Abstract
Endogenous thymic regeneration is a crucial function that allows for renewal of immune competence after stress, infection or immunodepletion. The mechanisms governing this regeneration, however, remain poorly understood. Here we detail a framework of thymic regeneration centred on IL-22 and triggered by depletion of CD4+CD8+ double positive (DP) thymocytes. Intrathymic levels of IL-22 were increased following thymic insult, and thymic recovery was impaired in IL-22-deficient mice. IL-22, which signalled through thymic epithelial cells (TECs) and promoted their proliferation and survival, was upregulated by radio-resistant RORγ(t)+CCR6+NKp46− lymphoid tissue-inducer cells (LTi) after thymic injury in an IL-23 dependent manner. Importantly, administration of IL-22 enhanced thymic recovery following total body irradiation (TBI). These studies reveal mechanisms of endogenous thymic repair and offer innovative regenerative strategies for improving immune competence.
Despite being exquisitely sensitive to insult, the thymus is remarkably resilient in young healthy animals. However, thymic renewal after immune depletion is a prolonged process, particularly in elderly patients, which substantially impairs the recovery of adaptive immunity (1, 2). This period of prolonged immune deficiency leads to an increase in opportunistic infections and higher treatment-associated morbidity and mortality (2, 3).
Thymopoiesis is a complex process involving cross-talk between developing thymocytes and the non-hematopoietic supporting stromal microenvironment, which is comprised of specialized thymic epithelial cells (TECs), endothelium, fibroblasts and dendritic cells (DCs) (4, 5). TECs can be separated into two populations, cortical TECs (cTECs) and medullary TECs (mTECs), which differ in their spatial location and function within the thymus (4–6). IL-22 is primarily associated with the maintenance of barrier function and induction of innate antimicrobial molecules at mucosal surfaces (7, 8). The principal sources of IL-22 are T helper 17 cells and innate lymphoid cell (ILC) subsets (9–12). Given its role in both promoting and reducing autoimmune pathology within epithelial compartments (8), we hypothesized that IL-22 would mediate epithelial regeneration after thymic injury.
At baseline, untreated wildtype (WT) and mice genetically deficient in IL-22 (Il22−/−) (13) demonstrated no difference in total thymic cellularity or in numbers of the various thymic cell populations (fig. S1A–D). To explore the effects of IL-22 deficiency on thymic regeneration after insult (14), WT or Il22−/− mice were given sublethal total body irradiation (SL-TBI). Il22−/− mice demonstrated significantly impaired thymic regeneration for up to 28 days after SL-TBI (fig. 1A) with significantly reduced numbers of all developing thymocyte subsets, TECs and non-TECs (including endothelial cells and fibroblasts) (fig. 1B–D). We also performed syngeneic hematopoietic stem cell transplantation (HSCT) or allogeneic HSCT and in both cases observed significantly reduced thymic cellularity and reduced numbers of all thymic cell subsets in Il22−/− hosts (fig S2). Upon long-term follow-up, we found that impaired thymic regeneration in Il22−/− mice persisted for up to 98 days after TBI (fig. 1E). Il22−/− mice given a targeted dose of radiation to the thymus also exhibited significantly reduced thymic regeneration compared to WT controls at day 7 (fig. 1F), suggesting that the systemic damage of TBI is not required for the impacts of IL-22 deficiency on thymic regeneration.
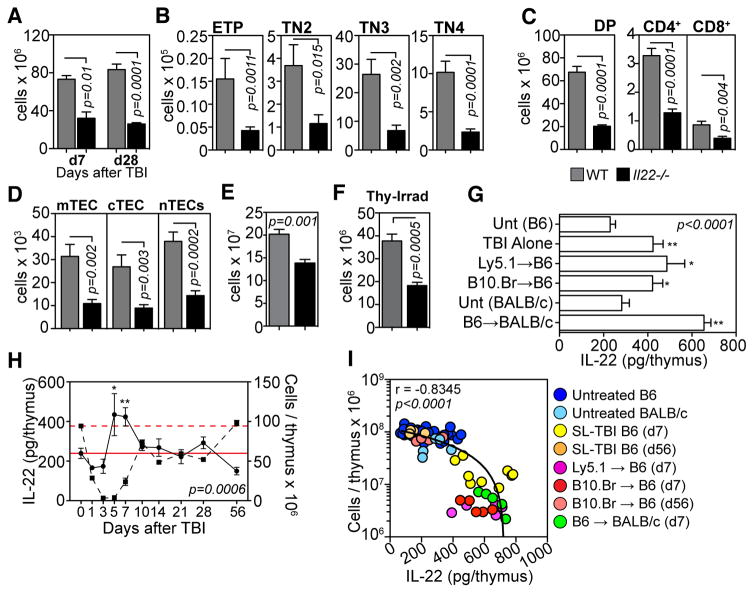
Fig. 1.
