory joint disease in the absence of overt clinical findings of IA (phase 3)—a period defined as arthralgia in absence of IA by several European investigative groups [39]—that is later followed by clinically apparent IA that may, at first, be undifferentiatedand then later evolve into fully classifiable RA (phase 4). Importantly, in terms of prediction and potential prevention of RA, it appears that abnormalities of RA-related autoantibodies and, in particular, the highly RA-specific ACPAs in individuals without apparent IA are highly predictive of future onset of disease [36, 37, 40]. On the basis of this, there is hope that these biomarkers can be used to identify subjects who are at sufficiently high risk for future RA that they would be candidates for receiving interventions to prevent disease while they are still in the early phase of disease development prior to substantial joint injury.
Fig. 1.
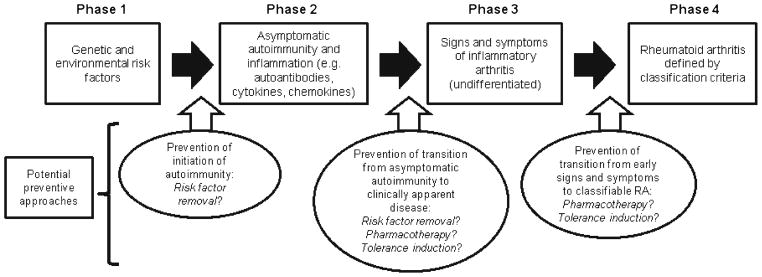
Model of rheumatoid arthritis (RA) development and potential interventions to prevent progression of disease. In this model of RA development, disease likely begins with genetic risk and exposure to environmental risk factors (phase 1) that trigger asymptomatic inflammation and autoimmunity (phase 2). Over time, autoimmunity progresses to symptomatic inflammatory arthritis (phase 3) that may further progress to classifiable RA (phase 4). The mechanisms of transition between these phases are not well understood but likely involve complex relationships between host and environmental factors that may differ between phases. Prevention of the progression of disease may be implemented at several points along this pathway of disease development
However, prevention of RA may mean different things to different investigators. It may mean prevention of progression from a phase of undifferentiated IA to that of classifiable RA (Fig. 1). It could mean identification of individuals who have developed RA-related autoimmunity in the absence of overt IA and implementation of therapies to prevent the further development of disease. Finally, it could mean modulation of RA-related risk factors on an individual or population level to prevent the future occurrence of disease. Importantly, because, as was discussed above, tissue injury may occur even very soon after the symptomatic onset of RA, the latter approach is most like primary disease prevention—that is, alteration of risk factors for disease so that RA-related tissue injury never occurs. Approaches to each of these types of prevention are outlined in Fig. 1 and will be discussed in more detail below.
Prevention of Progression From Undifferentiated IA to Classifiable RA
To a large extent, the progression of undifferentiated IA to classifiable RA is related to the definitions of disease that are used. For example, if the 1987 ACR classification criteria are used, it may take many months for someone to progress from undifferentiated IA to classifiable RA [6]. However, with the 2010 ACR/EULAR criteria, in an individual with IA and high-titer RA-related autoantibody positivity, progression from IA to classifiable RA may be only a matter of having three swollen small joints evolve to four swollen small joints (Table 2) [8]. By these definitions of RA, in a patient with undifferentiated IA, it may be relatively easy to prevent progression to RA as defined by 1987 criteria, although difficult to prevent progression to RA as defined by 2010 criteria.
Table 2.
Comparison of the 1987 ACR RA and 2010 ACR/EULAR Classification Criteria for RA
| 1987 ACR classification criteria | 2010 ACR/EULAR classification criteria | |
|---|---|---|
| 1) Morning stiffness >1 h | Who should be tested? Patients with ≥1 swollen joint consistent with synovitis not better explained by another disease. If the patient meets these initial criteria with a score of ≥6/10, he or she can be classified as having “definite RA”: | |
| 2) Arthritis of ≥3 joint areas | A. Joint involvement* | |
| 3) Hand arthritis | 1 large joint | 0 |
| 4) Symmetric arthritis | 2–10 large joints | 1 |
| 5) Nodules | 1–3 small joints | 2 |
| 6) Elevation of rheumatoid factor | 4–10 small joints | 3 |
| 7) Radiographic changes | >10 joints (at least 1 small) | 5 |
| Findings 1–4 must be present for ≥6 weeks. Arthritis must be observed by a physician. | B. Serology (at least 1 test needed) | |
| Negative RF and ACPA | 0 | |
| Low positive RF or ACPA | 2 | |
| High positive RF or ACPA** | 3 | |
| C. Acute-phase reactants (at least one test needed) | ||
| Normal CRP and ESR | 0 | |
| Abnormal CRP or ESR | 1 | |
| D. Duration of symptoms | ||
| <6 weeks | 0 | |
| ≥6 weeks | 1 | |
Categories of joint distribution are classified according to the location and number of involved joints, with placement into the highest category possible based on the pattern of joint involvement.
