Abstract
Invariant natural killer T (iNKT) cells are innate lymphocytes with unique specificity for glycolipid antigens and remarkable immunomodulatory properties. The role of costimulatory interactions in iNKT cell responses has recently come under scrutiny. Although iNKT cells and their prototype glycolipid agonist α-galactosylceramide (α-GalCer) have shown promise in several clinical trials conducted in patients with cancer or viral diseases, current iNKT cell-based therapies are far from effective. The concomitant targeting of T cell receptors (TCRs) and costimulatory molecules on iNKT cells represents an exciting new opportunity to optimize such therapeutic approaches. Here, we review recent advances in our understanding of iNKT cell costimulation and discuss potential treatment modalities based on the responsiveness of iNKT cells to disease-tailored glycolipids and select costimulatory ligands.
NKT cells: a brief overview
Definition, subsets and localization
NKT cells constitute a numerically minor but functionally prominent subpopulation of lymphocytes that were initially defined based on their simultaneous expression of NK cell markers (e.g. mouse NK1.1 or DX5 and human CD161) and TCRs [1,2]. Although this definition still holds true for the vast majority of NKT cells, it is no longer considered precise because certain conventional T cell populations such as CD8+ T cells can also express NK cell markers upon activation [3]. In addition, the expression level of NK cell markers by NKT cells varies in accordance to their maturation and activation states [4]. NKT cells are now defined based on the unique restriction of their TCRs by CD1d, a nonpolymorphic major histocompatibility complex (MHC) class I-like glycoprotein that presents glycolipid molecules to NKT cells.
Similar to their conventional counterparts, NKT cells develop in the thymus and express an αβ TCR [2]. However, unlike conventional T cells that are positively selected by cortical thymic epithelial cells and recognize peptide:MHC complexes, the positive selection of NKT cells depends on CD4+CD8+ thymocytes that express CD1d. The identity of endogenous CD1d-restricted glycolipid antigens participating in the positive selection of NKT cells and possibly contributing to the maintenance of their partially activated phenotype remains elusive. Furthermore, whether cells of the NKT lineage undergo negative selection in the thymus to eliminate autoaggressive cells has yet to be established.
CD1d restriction is the cornerstone of NKT cell development and responsiveness. The advent of CD1d tetramer reagents loaded with NKT cell glycolipid ligands has allowed the accurate detection, enumeration and functional characterization of these cells [5,6]. Most CD1d-restricted NKT cells express an invariant TCRα chain exhibiting the characteristic Vα14-Jα18 and Vα24-Jα18 gene rearrangements in mice and humans, respectively [1]. This α chain pairs with a limited set of TCRβ chains (Vβ8.2, Vβ2 or Vβ7 in mice and Vβ11 in humans), thereby defining type I or “invariant” NKT (iNKT) cells. iNKT cells recognize and respond to the marine sponge-derived glycolipid α-GalCer. A smaller and relatively poorly studied subset of CD1d-restricted NKT cells, which are known as type II or variant NKT (vNKT) cells, express a diverse TCRαβ repertoire and fail to recognize α-GalCer [2]. In this review, we focus on iNKT cells and their potential for immunotherapy.
Mouse iNKT cells are categorized into CD4+CD8– and CD4–CD8– double negative (DN) subsets. An additional CD4–CD8+ subset exists in humans [7]. iNKT cells occur in low abundance in blood and in various tissues including the thymus, bone marrow, spleen and lymph nodes. Exceptions include the mouse liver and human omentum, which house unusually large numbers of iNKT cells [8]. Importantly, iNKT cell subsets found in different locations are functionally heterogeneous [7,9–12].
Means and modes of activation
The canonical TCR of iNKT cells (iTCR) recognizes glycolipid antigens in the context of CD1d, which is expressed by a variety of cell types including professional antigen-presenting cells (pAPCs). Certain microbial glycolipids such as those derived from Novosphingobium (formerly Sphingomonas) spp., Ehrlichia spp. and Borrelia burgdorferi induce iNKT cell activation in a TCR-dependent fashion [13]. However, α-GalCer is the most widely studied ligand for mouse and human iNKT cells. Initially found in an extract of Agelas mauritanius, α-GalCer might have originated from microorganisms symbiotic with this marine sponge [14]. Although α-GalCer is not a natural mammalian product, it has been employed extensively as an experimental tool to study iNKT cells and has also been used in clinical trials.
Almost all iNKT cell antigens have a lipid tail that is buried deep within the hydrophobic pocket of CD1d as well as a sugar head that protrudes out of CD1d and is accessed by the iTCRα chain [15]. Unlike conventional TCRs, whose α and β chains are both involved in cognate peptide recognition near the centre of the MHC platform, iTCR is rotated clockwise and pushed laterally, thereby allowing only the α chain to make contact with the galactose ring of α-GalCer; the β chain helps stabilize the iTCR–CD1d interaction [15]. The relative diversity of the iTCRβ chain allows iNKT cells to detect distinct structural features of various CD1d-restricted glycolipid antigens [16]. The length of both acyl and phytosphingosine chains of α-GalCer analogs controls the stability of CD1d binding [17]; however, the binding affinity of the iTCR for α-Gal-Cer:CD1d complexes is influenced by the length of the phytosphingosine chain [17]. This might at least partially explain the distinct cytokine responses elicited by iNKT cells stimulated with glycolipid antigens containing the same sugar head but different lipid tails.
iNKT cells can also be activated by bacteria lacking iTCR ligands [18]. This indirect activation mode is typically mediated by dendritic cells (DCs) secreting proinflammatory cytokines in response to bacterial components such as Toll-like receptor (TLR) ligands and might also require the engagement of iTCR by endogenous glycolipids. Interleukin (IL)-12 and IL-18 can also directly activate iNKT cells in a truly TCR-independent manner [19].
Roles in immune responses and regulation
iNKT cells are armed with a lethal arsenal of molecular weapons including perforin, granzymes, tumor necrosis factor (TNF)-α, Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL), and are likely to be directly involved in destroying malignant cells and clearing microbial pathogens [20–22]. The immunomodulatory properties of iNKT cells are mainly attributable to their ability to transactivate a wide range of downstream effector cells including DCs, macrophages and NK, T and B cells. As such, iNKT cells not only participate in innate host defense but also assist in adaptive immune responses [1].
iNKT cells contain preformed mRNAs encoding T helper (TH)1-type cytokines typified by interferon (IFN)-γ as well as TH2-type cytokines (e.g. IL-4 and IL-13), and quickly secrete enormous quantities of these cytokines following antigenic stimulation [23]. In mice and humans, iNKT cell subsets demonstrate distinct cytokine profiles. For instance, although CD4+ iNKT cells can secrete both TH1-and TH2-type cytokines, DN and CD8+ subsets in humans preferentially produce TH1-type cytokines [7,9,10].
The pro- versus anti-inflammatory nature of immune responses promoted or modulated by iNKT cells depends on the type of cytokines they secrete, which is in turn influenced by the structure and pharmacokinetic properties of iNKT cell glycolipid ligands and the cell membrane location of their CD1d-mediated presentation [24], the binding affinity of iTCR for these ligands, the costimulatory and danger signals received by iNKT cells and the cytokine milieu and anatomical sites where iNKT cell subsets are primed.