IL-22 is critical for endogenous thymic regeneration and is upregulated upon thymic damage. A–D, WT (grey bars, n=11) and Il22−/− (black bars, n=11) C57Bl/6 mice were given SL-TBI (550 cGy) with no hematopoietic rescue and enzyme-digested thymus analyzed. Total thymic cellularity at days 7 and 28 after TBI (A), and developing thymocyte (B–C) and stromal cell subsets (D) 28 days after SL-TBI. E, Total thymus cellularity in WT (n=5) or Il22−/− (n=6) mice 98 days after SL-TBI. F, Total thymus cellularity seven days after targeted thymic-irradiation (850 cGy) of WT (n=10) or Il22−/−(n=7) mice. G, Absolute amounts of intrathymic IL-22 were measured by ELISA in untreated C57BL/6 (n=22), untreated BALB/c (n=5) or 7 days after SL-TBI without HSCT (550 cGy, n=15) or L-TBI and syngeneic HSCT (C57Bl/6 HSCs into congenic C57Bl/6 hosts, 2 × 550 cGy, n=10) or T cell depleted allogeneic-BMT (B10.BR HSCs into MHC-mismatched C57Bl/6 hosts, 2 × 550 cGy, n=10; or C57Bl/6 HSCs into MHC-mismatched BALB/c hosts, 2 × 425 cGy, n=5). H, Absolute amounts of IL-22 (solid circle) plotted with total thymic cellularity (dashed square) over time following SL-TBI (n=5–10/timepoint). Dashed and solid red lines represent mean cellularity and IL-22 amounts respectively at baseline. I, Spearman correlation between absolute amounts of intrathymic IL-22 and total thymic cellularity in various models of thymic insult. Bar graphs represent mean ± SEM of at least 2–3 independent experiments.
Thymic IL-22 production was measured in mice seven days after SL-TBI without HSCT, lethal TBI and syngeneic HSCT or T cell depleted allogeneic-HSCT. In each of these models we found a 2–3-fold increase in absolute amounts of IL-22 compared to control mice that were not irradiated (fig. 1G). This was striking given the significant decrease in thymic cellularity seen in irradiated mice (fig. S3A) leading to a profound increase in the amount of IL-22 on a per cell basis (fig. S3B). Absolute amounts of IL-22 peaked on day 5, corresponding closely with the lowest point of thymic cellularity, and returned to normal amounts by day 10 as thymic cellularity returned to baseline (fig. 1H). These findings revealed an inverse correlation (r=−0.8345) between thymic size and absolute amount of intrathymic IL-22 (fig. 1I). We next titrated the radiation dose to further explore the coupling between thymic cellularity and IL-22. Although increasing doses of radiation led to more severe thymic insult (fig. S3C), peak absolute amounts of IL-22 were achieved at the lowest TBI dose (fig. S3D), which suggests that only a partial loss of thymic cellularity is necessary for increased expression of IL-22. Importantly, mice given a range of radiation doses targeted directly to the thymus also significantly increased their intrathymic amounts of IL-22 (fig. S3E). In these same mice there was no change in the amounts of IL-22 in the spleen after thymic irradiation suggesting that upregulation of intrathymic IL-22 is an intrinsic local response to thymic injury.