High positive is equivalent to >3 times the upper limit of normal based on the reference range of the laboratory that assesses the biomarker
With these issues in mind, several studies have evaluated the efficacy of interventions in preventing progression of undifferentiated IA to classifiable RA. In the PRObable Rheumatoid Arthritis: Methotrexate Versus Placebo Treatment (PROMPT) study, 110 patients with undifferentiated IA of <2-year duration were randomized to receive MTX versus placebo, and they were followed for ~18 months for the primary outcome of fulfillment of the 1987 ACR RA classification criteria [41]. Of the MTX-treated patients, 40 % developed RA, as compared with 53 % in the placebo group, although importantly the majority of benefit of MTX in delaying or preventing development of classifiable RA was seen in the anti-CCP positive subgroup of patients. Furthermore, the onset of classifiable RA was later in the MTX-treated group, and radiographic joint damage was also less in MTX-treated subjects.
In the Stop Arthritis Very Early (SAVE) trial, patients with IA of <16-week duration were given a single intramuscular (IM) dose of 120 mg of methylprednisolone versus placebo and were followed for 12 months for the development of RA according to the 1987 criteria [42]. This intervention did not result in any decrease in progression to RA between the study groups. In the STIVEA trial, patients with early IA (4- to 10-week duration) were given three weekly IM injections of 80 mg of methylprednisolone versus placebo and were followed for 12 months [43]. This intervention resulted in the delay of prescription of DMARDs and prevented the development of RA (1987 criteria) in 1 of 10 subjects treated. In the ADJUST trial, 50 patients with undifferentiated IA (two or more swollen joints not fulfilling 1987 RA criteria) were randomized to receive abatacept versus placebo for 6 months, with the primary outcome evaluated being the development of RA (1987 criteria) at 12 months. At 12 months, 12/26 (46 %) of abatacept-treated patients had progressed to RA, as compared with 16/24 (67 %) of placebo-treated subjects, although no statistical comparison was provided for these results.
The results of these studies in preventing the progression from undifferentiated IA to classifiable RA are mixed; however, overall, if the SAVE trial is excluded, it appears that a sustained intervention with an immunomodulatory agent appears to reduce some of the progression of RA. Further studies of interventions in this very early period of arthritis development need to be performed to understand the optimal approach to these patients. Importantly, fulfillment of the 2010 ACR/EULAR criteria also needs to be evaluated, since it is likely that many of the subjects in these studies fulfilled these criteria at baseline.
Prevention of Initial Onset of IA in Subjects Who Have Developed RA-Related Autoimmunity
Moving even further back into the evolution of RA, it may be possible to prevent the future onset of RA in subjects who have developed abnormalities of disease-specific auto-antibodies in the absence of overt IA (Fig. 1). Bos and colleagues attempted such an approach by treating 83 anti-CCP positive subjects with “arthralgias” but no IA based on examination by two rheumatologists with two doses (baseline and 6 weeks) of IM 100 mg dexamethasone versus placebo [44]. Dexamethasone reduced autoantibody titers (ACPAs and rheumatoid factor); however, it did not delay the progression to clinically apparent IA. While this study was not successful in reducing outcomes of IA, it is compelling to think that such an approach may be used to identify individuals at high risk for future RA and then intervene to prevent progression of disease. Perhaps such an intervention may be not pharmacologic, but removal of environmental risk factors? For example, exposure to tobacco smoke is strongly associated with RA, with some estimates that it explains ~30 % of the risk for seropositive RA [45]. On the basis of this, some have proposed that broadly implemented programs for smoking cessation would result in a significant reduction of RA [45]. In addition, recent attention has focused on the potential role of periodontal inflammation and infection with the organism Porphyromonas gingivals in the pathogenesis of RA [46]. If this relationship is truly causal, perhaps treatment of periodontal disease/infection may result in reduced risk for future RA.
However, while autoantibodies seem, in published case–control studies, to predict future RA with a high degree of accuracy, our knowledge is limited regarding the diagnostic accuracy of these antibodies for future RA if testing is implemented in large-scale healthy populations in whom the overall risk for RA is low. Furthermore, we have limited understanding of the mechanisms of early development of RA, and in particular, it is not yet clear what mechanisms of disease development would be most amenable to targeting with specific interventions to prevent the progression of RA-related autoimmunity prior to the first appearance of IA. Going forward, we will need detailed studies of the natural history of RA development in order to develop accurate predictive models for future disease and to identify specific mechanisms of disease development so that these factors can be utilized in preventive approaches for RA.