Costimulatory interactions in iNKT cell responses
General concepts and functional outcomes
At least two signals are needed for conventional T cell activation leading to their proliferation, extended survival, cytokine secretion and differentiation into effector cells [25–27]. Signal 1 is antigen-specific and emanates from peptide:MHC–TCR interactions. This signal might trigger a response by effector and memory T cells but is not sufficient for the optimal activation of naïve T cells. In fact, TCR engagement on naïve T cells in the absence of a costimulatory signal (also known as signal 2) might lead to anergy or apoptotic death. Anergic T cells fail to mount productive responses to subsequent encounters with their cognate antigen even under optimal conditions.
Signal 2 is generated when CD28 on T cells is engaged by B7.1 and/or B7.2, which are abundantly expressed by pAPCs [25]. Cell surface molecules other than CD28 can also contribute to T cell costimulation. The ligation of costimulatory molecules, typically of CD28, can result in lipid raft aggregation and immunological synapse optimization [28,29], the upregulated expression of antiapoptotic proteins such as Bcl-xL [30], enhanced IL-2 transcription and mRNA stability [31] and increased glucose uptake and glycolysis [32]. These changes are consistent with these molecules promoting T cell survival, growth and sustained responsiveness and they help T cells meet a suddenly increased demand for energy. By contrast, several cell surface proteins function as negative costimulatory molecules (also termed coinhibitory molecules) to prevent or dampen T cell responses [33]. These coinhibitors are best exemplified by cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed death (PD)-1 and B and T lymphocyte attenuator (BTLA).
Although iNKT cells express several costimulatory and coinhibitory molecules shared by conventional T cells, the engagement of these molecules on the two cell types might not yield similar functional outcomes. The pre-activated or “memory-like” phenotype of iNKT cells, which is evident even in germ-free animals [34] and human cord blood [35], together with their expression of preformed mRNAs for various cytokines [23] suggests that they might have a lower threshold for activation compared with naïve T cells. Also importantly, anergy is defined differently in iNKT cells than in conventional T cells. In iNKT cells, a single dose of α-GalCer induces rapid cytokine secretion followed by a robust proliferative burst and then a homeostatic contraction phase when most iNKT cells die; the remaining cells acquire a long-term anergic state [36]. Anergic iNKT cells neither proliferate nor release IFN-γ upon re-exposure to α-GalCer, but they partially retain the ability to produce IL-4. Although cytokine secretion by α-GalCer-activated iNKT cells is likely to depend on the balanced delivery of costimulatory and coinhibitory signals [37], whether the presence of such signals during the initial glycolipid priming dictates the course and extent of subsequent iNKT cell anergy is unknown.
Several members of the immunoglobulin (Ig) superfamily, the TNF receptor (TNFR)/TNF superfamily and the transmembrane (or T cell) Ig and mucin domain (TIM) family costimulate iNKT cells either positively or negatively (Table 1 and Figure 1). Understanding how these molecules control the regulatory performance of iNKT cells might reveal attractive targets for therapeutic intervention.
Table 1.
Expression of costimulatory and coinhibitory molecules by conventional T cells and iNKT cells
| Family | Type | Molecule | Cell Surface Expression |
Ligand(s) | Ligand Expression | References | |
|---|---|---|---|---|---|---|---|
| iNKT cells | Conventional T cells | ||||||
| IgSF | Costimulatory | CD28 | + | + | B7.1 (CD80) B7.2 (CD86) |
APCs (↑) Activated conventional T cells (↑) APCs (+,↑) |
[25,38,41,43] |
| ICOS (CD278) | +, ↑ (CD4+>DN) | ↑ | ICOSL (LICOS, B7h, B7RP-1, B7-H2, GL50, CD275) | B cells (+) Macrophages (+) DCs (+) Non-lymphoid cells (↑) |
[25,39,42,47,48] | ||
| Coinhibitory | CTLA-4 (CD152) | ? | ↑ | B7.1 (CD80) B7.2 (CD86) |
APCs (↑) Activated T cells (↑) APCs (+, ↑) |
[25,45] | |
| PD-1 (CD279) | +, ↑ | ↑ | PD-L1 (CD274, B7-H1) | Resting and activated conventional T cells (+, ↑) B cells (+, ↑) NK cells (+, ↑) Macrophages (+, ↑) DCs (+, ↑) Non-hematopoietic cells (+, ↑) |
[40,49,54,55] | ||
| PD-L2 (CD273, B7-DC) | Activated DCs (↑) Macrophages (↑) |
||||||
| BTLA (CD272) | + | ↑ | HVEM (CD270) | Most immune cells and in all internal organs (+) | [49,57,58] | ||
| TNFSF | Costimulatory | CD40L (CD154) | ↑ (CD4+>DN) | ↑ (CD4+>CD8+) | CD40 | B cells (+) DCs (+) Macrophages (+) Non-hematopoietic cells (fibroblast, endothelial, epithelial) (↑) |
[59–61] |
| TNFRSF | Costimulatory | OX40 (CD134) | + | ↑ (CD4+>CD8+) | OX40L (CD252) | B cells (↑) DCs (↑) Macrophages (↑) Conventional T cells (↑) Endothelial cells (↑) |
[63–66] |
| 4-1BB (CD137) | ↑ | ↑ (including memory CD8+) | 4-1BBL (CD137L) | DCs (↑) B cells (↑) Macrophages (↑) |
[68–71] | ||
| Coinhibitory | GITR (CD357) | +, ↑ | +, ↑ | GITRL | B cells (+, ↑) Macrophages (+, ↑) BMDCs (+, ↑) Endothelial cells (+, ↑) |
[72–74] | |
| TIM Family | Costimulatory | TIM-1 (KIM-1) | + | ↑ (TH2>TH1) | TIM-1, TIM-4, Ptd-L-Ser | APCs (+, ↑) | [77] |
+: Constitutive; ↑: Inducible.
Abbreviations: BMDC: bone marrow-derived dendritic cell; BTLA: B and T lymphocyte attenuator; CD: cluster designation; cDC: conventional dendritic cell; CTLA-4: cytotoxic T lymphocyte-associated antigen-4; DN: double negative; GITR(L): glucocorticoid-induced TNFR family-related (ligand); HVEM: herpes virus entry mediator; ICOS(L): inducible costimulator (ligand); IgSF: immunoglobulin superfamily; iNKT cell: invariant natural killer T cell; KIM: kidney injury molecule; NK: natural killer; nTreg cell: naturally occurring regulatory T cell; (p)APC: (professional) antigen-presenting cell; PD-1: programmed death-1; pDC: plasmacytoid dendritic cell; Ptd-L-Ser: phosphatidylserine; TH: T helper; TIM: transmembrane (or T cell) immunoglobulin and mucin; TNFSF: tumor necrosis factor (TNF) superfamily; TNFRSF: tumor necrosis factor receptor superfamily.