ILCs that express the transcription factor RORγ(t) have been identified as potent producers of IL-22 (11, 15). Moreover, CD4+CD3− thymic LTi contribute towards TEC development and maturation (16). Three days after L-TBI (with no hematopoietic rescue) we identified a population of CD45+IL-7Rα+CD3−CD8−RORγ(t)+ thymic ILCs (tILC) that upregulated their production of IL-22 (fig. 2A). No IL-22 expression was found by CD3+ or CD45− populations (fig. S4A–C). Closer examination revealed that IL-22-producing tILCs in both untreated and TBI-treated mice uniformly expressed CD4 and CCR6 but not NKp46 (fig. 2B), a phenotype consistent with that of LTi cells (15). Apart from its role in ILC function, RORγ(t) is critical for thymocyte development and is widely expressed in the thymus (17). Mice deficient for Rorc, the gene encoding for RORγ(t), contain normal amounts of intrathymic IL-22 (fig. 2C) at baseline indicating that steady-state amounts of intrathymic IL-22 do not require RORγ(t) or LTi. However, in contrast to WT mice, Rorc−/− mice do not significantly increase their intrathymic amounts of IL-22 in response to TBI (fig. 2C) suggesting that RORγ(t)+ LTi are critical for intrathymic upregulation in the production of IL-22 after thymic damage. Thymic LTi were present immediately after radiation (fig. 2D), indicating they are radio-resistant for the period when the upregulation of IL-22 is crucial for thymic regeneration, and could persist for up to three months after L-TBI and HSCT (fig. S4D). Furthermore, given the severe depletion of thymus cellularity early after TBI, their frequency increased significantly after L-TBI (fig. 2E). After TBI, LTi also increased their expression of RANKL (figs. 2F and S4E), which has been reported to aid TEC maintenance and regeneration (16).
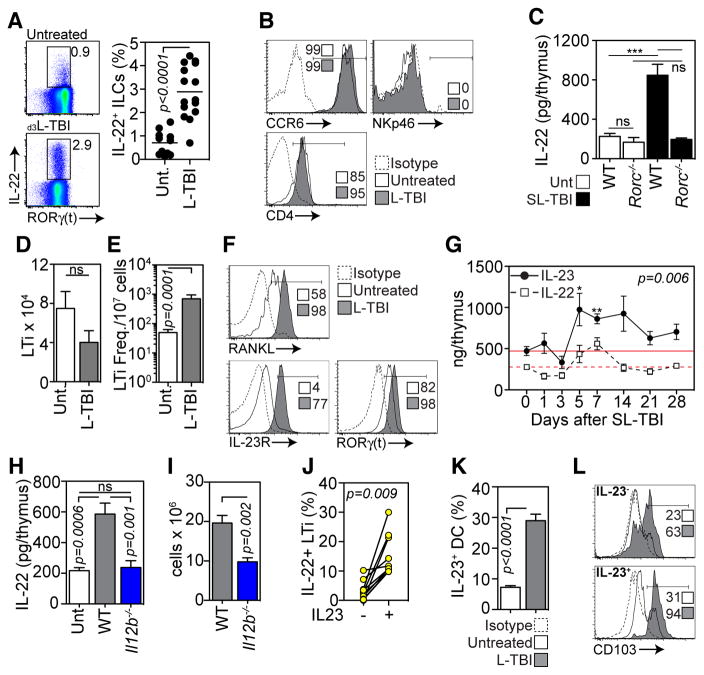
Fig. 2.
IL-22 is produced by intrathymic ILCs under the control of IL-23. A–B, Enzyme-digested thymus from untreated (n=11) or three-days after L-TBI (n=15) was incubated with Brefeldin A (3μg/ml) for 4 hours, but otherwise remained unstimulated. A, Intracellular expression of IL-22 and RORγ(t) by CD45+IL-7Rα+CD3−CD8− tILCs in untreated or L-TBI animals. B, Expression of CCR6, NKp46 and CD4 on IL-22 producing tILCs. C, IL-22 levels measured by ELISA in thymus of untreated mice or 7 days after SL-TBI in WT or Rorc−/− mice. Absolute number (D) and frequency (E) of CD45+IL-7Rα+CD3−CD8−CD4+RORγ(t) LTi in untreated mice (n=25) or 3 days after L-TBI (n=10). F, Expression of RANK ligand (RANKL), IL-23R and RORγ(t) in LTi from untreated mice or 3 days after L-TBI. G, C57Bl/6 mice were given SL-TBI (550 cGy) and absolute levels of IL-23 (solid circle) were measured by ELISA at days 1, 3, 5, 7, 10, 14 and 21 (n=5/timepoint). Compared with IL-22 kinetics (dashed square) taken from fig. 1H. H–I, Absolute IL-22 levels measured by ELISA (G) and total thymic cellularity (H) in untreated mice (n=11) or 7 days after SL-TBI in WT (n=10) or Il12b−/− (n=8) animals. J, Untreated WT thymus was enzyme-digested and incubated +/− IL-23 (60ng/ml) for 4 hours. After 1 hour of IL-23 incubation, Brefeldin A was added to all wells. IL-22 expression was examined in CD45+CD3−CD8−CD4+IL7Rα+RORγ(t)+ LTi. K, Untreated (n=10) or 3 days after L-TBI (n=10) thymus cells were incubated for 4 hours in Monensin (2μM), but otherwise remained unstimulated. Intracellular IL-23 expression in thymic DCs (CD45+CD11c+MHCII+) was measured. L, Expression of CD103 on IL-23-and IL-23+ thymic DCs in untreated and L-TBI mice. Bar graphs represent mean ± SEM of 2–3 independent experiments. FACS plots were generated by concatenation of at least 5 individual observations from one of at least 2 independent experiments.