Conclusions
Growing evidence suggests that early identification and treatment of RA leads to improved outcomes and even improved rates of drug-free remission. The optimal time to identify and treat RA is not known; however, less than 3–6 months of symptoms of IA appears to be a good time period to target for initiation of DMARD therapy, although this target may be difficult to reach due to multiple factors that can affect early diagnosis of RA. The 2010 ACR/EULAR classification criteria appear to identify RA earlier than the 1987 criteria, although the effectiveness of these new criteria in leading to improved outcomes in RA needs further investigation. Additionally, the optimal drug therapy in early RA is not known, although early use of DMARD monotherapy with rapid escalation to combination therapy that may include biologics if disease activity is not controlled and, perhaps, initial combination therapy in patients with severe disease are reasonable therapeutic approaches at this time, although further studies are needed to define what treatment regimens are best for individual patients with early RA.. With the growing understanding of the early natural history of RA and the ability of biomarkers to predict those at future risk for RA, screening programs to identify subjects at high risk for future RA and implementation of preventive strategies for RA that may target specific pathogenic mechanisms of disease development may be in use in the near future.
Acknowledgments
Grant Funding Dr. Demoruelle is supported by grant funding from the NIH (T32AR07534), the Artritis Foundation, and the American College of Rheumatology. Dr. Deane is supported by the NIH (U19AI50864), the American College of Rheumatology, Abbott Laboratories, Inc., and the Walter S. and Lucienne Driskill Foundation.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007;56:433–40. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 2.Aletaha D, Eberl G, Nell VP, Machold KP, Smolen JS. Attitudes to early rheumatoid arthritis: changing patterns: results of a survey. Ann Rheum Dis. 2004;63:1269–75. doi: 10.1136/ard.2003.015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–39. doi: 10.1002/acr.21641. This article provides an expert opinion based on a literature review regarding the management of RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Garcia C, Vargas E, Abasolo L, et al. Lag time between onset of symptoms and access to rheumatology care and DMARD therapy in a cohort of patients with rheumatoid arthritis. J Rheumatol. 2000;27:2323–8. [PubMed] [Google Scholar]
- 5.Amjadi S, Khanna D, Park GS, Bulpitt KJ, Wong WK, Paulus HE. Dating the “window of therapeutic opportunity” in early rheumatoid arthritis: accuracy of patient recall of arthritis symptom onset. J Rheumatol. 2004;31:1686–92. [PubMed] [Google Scholar]
- 6.Aletaha D, Breedveld FC, Smolen JS. The need for new classification criteria for rheumatoid arthritis. Arthritis Rheum. 2005;52:3333–6. doi: 10.1002/art.21410. [DOI] [PubMed] [Google Scholar]
- 7.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 9.Zeidler H. The need to better classify and diagnose early and very early rheumatoid arthritis. J Rheumatol. 2012;39:212–7. doi: 10.3899/jrheum.110967. [DOI] [PubMed] [Google Scholar]
- 10••.Cader MZ, Filer A, Hazlehurst J, de Pablo P, Buckley CD, Raza K. Performance of the 2010 ACR/EULAR criteria for rheumatoid arthritis: comparison with 1987 ACR criteria in a very early synovitis cohort. Ann Rheum Dis. 2011;70:949–55. doi: 10.1136/ard.2010.143560. This retrospective study reports that, in comparison with the 1987 ACR RA criteria, the 2010 ACR/EULAR criteria for RA classifies more patients with early synovitis as having RA; however, the 2010 criteria may also identify subjects as having RA that will have spontaneous disease remission or who will ultimately have an alternative arthritis diagnosis. [DOI] [PubMed] [Google Scholar]
- 11.van der Linden MP, Knevel R, Huizinga TW, van der Helmvan Mil AH. Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheum. 2011;63:37–42. doi: 10.1002/art.30100. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43:22–9. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Cush JJ. Early rheumatoid arthritis—is there a window of opportunity? J Rheumatol Suppl. 2007;80:1–7. [PubMed] [Google Scholar]
- 14.Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004;43:906–14. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 15.van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum. 2009;60:2262–71. doi: 10.1002/art.24661. [DOI] [PubMed] [Google Scholar]
- 16.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lard LR, Visser H, Speyer I, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446–51. doi: 10.1016/s0002-9343(01)00872-5. [DOI] [PubMed] [Google Scholar]
- 18.van der Linden MP, Boja R, Klarenbeek NB, Huizinga TW, van der Heijde DM, van der Helm-van Mil AH. Repair of joint erosions in rheumatoid arthritis: prevalence and patient characteristics in a large inception cohort. Ann Rheum Dis. 2010;69:727–9. doi: 10.1136/ard.2009.108332. [DOI] [PubMed] [Google Scholar]