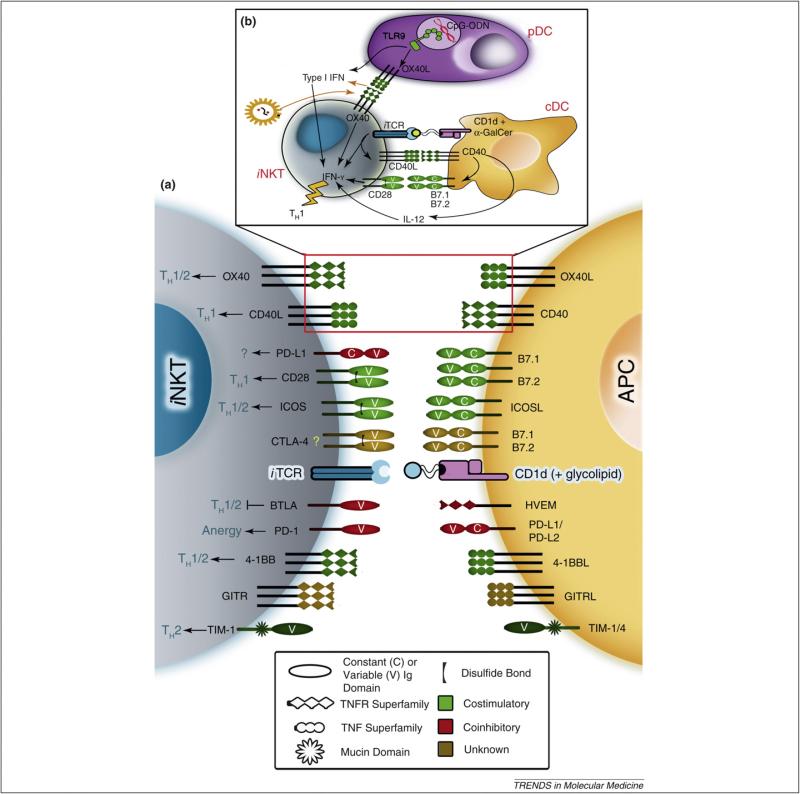
Figure 1.
Costimulatory and coinhibitory interactions between iNKT cells and APCs. iNKT cells recognize and respond to glycolipid antigens (e.g. α-GalCer) presented in the context of CD1d expressed by APCs. These responses are regulated by several costimulatory (green) and coinhibitory (red) molecules belonging to the Ig superfamily, the TNFR/TNF superfamily or the TIM family (a). Cell surface molecules represented in brown have yet to be firmly classified as costimulatory or coinhibitory in iNKT cell responses. For instance, the expression and function of CTLA-4 in iNKT cells have not been established. Costimulatory interactions affect TH1- and/or TH2-type cytokine production by iNKT cells. These interactions might also mediate crosstalk between iNKT cells and DC subsets (b). TLR-9 triggering by CpG-ODNs stimulates pDCs to produce type I IFN. This effect together with the OX40–OX40L interaction contributed by pDCs synergizes with an iTCR signal to promote IFN-γ production by iNKT cells [66]. The iTCR signal is dependent on α-GalCer presentation by conventional DCs (cDCs) to iNKT cells. This signal also leads to CD40L upregulation in iNKT cells, which engages with CD40 in cDCs and stimulates their IL-12 secretion and enhanced B7 expression [59,61]. IL-12 and CD28–B7 interactions in turn act on iNKT cells and modulate their cytokine responses. Infection with viral pathogens such as LCMV might result in the enhanced expression of OX40 in iNKT cells [63]. This represents yet another mechanism by which the OX40–OX40L-mediated interaction between iNKT cells and pDCs can stimulate type I IFN secretion by pDCs. Ultimately, this three-way communication results in a TH1-type iNKT cell response.
Costimulatory molecules of the Ig superfamily
These are type I transmembrane glycoproteins containing the characteristic extracellular Ig variable-like (IgV) domain and a cytoplasmic tail that participates in signal transduction.
CD28/CTLA-4/B7 pathway
At least in mice, the development of iNKT cells in the thymus depends heavily on CD28–B7 interactions. CD28 knockout and B7.1/B7.2 double knockout mice exhibit a greater than 50% reduction in the frequency and absolute number of thymic iNKT cells, which manifests not only in early postnatal life but also in adult animals [38–40]. A similar numerical decrease in splenic and hepatic iNKT cells has also been noted in young CD28–/– and B7–/– mice but not in older animals [38], suggesting that homeostatic mechanisms involved in the maintenance of peripheral iNKT cells might be less dependent on the CD28–B7 pathway. This is further supported by the observation that iNKT cell-enriched thymocytes exhibit comparable homeostatic proliferation in sublethally irradiated B7-sufficient and -deficient hosts [39].
Signaling through CD28 seems to be dispensable for early lineage commitment and the positive selection of iNKT cells but is essential for their subsequent intrathymic maturation as judged by membrane expression levels of NK1.1, CD44, CD69 and CD122, the intracellular expression of the transcription factor T-bet and their capacity to synthesize IFN-γ [38,39]. Interestingly, transgenic over-expression of CD28 or B7 on thymocytes leads to defective rather than augmented iNKT cell development [38], indicating a delicate requirement for a physiological level of CD28 signaling during this process.
CD28 costimulation is crucial for the optimal responses of iNKT cells to glycolipid antigens. CD28 is constitutively expressed on peripheral iNKT cells and is thus readily available to B7.1 and/or B7.2 [41,42]. The disruption of CD28–B7 interactions impairs various effector functions elicited by iNKT cells. In combination, blocking monoclonal antibodies (mAbs) to B7.1 and B7.2 partially inhibit IFN-γ and IL-4 responses to α-GalCer in vitro and in vivo [42]. Similarly, diminished levels of IFN-γ and IL-4 have been detected in cultures of α-GalCer-stimulated CD28–/– splenocytes in comparison with wild-type splenocytes incubated with the same glycolipid antigen [41]. Furthermore, treatment with α-GalCer induces lower serum levels of IFN-γ and IL-4 in CD28–/– mice compared with wild-type animals [41]. Interestingly, the production of IFN-γ by α-GalCer-stimulated splenocytes was inhibited to a greater extent than that of IL-4 when B7.2 but not B7.1 was blocked [43], implicating the CD28–B7.2 costimulation axis in TH1-skewed responses to α-GalCer.
Splenic and hepatic mononuclear cells obtained from α-GalCer-primed mice exert substantial cytotoxic activities against both NK cell-sensitive and -resistant target cells [20,41]. α-GalCer administration also reduces lung metastasis in a mouse melanoma model [20]. These are examples of iNKT cell-mediated TH1-type responses that are abrogated or greatly reduced in the absence of CD28 signaling [41,42]. Importantly, experimental autoimmune encephalomyelitis (EAE), a widely accepted mouse model of multiple sclerosis, can be suppressed by α-GalCer-pulsed, B7.2-blocked APCs that favor a TH2-type response [43].