Regulation of IL-22 production has been closely associated with DC-produced IL-23, and ex vivo incubation of ILCs with IL-23 stimulates production of IL-22 (13, 18–20). Three days after L-TBI we found increased expression by LTi of IL-23R and RORγ(t) (figs. 2F and S4E), consistent with its importance in regulating IL-22 (21, 22). We then assessed intrathymic amounts of IL-23 in vivo and observed increased IL-23 production after SL-TBI, mirroring the kinetics of IL-22 (fig. 2G). Mice genetically deficient in Il12b, the gene that encodes the p40 subunit of IL-12 and IL-23, showed no change in IL-22 production (fig. 2H) and exhibited a defect in thymic regeneration after SL-TBI (fig. 2I), demonstrating that intrathymic TBI-induced production of IL-22 requires p40. Consistent with this, IL-22 expression was increased by thymic LTi after IL-23 stimulation in vitro (fig. 2J).
We next sought to identify the source of elevated intrathymic IL-23 after TBI. Although some thymic DCs expressed IL-23 at baseline, a greater frequency expressed IL-23 after L-TBI (fig. 2K). IL-23 expression was found in both CD103+ and CD103− thymic DCs in untreated mice; however, there was significant enrichment of IL-23+ thymic DCs expressing CD103 (fig. 2L) in irradiated animals. This is consistent with the finding that mucosal CD103+ DCs are potent IL-23 producers (23).
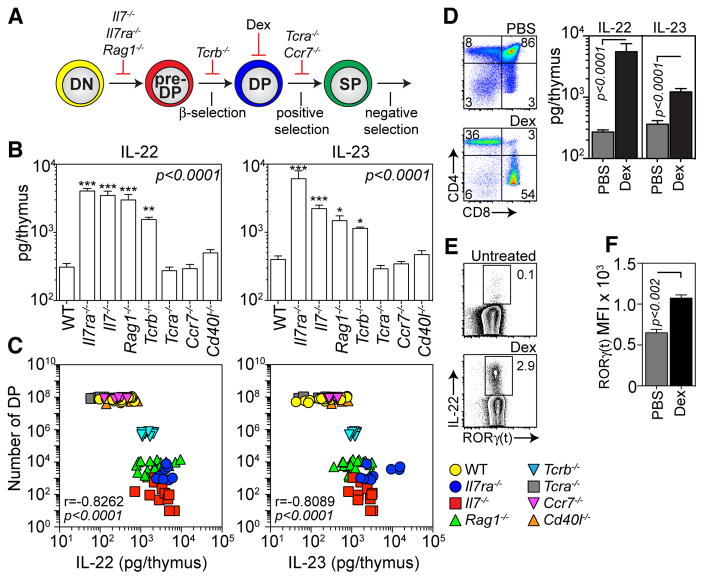
To further explore the relationship between IL-22 and thymocyte cellularity (fig. 1G), mutant animals with well-defined blocks in intrathymic T cell development (24) were examined for production of IL-22 and IL-23 (fig. 3A). Mice blocked within the CD4-CD8− double negative (DN) stage of thymocyte differentiation, prior to developing CD4+CD8+ double positive (DP) thymocytes, expressed significantly more intrathymic IL-22 and IL-23 than WT controls (fig. 3B). In contrast, mice deficient for TCRα or CCR7, which lack mature CD4 or CD8 single positive (SP) thymocytes but have no loss of DP thymocytes (5, 24), exhibited no upregulation of IL-22 and IL-23 (fig. 3B). Stable intrathymic IL-22 and IL-23 were also observed in mice deficient for CD40 ligand (Cd40l−/−) (fig. 3B), which have a defect in mTECs, but normal numbers of DP and SP thymocytes (25). Consequently, there was a strong inverse correlation between the number of DP thymocytes and amounts of intrathymic IL-22 and IL-23 (fig. 3C), further suggesting that depletion or absence of DP leads to upregulation of IL-22 and IL-23. This was confirmed by treatment with dexamethasone (Dex), which specifically depletes DP thymocytes (fig. 3D) (26), and led to upregulation of IL-22 and IL-23 in WT mice (fig. 3D). Strikingly, increased IL-22 expression was detected in freshly isolated LTi from Dex-treated mice without incubation, in stark contrast to the low/un-detectable