- 19.Mottonen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46:894–8. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 20.Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 2006;55:864–72. doi: 10.1002/art.22353. [DOI] [PubMed] [Google Scholar]
- 21.van der Linden MP, le Cessie S, Raza K, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62:3537–46. doi: 10.1002/art.27692. [DOI] [PubMed] [Google Scholar]
- 22.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2008;58:S126–35. doi: 10.1002/art.23364. [DOI] [PubMed] [Google Scholar]
- 23.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 24.Emery P, Kvien TK, Combe B, et al. Combination etanercept and methotrexate provides better disease control in very early (<=4 months) versus early rheumatoid arthritis (>4 months and <2 years): post hoc analyses from the COMET study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201066. [DOI] [PubMed] [Google Scholar]
- 25.Mottonen T, Hannonen P, Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353:1568–73. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 26.Mottonen TT, Hannonen PJ, Boers M. Combination DMARD therapy including corticosteroids in early rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:S59–65. [PubMed] [Google Scholar]
- 27.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 28•.Moreland LW, O’Dell JR, Paulus HE, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early, aggressive rheumatoid arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.34498. This study suggests that ~28 % of patients with early RA may have adequate disease response to monotherapy with MTX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374:459–66. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]
- 30•.Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70:469–75. doi: 10.1136/ard.2010.139212. This study suggests that ~34 % of patients with early RA (<1-year duration) may have a good response (as defined by a DAS28) to monotherapy with MTX, although a longer duration of symptoms prior to initiation of MTX resulted in decreased efficacy. [DOI] [PubMed] [Google Scholar]
- 31.Rezaei H, Saevarsdottir S, Forslind K, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2-year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis. 2011 doi: 10.1136/annrheumdis-2011-200038. [DOI] [PubMed] [Google Scholar]
- 32.Raza K, Stack R, Kumar K, et al. Delays in assessment of patients with rheumatoid arthritis: variations across Europe. Ann Rheum Dis. 2011;70:1822–5. doi: 10.1136/ard.2011.151902. [DOI] [PubMed] [Google Scholar]
- 33.Breedveld F. The value of early intervention in RA—a window of opportunity. Clin Rheumatol. 2011;30 (Suppl 1):S33–9. doi: 10.1007/s10067-010-1638-5. [DOI] [PubMed] [Google Scholar]
- 34.Ospelt C, Reedquist KA, Gay S, Tak PP. Inflammatory memories: is epigenetics the missing link to persistent stromal cell activation in rheumatoid arthritis? Autoimmun Rev. 2011;10:519–24. doi: 10.1016/j.autrev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Aho K, Palosuo T, Heliovaara M, Knekt P, Alha P, von Essen R. Antifilaggrin antibodies within “normal” range predict rheumatoid arthritis in a linear fashion. J Rheumatol. 2000;27:2743–6. [PubMed] [Google Scholar]
- 36.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 37.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 38.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36:213–41. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bos WH, Wolbink GJ, Boers M, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis. 2010;69:490–4. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 40.Deane KD, O’Donnell CI, Hueber W, et al. The number of elevated cytokines/chemokines in pre-clinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010 doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Dongen H, van Aken J, Lard LR, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424–32. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 42.Machold KP, Landewe R, Smolen JS, et al. The Stop Arthritis Very Early (SAVE) trial, an international multicentre, randomised, double-blind, placebo-controlled trial on glucocorticoids in very early arthritis. Ann Rheum Dis. 2010;69:495–502. doi: 10.1136/ard.2009.122473. [DOI] [PubMed] [Google Scholar]
- 43.Verstappen SM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DP. Beneficial effects of a 3-week course of intramuscular glucocorticoid injections in patients with very early inflammatory polyarthritis: results of the STIVEA trial. Ann Rheum Dis. 2010;69:503–9. doi: 10.1136/ard.2009.119149. [DOI] [PubMed] [Google Scholar]
- 44.Bos WH, Dijkmans BA, Boers M, van de Stadt RJ, van Schaardenburg D. Effect of dexamethasone on autoantibody levels and arthritis development in patients with arthralgia: a randomised trial. Ann Rheum Dis. 2010;69:571–4. doi: 10.1136/ard.2008.105767. [DOI] [PubMed] [Google Scholar]
- 45.Klareskog L, Gregersen PK, Huizinga TW. Prevention of autoimmune rheumatic disease: state of the art and future perspectives. Ann Rheum Dis. 2010;69:2062–6. doi: 10.1136/ard.2010.142109. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg K, Kinloch A, Fisher BA, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–19. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]