Although the above studies demonstrate a requirement for CD28 signaling in α-GalCer-induced responses, it is not clear to what extent the direct ligation of CD28 on iNKT cells contributes to these responses. CD28 costimulation enhances cytokine production by anti-CD3-stimulated splenic NKT cells in vitro [44]. Interestingly, however, in vivo disruption of the CD28–B7 pathway in α-GalCer-treated mice leads to little or no decrease in intracellular cytokine levels in iNKT cells, whereas serum cytokine concentrations can drop substantially [37,45]. Nevertheless, from a therapeutic standpoint, the established role of CD28 costimulation in α-GalCer-mediated downstream effector responses should remain a primary focus.
The CD28 homolog CTLA-4 is a coinhibitory receptor that binds to B7.1 and B7.2 and inhibits T cell activation [25]. Temporal differences in the expression of CD28 and CTLA-4 by conventional T cells orchestrate their timely participation in signal transduction. With the exception of naturally occurring regulatory T (nTreg) cells, resting T cells do not express CTLA-4. The expression of CTLA-4 is, however, induced following T cell activation. CTLA-4 has a high binding avidity for B7 and efficiently outcompetes CD28 in this respect. The consequent low availability of B7 for binding to CD28, together with a T cell-intrinsic negative signal transmitted through CTLA-4, ensures the suppression of excessive and unnecessarily persistent T cell responses.
CTLA-4 is not detectable on the surface or within the cytoplasm of resting NKT cells [41,45,46]. α-GalCer-stimulated iNKT cells also fail to express detectable CTLA-4. In light of these observations, it is puzzling that an anti-CTLA-4 mAb inhibited iNKT cell proliferation induced by α-GalCer-pulsed DCs [14]. Responder cells in this study were obtained from the spleens of RAG–/–Vα14 transgenic mice, which have many iNKT cells but no T, B or NK cells. Whether CTLA-4 is expressed by and operates in the various NKT cell subsets located in different tissues under physiological and pathological conditions warrants comprehensive investigation.
Taken together, the CD28–B7 pathway is essential for the normal thymic development of mouse iNKT cells but apparently not for their peripheral maintenance. While optimal iNKT cell responses to glycolipid antigens depend on CD28–B7 interactions, the contribution, if any, of CTLA-4–B7 interactions to these responses remains unclear.
ICOS/ICOSL pathway
Unlike naïve conventional T cells, resting iNKT cells constitutively express inducible costimulator (ICOS) [39,42,47]. Mouse CD4+ iNKT cells express higher levels of ICOS in comparison with DN cells [47], suggesting a more critical role for ICOS costimulation in the former subset. ICOS levels are further upregulated on iNKT cells upon stimulation with α-GalCer. Unlike conventional T cells, the expression of ICOS in iNKT cells is independent of CD28 signaling and vice versa such that iNKT cells isolated from CD28–/– mice have normal levels of ICOS, and iNKT cells found in ICOS–/– animals show an intact expression of CD28 [42].
The interaction between ICOS and its ligand ICOSL is important for normal iNKT cell homeostasis. A lack of ICOS signaling in both C57BL/6 and Balb/c mouse strains results in reduced iNKT cell numbers in the spleen and liver [39,47,48]. Signaling through ICOS but not CD28 is required for iNKT cell survival in the periphery [47]. Whether ICOS costimulation is needed for the intrathymic development of iNKT cells is not completely clear. The absence of either ICOS or ICOSL dramatically decreases iNKT cell numbers in the thymus of C57BL/6 mice [39] but not in Balb/c mice [47]. Moreover, C57BL/6 radiation chimeras harboring bone marrow cells from ICOS+/+ and ICOS–/– mice exhibit lower percentages of ICOS–/– iNKT cells but normal proportions of T cells, NK cells and other major immunocyte populations [48]. These findings also highlight the role of genetic factors in iNKT cell costimulation.
Recent studies using stimulatory or blocking mAbs and gene knockout approaches have established the importance of the ICOS–ICOSL pathway in the effector responses of iNKT cells. In the presence of a suboptimal dose of anti-CD3 mAb, bead-coated anti-ICOS costimulated IL-4 secretion by hepatic iNKT cells [48]. Anti-ICOSL and anti-B7.1/B7.2 mAbs could each partially inhibit IL-4, IL-10, IL-13 and IFN-γ production by α-GalCer-stimulated iNKT cells [42]. In combination, these mAbs abolished cytokine production in an additive fashion, suggesting that ICOS and CD28 costimulate iNKT cells independently of each other. This was also true for α-GalCer-induced in vivo responses, including cytokine release, bystander cytotoxic activity and the prevention of metastasis. In a separate study, interfering with the ICOS–ICOSL pathway decreased the production of IL-4, IL-5, IL-10, IL-13 and IFN-γ by iNKT cells, whereas IL-2 production remained intact [47]. Both the CD4+ and DN subsets of iNKT cells seem to require ICOS signaling for optimal cytokine production.
Signaling through ICOS might also play a role in iNKT cell-induced pathology. In a mouse model of asthma, airway hyperreactivity (AHR) mediated by CD4+ iNKT cells depended on ICOS–ICOSL interactions [47]. These interactions also contribute to liver injury associated with concanavalin A (ConA)-induced hepatitis, a commonly used mouse model of human autoimmune hepatitis with documented involvement of IL-4 produced by iNKT cells [48].
Collectively, the above observations indicate that intact costimulation through ICOS plays an important role in normal homeostasis and the peripheral survival of iNKT cells and might influence their cytokine responses under physiological and pathological conditions.
PD-1/PD-L pathway
PD-1 is a monomeric receptor with a coinhibitory function in T cells [49]. The selective upregulation of PD-1 is associated with the functional exhaustion of virus-specific CD8+ T cells in mice and humans with chronic viral infections [50,51].
PD-1 binds to two separate ligands PD-L1 and PD-L2 and has a higher affinity for the latter [49]. B7.1 was recently discovered to serve as an additional binding partner for PD-L1 in both mouse and human cells [52,53]. iNKT cell numbers in the thymus, spleen and liver of PD-L1–/–, PD-L2–/– and PD-L1–/– PD-L2–/– mice are similar to those in wild-type animals [54], suggesting that PD-1–PD-L interactions are dispensable for iNKT cell development.
PD-1 expression is low in resting iNKT cells, rapidly upregulated upon treatment with a single dose of α-GalCer and maintained throughout the anergic phase of α-GalCer-experienced iNKT cells [46,55]. The same treatment can transiently increase the expression of PD-L1 and PD-L2 on APCs [46] and that of PD-L1 on iNKT cells[55]. Importantly, α-GalCer-induced anergy is not observed in PD-1–/– mice and can be prevented in wild-type animals receiving a combination of anti-PD-L1 and anti-PD-L2 mAbs [46]. The blockade of PD-L1 but not PD-L2 reversed α-GalCer-induced anergy ex vivo [55]. Although a potential role for PD-L1–B7.1 interactions in iNKT cell anergy has not been ruled out, these observations collectively suggest that the PD-1/PD-L1 axis is a major contributor to this phenomenon. It is noteworthy that the PD-1–PD-L pathway seems to be selectively required for α-GalCer-mediated anergy because interfering with this pathway does not prevent iNKT cell anergy induced by heat-inactivated Escherichia coli or the vNKT cell glycolipid agonist sulfatide [46].