levels in untreated mice (fig. 3E). Furthermore, consistent with our findings in the TBI model, we observed significantly increased expression of RORγ(t) in LTi isolated from Dex-treated mice compared to untreated controls (fig. 3F). Although IL-7 signaling has been implicated in LTi maintenance (27), similar numbers of LTi were found in Il7−/− and Rag1−/− mice, and there was an increase in Il7ra−/− mice (fig. S4F). Furthermore, both the frequency of LTi (fig. S4G) and their baseline production of IL-22 (fig. S4H) was increased compared to WT controls. In all our models of thymic damage and mutant mouse strains there was a strong correlation (r=0.9554) between amounts of thymic IL-22 and IL-23 (fig. S5).
Fig. 3.
Absence of CD4+CD8+ double positive thymocytes triggers the upregulation of IL-23 and IL-22. A–C, Mutant mouse strains with blocks at different stages of T cell development were assessed for their production of IL-22 and IL-23. A, Schematic of T cell developmental stage blocked in various mutant strains/methods used. B, Absolute IL-22 and IL-23 at baseline in thymus of untreated WT (n=15), Il7Ra−/− (n=9), Il7−/− (n=11), Rag1−/− (n=22), Tcrb−/− (n=10), Tcra−/− (n=18), Ccr7−/− (n=6) and Cd40l−/− (n=10) mice. Statistical comparisons were made with the Kruskal-Wallis test with post-test comparison to WT controls. C, Spearman correlation between number of DP thymocytes and amounts of IL-22 or IL-23 in various mutant mouse strains. D–F, C57Bl/6 mice were treated with PBS (n=10) or Dex (20mg/kg, n=11). D, Thymocyte profiles and absolute amounts of thymus IL-22 and IL-23 were assessed 5 days after treatment. E, Freshly isolated LTi from untreated WT (n=12) or Dex-treated (n=13) mice were analyzed for intracellular IL-22 with no incubation period. F, Mean Fluorescence Intensity (MFI) of RORγ(t) in LTi isolated from untreated or Dex-treated mice. Bar graphs represent mean ± SEM and all data is generated from 2–3 independent experiments. FACS plots were generated by concatenation of at least 5 individual observations from one of at least 2 independent experiments
IL-22R is a heterodimer of IL-10Rβ and IL-22Rα (8). Its expression has been reported to be restricted to non-hematopoietic cells (8). No IL-22Rα was detectable on developing thymocytes or non-epithelial stromal cells (fig. S6A). In contrast, IL-22Rα was expressed on cTECs as well as MHC class II high and low expressing mTECs(mTEChi and mTEClo respectively), a marker of TEC maturation (25) (Fig. 4A). To test if IL-22 could functionally signal through IL-22R expressed by TECs, the TE-71 TEC cell line was stimulated with IL-22. Consistent with mucosal epithelia (28), IL-22 stimulation of TE-71 cells led to activation-induced phosphorylation of STAT-3 and STAT-5 (fig. S6B, 29).
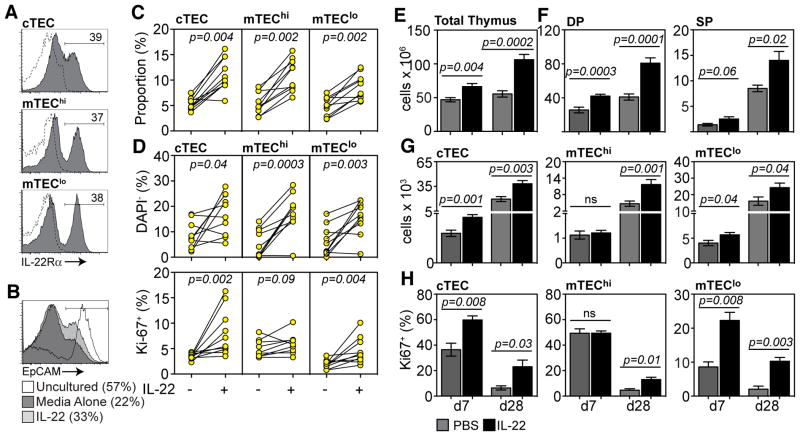
Fig. 4.