In a mouse melanoma model, the antimetastatic activity of α-GalCer, which was otherwise hampered by a prior injection of the same glycolipid, could be rescued by blocking PD-1–PD-L interactions during the initial glycolipid priming [46]. Coadministration of anti-PD-L mAbs and α-GalCer starting a few days after the melanoma challenge also reduced metastatic burden. This mimics an effective treatment regimen for established cancer.
A recent investigation has uncovered important but opposing roles for PD-L1 and PD-L2 in two mouse models of iNKT cell-mediated asthma [54]. PD-L1–/– mice developed mild AHR in response to model allergenic challenges and their iNKT cells secreted high concentrations of IFN-γ in response to α-GalCer. By contrast, the development of AHR and airway inflammation was exacerbated in PDL2–/– mice, and iNKT cells from these animals produced elevated levels of IL-4 compared with iNKT cells obtained from wild-type or PD-L1–/– animals. Consistently, while the adoptive transfer of iNKT cells purified from wild-type or PD-L2–/– mice into iNKT cell-deficient Jα18–/– mice restored allergen-induced AHR in these animals, Jα18–/– recipients reconstituted with PD-L1–/– iNKT cells did not develop severe AHR.
Altogether, the PD-1–PD-L pathway does not seem to be required for the intrathymic development and peripheral homeostasis of iNKT cells but mediates α-GalCer-induced anergy. Furthermore, PD-L1 and PD-L2 might differentially regulate iNKT cell responses at least in allergic asthma.
BTLA/HVEM pathway
BTLA is a polymorphic molecule exhibiting allelic variation across mouse strains [49]. Herpes virus entry mediator (HVEM), which binds to herpes simplex virus glycoprotein D and mediates viral entry into host cells, was recently identified as the ligand for BTLA [56]. HVEM is a member of the TNFR superfamily, and the BTLA–HVEM interaction constitutes the first example of crosstalk between costimulatory members of the Ig and TNFR superfamilies.
BTLA signaling seems to be dispensable for iNKT cell development and maintenance [57]. Thymic, splenic and hepatic iNKT cells express BTLA at levels similar to those found in conventional T cells. BTLA–/– mice secrete heightened levels of cytokines including IL-4 and IFN-γ following α-GalCer injection [58], and NKT cells obtained from these animals hyperproliferate and produce more cytokines than wild-type NKT cells in response to α-GalCer. Two independent studies have reported that BTLA inhibits iNKT cell-induced pathology associated with ConA hepatitis [57,58]. Compared with wild-type controls, BTLA–/– mice mounted a more vigorous cytokine response and showed higher mortality following ConA injection. In addition, Jα18–/– mice reconstituted with hepatic BTLA–/– iNKT cells were moderately more susceptible to ConA-inflicted liver damage than those receiving wild-type iNKT cells. Therefore, signaling through BTLA may negatively regulate iNKT cell responses in health and disease.
Costimulatory molecules of the TNFR/TNF superfamily
TNFR and TNF family members are type I and type II transmembrane proteins, respectively and participate in various biological processes including T and iNKT cell costimulation. Ligation and trimerization of TNFR family members can recruit adapter proteins called TNFR-associated factors (TRAFs) that activate several signaling cascades, notably those involving nuclear factor (NF)-κB.
CD40L/CD40 pathway
CD40L–CD40 interactions result in bidirectional signaling with important consequences not only for T or iNKT cells but also for their engagement partners.
Activated iNKT cells express functional CD40L [41,59] and provide “innate help” to B cells [60]. They also engage in productive crosstalk with DCs [61] (Figure 1). In fact, reciprocal iNKT cell–DC interaction is a prerequisite for α-GalCer-induced effector responses. Following glycolipid presentation by DCs, iNKT cells, predominantly the CD4+ subset in mice, express CD40L, which then cross-links CD40 on DCs [59,61]. This results in the production of IL-12 by DCs, which in turn stimulates IFN-γ secretion by iNKT cells. CD40 costimulation and IL-12 are apparently not essential for α-GalCer-mediated IL-4 production. It is therefore not surprising that CD40L–CD40 interactions skew iNKT cell responses towards a TH1 phenotype. In mice, antibody blockade or genetic disruption of this pathway inhibits the production of IFN-γ but not IL-4 in response to α-GalCer [41,61,62]. TH1-type antimetastatic properties of α-GalCer and its enhancement of cytotoxic activities of splenic and hepatic mononuclear cells are also missing in CD40–/– mice [41]. In addition, the presentation of α-GalCer by APCs pretreated with an agonistic anti-CD40 mAb induced TH1-biased iNKT cell responses in vitro and aggravated EAE in vivo [43]. Therefore, the CD40–CD40L pathway plays an important role in the generation of TH1-type iNKT cell responses.
OX40/OX40L pathway
OX40 serves as a “second-wave” costimulatory receptor supporting the continued survival, effector function and memory responses of conventional T cells, notably CD4+ T cells. Although a TCR-mediated signal 1 is sufficient for OX40 induction, CD28–B7 interactions augment and sustain the subsequent expression of OX40. The sequential expression/function of CD28 and OX40 in T cells reiterates the concept that effector and memory T cells are reliant on inducible costimulatory molecules such as OX40, and clearly disputes the previously popular view that these cells are completely costimulation-independent.
Steady-state iNKT cells homing to certain peripheral organs (e.g. liver and pancreas) express substantial levels of OX40 [63]. OX40–/– mice show diminished serum concentrations of IFN-γ following α-GalCer injection, and the disruption of the OX40–OX40L pathway partially blunts IFN-γ response to α-GalCer in vitro [64].
OX40–OX40L interactions might mediate the crosstalk between iNKT cells and DCs during antitumor and antiviral immune responses. In a melanoma model, the intratumoral administration of DCs genetically modified to express OX40L recruited iNKT cells and slowed tumor growth [64]. The success of this vaccination strategy was dependent on CD1d expressed by DCs and the engagement of OX40 on tumor-infiltrating iNKT cells that produce IFN-γ. The protective role of iNKT cells in this setting was confirmed by the observation that OX40L+ DCs suppress tumor growth in iNKT-deficient mice reconstituted with wild-type but not OX40–/– iNKT cells.
The OX40–OX40L pathway also promotes the crosstalk between iNKT cells and plasmacytoid DCs (pDCs), which are specialized in type I IFN production [65]. CpG-containing oligodeoxynucleotides, which mimic microbial DNA binding to TLR-9 in pDCs, synergized with α-GalCer to induce IFN-γ production by human iNKT cells [66]. This effect required the CD1d presentation of α-GalCer by myeloid DCs, the secretion of IFN-α by CpG-stimulated pDCs and the engagement of OX40 on iNKT cells by pDC-expressed OX40L. Reverse signaling through OX40L can also promote pDC function in the context of iNKT–pDC cooperation. Pancreatic and hepatic iNKT cells upregulated OX40 following the infection of mice with lymphocytic choriomeningitis virus (LCMV) and controlled early viral replication in these organs [63]. This effect was mediated by IFN-α secreted by pDCs after they interacted with iNKT cells via the OX40–OX40L pathway.
Signaling through OX40 has also been implicated in pathogenic TH2-type responses. In a mouse model of house dust mite (HDM)-induced allergy, eosinophilic airway inflammation, TH2-type cytokine responses (IL-4, IL-5 and IL-13) and elevated HDM-specific IgE levels were dependent on OX40 costimulation [67]. These responses required OX40 engagement on CD4+ and iNKT cells during the sensitization and re-exposure phases, respectively. Allergic inflammation in this model was attenuated in iNKT cell- and OX40-deficient mice or by the instillation of a blocking anti-OX40L mAb during intranasal challenge with HDM. Moreover, adoptive transfer of OX40+/+ but not OX40–/– iNKT cells into HDM-sensitized, iNKT-deficient animals restored allergic responses following a subsequent challenge with HDM. Therefore, interfering with OX40–OX40L interactions might be an attractive approach in the treatment of respiratory allergy.
4-1BB/4-1BBL pathway
4-1BB has emerged as a crucial costimulator of survival signaling, predominantly in activated and memory CD8+ T cells [68]. The 4-1BB–4-1BBL pathway seems to be dispensable at early stages of T cell activation when other costimulatory signals are abundant but plays an important part in augmenting TCR signals and sustaining effector functions when other costimulatory signals are limiting. 4-1BB can also be induced in memory CD8+ T cells by IL-2 and IL-15, thus potentially contributing to their survival in the absence of overt antigenic stimulation.
NKT cell development and/or peripheral maintenance depend on 4-1BB because 4-1BB–/– mice have decreased frequencies and absolute numbers of CD3+DX5+ NKT cells in their thymuses, spleens and livers [69]. It is noteworthy that this cell population contains but is not necessarily equal to CD1d tetramer+ iNKT cells. 4-1BB expression is induced on splenic and hepatic iNKT cells following TCR stimulation [70]. 4-1BB–/– mice produce less IL-4 soon after injection with anti-CD3 mAb, a response mediated by NKT cells [69]. In addition, an agonistic mAb to 4-1BB could augment α-GalCer-induced cytokine production by iNKT cells in vitro and in vivo [70].
In a lipopolysaccharide model of toxic shock where NKT cells contribute to the pathogenesis of fulminant hepatitis, a blocking anti-4-1BB mAb was protective and curtailed cytokine upregulation in NKT cells [69]. In a mouse model of pulmonary inflammation, an agonistic anti-4-1BB mAb enhanced the detrimental effect of α-GalCer and worsened AHR and inflammatory cell accumulation in an IL-4 receptor-dependent fashion [70]. Interestingly, administering a therapeutic cocktail containing the same mAb and α-GalCer eradicates established mammary and renal carcinomas in mice in an IFN-γ-dependent manner [71]. Therefore, the outcomes of 4-1BB triggering might vary depending on the experimental model used.
GITR/GITRL pathway
Glucocorticoid-induced TNFR family-related gene (GITR) is a relatively new member of the TNFR superfamily with low expression on resting conventional T cells and upregulated levels on activated T cells [72]. The constitutively high expression of GITR is detectable on nTreg cells. GITR ligation promotes the proliferative and cytokine production capacities of effector T cells and regulates the suppressor function of nTreg cells [72].
GITR–/– mice have intact thymic, splenic and hepatic iNKT cell compartments [73]. GITR expression on iNKT cells is constitutive and further enhanced upon TCR ligation [73,74]. The role of GITR in the regulation of iNKT cell responses is controversial because both costimulatory and coinhibitory properties have been reported. Although an agonistic anti-GITR mAb has been shown to augment the cytokine responses of an iNKT hybridoma and primary TCRb+NK1.1+ cells to α-GalCer and anti-CD3, respectively [74], a later study found that the same mAb inhibits α-GalCer-induced proliferation and cytokine production by CD1d tetramer+ NKT cells [73]. The TCRβ+NK1.1+ fraction contains both iNKT and vNKT cells with potentially opposing characteristics [75], whereas CD1d tetramer-positive cells more accurately represent iNKT cells [5,6]. Moreover, GITR–/– mice used in the latter study exhibited boosted cytokine responses to α-GalCer and prolonged survival following glycolipid therapy in a metastatic tumor model in comparison with wild-type animals. Therefore, we favor a coinhibitory role for GITR in the context of iNKT cell activation.
The TIM domain family
TIM family members are type I transmembrane glycoproteins with a wide range of ligands and diverse roles in immunity [76]. They contain a single IgV domain and a glycosylated mucin domain that distinguishes them from other costimulatory molecules. Eight predicted tim genes exist in the mouse genome, four of which encode functional proteins (TIM-1, TIM-2, TIM-3 and TIM-4), whereas the human TIM gene family has only three members encoding TIM-1, TIM-3 and TIM-4. We will discuss TIM-1 [kidney injury molecule-1 (KIM-1)] because it is relevant to iNKT cell immunobiology.
Both TIM-1 and TIM-4 are constitutively expressed by iNKT cells [77]. Several ligands have been identified for TIM-1, including TIM-4, TIM-1 itself and phosphatidylserine. In the presence of a TCR signal, several agonistic anti-TIM-1 mAbs have been shown to suppress the IFN-γ secretion capacity of iNKT cells while increasing or not altering their IL-4 responses in vitro. Coinjection of mice with α-GalCer and an anti-TIM-1 mAb also lowers IFN-γ and increases IL-4, IL-10 and IL-13 secretion in comparison with animals receiving α-GalCer only, which also correlates with the intracellular cytokine content of iNKT cells. Although the potential effects of anti-TIM-1 mAbs in the absence of glycolipid treatment have not been investigated, these results implicate TIM-1 signaling in the induction of TH2-skewed iNKT cell responses.
In summary, the thymic ontogeny, peripheral maintenance and effector functions of iNKT cells, including their cytokine responses in health and disease, can be regulated by costimulatory members of the Ig superfamily, the TNFR/TNF superfamily and the TIM domain family. However, because the costimulation requirements of iNKT and conventional T cells are not identical, one cannot extrapolate the experimental results obtained from conventional T cell costimulation studies to iNKT cells.
Clinical implications
KRN7000, the prototype ligand for iNKT cells with a unique α-GalCer structure, was initially discovered in a screen for novel anticancer agents [78] and was demonstrated to trigger the antitumor and antimetastatic activities of iNKT cells in mouse models [79]. These activities are mainly attributed to the ability of iNKT cells to mature DCs and stimulate their IL-12 production, secrete IFN-γ and boost NK cell- and cytotoxic T lymphocyte-mediated cytotoxicity, and counteract or eliminate immunosuppressive or tolerogenic leukocytes within the tumor microenvironment.
The recognition mode of iNKT cells is evolutionarily conserved to the extent that human iNKT cells recognize mouse CD1d and vice versa [80]. In addition, iNKT cells from both species are responsive to α-GalCer. These observations prompted several clinical trials of α-GalCer or ex vivo-expanded iNKT cells in patients with cancer or viral diseases [81–89], which are summarized in Table 2.