Exogenous administration of recombinant murine IL-22 enhances thymopoiesis by promoting the proliferation and viability of TECs. A, WT thymus was enzyme-digested and enriched for CD45− cells. Expression of IL-22Rα on cTECs (UEA-1lo), mTEClo (UEA-1hiMHCIIlo) and mTEChi (UEA-1hiMHCIIhi). All populations gated on CD45−EpCAM+. B–D, CD45− or MHC-II+ enriched thymus cells were incubated for 24 hours +/− IL-22 (100ng/ml). B, Expression of EpCAM in uncultured CD45− cells (n=5) and in CD45− cells incubated for 24 hours with IL-22 (n=10) or media alone (n=10). C, Proportion of specific TEC subsets in CD45− cells incubated for 24 hours +/− IL-22. D, Expression of DAPI and Ki-67 on TEC subsets on MHCII-enriched thymus cells after 24 hours of incubation with IL-22 (n=10) or media alone (n=10). For in vitro experiments with enriched cells, each individual observation represents 3–4 pooled thymuses. E–H, C57Bl/6 mice were given SL-TBI (550 cGy), treated with PBS (grey bars, n=10) or IL-22 (black bars, 200μg/kg/day, n=10–15) at days −1, 0 and +1 and assessed at days 7 and 28. Total thymus cellularity (E) and absolute number of thymocyte (F) and TEC subsets (G). H, Proportion of Ki-67 expressing cTECs, mTEClo and mTEChi. Bar graphs represent mean ± SEM of at least 2 independent experiments. FACS plots were generated by concatenation of at least 5 individual observations from one of at least 2 independent experiments.
To assess the impact of IL-22 on primary TECs, CD45− cells were enriched and incubated with IL-22 or media alone for 24 hours. Although there was significant attrition of EpCAM expression in untreated cells, those treated with IL-22 maintained greater EpCAM expression, representing viability, in culture (fig. 4B–C). Indicative of this, the presence of IL-22 improved TEC survival and increased proliferation of cTECs and mTEClo (fig. 4D). There was no change in expression of apoptosis-related Annexin V or Bcl-2 proteins (fig. S6C). These findings demonstrate that IL-22 signals through IL-22R on the surface of TECs, and in particular in cTECs and mTEClo. It is within this latter population that immature mTEC populations are currently thought to reside (6). Although it is possible that IL-22 acts as a maturation signal for mTECs, it is more likely that IL-22 primarily functions to induce proliferation and viability, given the uniform expression of IL-22R on immature mTEClo and mature mTEChi, and given the preferential promotion of proliferation amongst mTEClo.
To examine the clinical effectiveness of IL-22 as a regenerative strategy, recombinant IL-22 was administered to mice after SL-TBI. We found significantly increased thymic cellularity at days 7 and 28, compared to controls, in mice receiving SL-TBI (fig. 4E). Increases were also observed in all developing thymocyte subsets (fig. 4F) and TEC subsets (fig. 4G). There was also a significant increase in the proliferation of cTECs and mTEClo at early timepoints after IL-22 treatment (fig. 4H). Importantly, IL-22-treated animals receiving L-TBI in combination with syngeneic HSCT showed significantly enhanced thymic recovery at day 7 (fig. S7). In otherwise untreated animals given IL-22, we observed no change in total thymic cellularity, although there was a small increase in cTEC and mTEClo proliferation (fig. S8).
These studies suggest that after thymic injury, and specifically the depletion of DP thymocytes, upregulation of IL-23 by radio-resistant CD103+ thymic DCs induces IL-22 production by tILCs. This cascade of events leads to regeneration of the supporting epithelial microenvironment and, ultimately, to enhanced thymopoiesis (fig. S9). We have previously demonstrated in knockout studies that keratinocyte growth factor is also required for thymic regeneration and is redundant for thymic ontogeny (30). In this instance, however, depletion of DP cellularity triggers a thymic molecular network to aid in its own regeneration. Interestingly, once the thymus has been restored, IL-22 production stabilizes. Consistent with this, administration of IL-22 was a highly effective regenerative strategy after radiation damage, but had little effect in untreated mice or those with significant recovery after syngeneic HSCT.