Table 2.
A summary of human clinical trials using iNKT-based therapeutic approaches in cancer and viral diseases
| Tumor/virus type(s) | No. of patients | Therapeutic agent/cells | Adverse events (one or more patients) | Clinical outcomes | References |
|---|---|---|---|---|---|
| Various refractory solid tumors | 24 | KRN7000 (i.v.) | Minora | 7 cases of stabilization 15 cases of tumor progression |
[81] |
| Various metastatic tumors | 12 | KRN7000-pulsed, immature MoDCs (i.v.) | Transient tumor-associated flares Minor systemic side effects |
↓ Serum tumor markers, Tumor necrosis ↓Serum levels of hepatic enzymes |
[82] |
| Advanced or recurrent non-small cell lung cancer | 11 enrolled 9 completed |
KRN7000-pulsed, immature MoDC-rich APCs (i.v.) |
Minora ↑ serum K+, creatinine, or total bilirubin |
No changes in 5 cases Progression in 4 cases |
[83] |
| Myeloma, anal squamous cancer, renal cell cancer | 6 enrolled 5 completed |
KRN7000-pulsed, mature MoDCs (i.v.) |
Positive rheumatoid factor Transient positive antinuclear antibodies |
Not evaluated | [84] |
| Advanced or recurrent non-small cell lung cancer | 6 | iNKT cells stimulated ex vivo with KRN7000-pulsed autologous PBMCs (i.v.) | Minora Transient arrhythmia ↑ LDH, γ-GTP, or total bilirubin |
6 cases of stabilization 2 cases of progression |
[85] |
| Unresectable or recurrent head and neck cancer | 9 | Activated autologous KRN7000-pulsed APCs (via nasal submucosa) | Temporary anemia | 1 partial tumor regression No changes in 5 cases Progressions in 2 cases |
[86] |
| Head and neck squamous cell carcinoma | 8 | In vitro expanded iNKT (intra-arterial infusion) and KRN7000-pulsed APCs (via nasal submucosa) | Minora Pharyngocutaneous fistula |
Partial response in 3 cases Stable disease in 4 cases Progressive disease in 1 case |
[87] |
| Hepatitis C virus | 40 enrolled; 38 completed |
KRN7000 (i.v.) | Minora | 1 case of ↓ viral RNA | [88] |
| Hepatitis B virus | 27 enrolled; 22 completed |
KRN7000 (i.v.) | Minora ALT flare Severe chills |
Transient ↓ viral DNA (3 cases) Sustained ↓ viral DNA (1 case) |
[89] |
Minor includes one or more of the following: fever, lethargy, malaise, headache, vomiting, chills, transient flush, fatigue, myalgia, rhinitis.
Abbreviations: ALT: alanine aminotransferase; APCs: antigen-presenting cells; γ-GTP: γ-glutamic-pyruvic transaminase; iNKT cells: invariant natural killer T cells; i.v.: intravenous; KRN7000: α-galactosylceramide; LDH: lactate dehydrogenase; MoDCs: monocyte-derived dendritic cells; PBMCs: peripheral blood mononuclear cells.
The goal of iNKT cell-based cancer immunotherapy is to expand iNKT cells, overcome their functional inadequacies, especially in terms of IFN-γ production, and potentiate TH1-type responses [79]. An ideal protocol should minimize toxicity and adverse immunological reactions without compromising the clinical response. Costimulatory manipulations might offer novel opportunities to achieve these objectives. Moreover, pAPCs from cancer patients are often dysfunctional [79], and this can be potentially reversed through optimized costimulation.
If a treatment regimen involves systemic α-GalCer administration, coinjection of agonistic mAbs to costimulatory molecules such as CD40, CD28 and 4-1BB might prove effective. This might not only strengthen iNKT cells and their downstream effects but also lower the therapeutic dose of α-GalCer or mAbs, thereby avoiding the potential toxicity associated with high-dose monotherapy. Such strategies might also benefit from the concomitant disruption of TH2-promoting costimulatory pathways or the blockade of coinhibitory molecules. The PD-1–PD-L pathway is of particular interest given its role in CD8+ T cell exhaustion resulting from persistent viral infections [50,51] and chronic antigenic stimulation in cancer [90] in addition to its involvement in α-GalCer-induced iNKT cell anergy [46,55]. Combining α-GalCer administration with a blockade of PD-1–PD-L interactions may improve treatment outcomes in chronic viral diseases or when repeated α-GalCer injections might be needed, for instance during disease relapse in cancer.
Direct injection of α-GalCer exerts a plethora of effects through transactivation of various cell types, some of which might not be desirable. Systemic administration of mAbs might target both iNKT and non-iNKT cells, and injection of some mAbs might even trigger detrimental responses. One classic example was the case of excessive toxicity and a severe systemic inflammatory response experienced by healthy volunteers receiving an agonistic anti-CD28 mAb [91], which had not been observed in animal models. Such adverse complications might be eliminated or minimized if “costimulation-optimized” α-GalCer-coated autologous pAPCs or iNKT cells expanded ex vivo using such pAPCs are infused back into patients.
iNKT cells infiltrate some tumors, and positive associations exist between the presence of iNKT cells within certain tumors and long-term survival in patients [92]. When injected intratumorally, DCs modified to express high OX40L levels recruit iNKT cells, provoke tumor-specific CTL responses and suppress tumor growth in a mouse model [64]. Therefore, in circumstances when tumors are readily accessible and have not yet metasta-sized, costimulation-optimized pAPCs that simultaneously display α-GalCer might harness intratumoral iNKT cell populations for cancer immunotherapy.
Other iNKT cell-based immunotherapies can be envisaged and pursued in future investigations. One option is to coadminister costimulatory mAbs and CD1d-transfected tumor cells coated with α-GalCer. Costimulation-optimized pAPCs copulsed with tumor lysate (or tumor-derived peptides) and α-GalCer might also be an attractive vaccine candidate for cancer.
In mouse models where tumor rejection is mediated by iNKT cells, hepatic DN cells are reportedly superior to their CD4+ counterparts and thymus-derived iNKT cells [12]. It is not currently understood whether these findings mimic anticancer iNKT cell responses in humans and whether/how costimulatory requirements might differ across the various iNKT subsets.
Recently, there has been increasing interest in synthesizing α-GalCer analogs that polarize immune responses towards either a TH1 or TH2 phenotype. One such compound is a C-glycoside analogue of α-GalCer (α-C-GalCer), which is a potent inducer of IFN-γ and IL-12 production in mice [93] and a candidate therapeutic for cancer and infectious diseases. A combination immunotherapy regimen utilizing iNKT cell glycolipid agonists, an anti-4-1BB mAb and a mAb against a TRAIL receptor was successful in rejecting mouse mammary and renal carcinomas [71]. Importantly, α-C-GalCer was more potent and less hepatotoxic than α-GalCer in “NKTMab therapy”.