Prolonged thymic deficiency after cytoreductive conditioning is a significant clinical challenge. These studies not only identify a mechanism governing endogenous thymic regeneration, but also offer an innovative therapeutic strategy for immune regeneration in patients whose thymus has been irrevocably damaged.
Supplementary Material
Summary.
Here we demonstrate a critical role for RORγ(t)+ lymphoid tissue-inducer cell-produced IL-22 in endogenous thymic repair.
Acknowledgments
We thank A. Farr for the gift of the TE-71 TEC cell line; D. Littman, W. Ouyang, A. Beaulieu, J. Sun and S. Prockop for the gift of mice; R. Essner and D. Schwartz for technical assistance; E. Velardi and Y. Shono for helpful discussion; L. Reilly, B. Morcerf and B. Rojas for administrative assistance. This work was supported by National Institutes of Health R01 awards HL069929, CA107096, AI080455 and HL095075. Support was also received from the US Department of Defense USAMRAA Award W81XWH-09-1-0294, the Radiation Effects Research Foundation (RERF-NIAID), The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center (funded by Mr. William H. Goodwin and Mrs. Alice Goodwin), The Lymphoma Foundation, Alex’s Lemonade Stand, and The Peter Solomon Fund. J.A.D. was supported by fellowships from the Australian National Health and Medical Research Council (CJ Martin Biomedical Research Fellowship, 1012401) and the Leukemia and Lymphoma Society (5534-11). A.M. Ha was supported by a Research Training Award for Fellows from the American Society of Hematology and a New Investigator Award from the American Society for Blood and Marrow Transplant. A.G. was supported by supported by a fellowship from the American Association of Cancer Research (Judah Folkman Fellowship). The data reported in this paper are tabulated in the main paper and in the Supporting Online Materials. A provisional patent application has been filed on the use of IL-22 as a thymopoietic growth factor (US 61/487,517) with J.A.D, A.M. Ha and M.R.M.vdB listed as inventors.
Footnotes
References and Notes
- 1.Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leuk Biol. 2008 Oct;84:915. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004 Nov;4:856. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 3.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995 Sep;16:413. [PubMed] [Google Scholar]
- 4.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006 Feb;6:127. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 5.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 6.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007 Oct 29;204:2521. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009 May;87:451. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 9.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011 Jan;12:21. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 10.Sawa S, et al. RORγ(t)+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 11.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009 Feb 5;457:722. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009 Jan 16;206:35. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supporting material on Science Online
- 15.Sawa S, et al. Lineage Relationship Analysis of RORγ(t)+ Innate Lymphoid Cells. Science. 2010 Oct 29;330:665. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 16.Rossi SW, et al. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007 Jun 11;204:1267. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000 Jun 30;288:2369. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4+ Lymphoid Tissue-Inducer Cells Promote Innate Immunity in the Gut. Immunity. 2011;34:122. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010 Apr 29;464:1371. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerosa F, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008 Jun 9;205:1447. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004 Jan;5:64. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 22.Sanos SL, et al. RORγ(t) and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009 Jan;10:83. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010 Apr 23;32:557. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak TW, Penninger JM, Ohashi PS. Knockout mice: a paradigm shift in modern immunology. Nat Rev Immunol. 2001;1:11. doi: 10.1038/3509551. [DOI] [PubMed] [Google Scholar]
- 25.Gray DH, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006 Dec 1;108:3777. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 26.Purton JF, et al. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J Immunol. 2004 Sep 15;173:3816. doi: 10.4049/jimmunol.173.6.3816. [DOI] [PubMed] [Google Scholar]
- 27.Kim MY, et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005 Jun 1;174:6686. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 28.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009 Jul 6;206:1465. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanai M, et al. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK-dependent pathway. Oncogene. 2003 Jan 30;22:548. doi: 10.1038/sj.onc.1206109. [DOI] [PubMed] [Google Scholar]
- 30.Alpdogan O, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006 Mar 15;107:2453. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray DH, et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008 Jan 1;329:56. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.