TH2-favoring glycolipids are potential therapeutic options for autoimmune disorders and transplant rejections resulting from pathogenic TH1-type responses. OCH, a sphingosine-truncated derivative of α-GalCer with TH2-promoting properties, prevented collagen-induced arthritis in a mouse model [94] as well as insulitis and type 1 diabetes in nonobese diabetic (NOD) mice [95]. When combined with rapamycin, OCH delayed TH1-mediated graft rejection in two mouse models of cardiac allotrans-plantation [96]. C20:2, an α-GalCer variant featuring a truncated fatty acyl side chain with two unsaturation sites at carbons 11 and 14, can also prevent autoimmune manifestations in NOD mice [97]. Importantly, this glycolipid is presumed to be superior to OCH in deviating human iNKT cell responses towards a TH2 phenotype [97]. Future investigations will address the efficiency of these glycolipids in combination therapies, including those targeting costimulatory molecules.
Concluding remarks and future directions
Recent years have witnessed increasing interest in iNKT cells and their immunomodulatory properties. The impressive adjuvanticity of iNKT cell ligands has led to many preclinical studies with promising results and perceived potential for benchtop-to-bedside translation. Despite recent advances in the field, many important questions remain regarding iNKT cell activation, costimulation and effector functions (Box 1). Addressing these questions will improve our understanding of iNKT cell biology and pave the way for effective iNKT-based therapies in a wide range of diseases. Several factors need to be considered for the rational design of such therapies. The choice of an α-GalCer derivative will have to be tailored to the specific disease. Combining glycolipid therapy with other agents (e.g. costimulatory or coinhibitory mAbs) or strategies (e.g. cancer chemotherapy) could induce fruitful clinical responses while minimizing the toxicity and adverse effects otherwise caused by each treatment alone. Manipulation of more than one costimulatory/coinhibitory pathway might further optimize these therapies; the timing of each intervention will be critical because the initial priming and effector responses of iNKT cells are regulated by first- and second-wave costimulatory molecules, respectively.
When glycolipid-pulsed pAPCs are employed instead of directly injected α-GalCer, the expansion protocol, maturation status and costimulatory capacities of these pAPCs need to be optimized first. For ex vivo iNKT cell expansion, it is imperative to use costimulation-optimized APCs and to determine which iNKT cell subsets from which tissues yield the most desirable response.
In treating cancer and infectious diseases, robust TH1-type immune responses induced by iNKT-based strategies might be associated with autoimmune sequelae requiring careful monitoring and timely management. Finally, although we depend heavily and inevitably on animal models for testing novel therapeutic approaches, mouse models or even nonhuman primate preclinical studies might not necessarily predict treatment outcomes in humans. Nevertheless, the promise of iNKT cell-based therapies, once optimized, is likely to outweigh the potential complications, and exciting scientific and clinical achievements are anticipated from future investigations in this active area of research.
Box 1. Outstanding questions.
Are CD4+, CD8+ and DN iNKT cell subsets regulated by similar or different costimulatory/coinhibitory signals provided by distinct tissue microenvironments?
How do costimulatory signals affect the activation threshold of iNKT cells and lipid raft-aggregation, as well as the stability and sustenance of the iNKT:APC immunological synapse [17]?
What are the costimulatory requirements for iNKT cells responding to TH1- and TH2-skewing α-GalCer analogs including α-C-GalCer, OCH and C20:2?
Is costimulation needed for the cytolytic functions of iNKT cells? iNKT cells are “naturally” cytotoxic, may detect endogenous glycolipids expressed by malignant cells [79] and lyse tumor cells pulsed with α-GalCer in vitro. They also express Fcγ receptors [98] and might thus be involved in antibody-dependent cell-mediated cytotoxicity.
Do “atypical” costimulatory molecules modulate iNKT cell responses? Cell surface proteins other than classical costimulatory molecules might regulate iNKT cell functions, with a good example being the human NK cell marker CD161 [99]. Whether/how other proteins shared by NK and NKT cells fulfill a similar costimulatory role for iNKT cells is not fully understood. Several glycosylphosphatidylinositol-anchored proteins (e.g. mouse Thy-1 and human CD55) costimulate conventional T cells [100,101] and might also regulate iNKT cell responses to glycolipid antigens.
When used in iNKT-based combination immunotherapies, how do mAbs to costimulatory molecules affect immunosuppression mediated by nTreg cells expressing the same targeted molecule(s)? This is an important question in light of the reported crosstalk between iNKT and nTreg cells [102].
How are iNKT cell functions influenced by promiscuous costimulatory molecules interacting with multiple ligands? For instance, the relative contributions of the PD-L1–PD-1 and PD-L1–B7.1 interactions to iNKT cell responses need to be explored. This will be facilitated by using 9G2 and 2H11 mAbs in parallel. The former blocks the interaction of PD-L1 with both PD-1 and B7-1, whereas the latter inhibits the PD-L1–B7-1 interaction only [54].
Are vNKT cells costimulation-dependent? iNKT and vNKT cells might counter-regulate each other in various diseases [75]. The identification of sulfatide as a vNKT cell ligand and its employment in CD1d tetramer reagents detecting vNKT cells [103] will help address this question. Mice solely deficient in vNKT cells do not yet exist. However, using CD1d–/– mice lacking both iNKT and vNKT cells in parallel with Jα18–/– mice selectively deficient in iNKT cells will be a useful approach to this question [79].
How do costimulatory/coinhibitory signals affect the responsiveness of lipid-reactive human T cells restricted by CD1 molecules other than CD1d?
Acknowledgments
S.M.M.H. is Canada Research Chair in Viral Immunity & Pathogenesis. This work was supported by grants from the Canadian Institutes of Health Research, The Cancer Research Society Inc. and the Natural Sciences and Engineering Research Council of Canada to S.M.M.H. We are indebted to Dr Tania H. Watts for her critical review of our manuscript. We apologize to our colleagues whose important work in this area could not be cited due to space limitation.
References
- 1.Bendelac A, et al. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, et al. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 3.Assarsson E, et al. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J. Immunol. 2000;165:3673–3679. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 4.McNab FW, et al. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J. Immunol. 2007;179:6630–6637. doi: 10.4049/jimmunol.179.10.6630. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda JL, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benlagha K, et al. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, et al. Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J. Immunol. 2002;168:3140–3144. doi: 10.4049/jimmunol.168.7.3140. [DOI] [PubMed] [Google Scholar]
- 8.Lynch L, et al. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 2009;39:1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 9.Gumperz JE, et al. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PT, et al. Distinct functional lineages of human V(alpha)24 natural killer T cells. J. Exp. Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe NY, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tupin E, et al. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 14.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 15.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 16.Florence WC, et al. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. EMBO J. 2009;28:3579–3590. doi: 10.1038/emboj.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J. Exp. Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, et al. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J. Clin. Invest. 2008;118:2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leite-De-Moraes MC, et al. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 1999;163:5871–5876. [PubMed] [Google Scholar]
- 20.Kawano T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, et al. Valpha24+ natural killer T-cell responses against T-acute lymphoblastic leukaemia cells: implications for immunotherapy. Br. J. Haematol. 2003;122:231–239. doi: 10.1046/j.1365-2141.2003.04429.x. [DOI] [PubMed] [Google Scholar]
- 22.Metelitsa LS, et al. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 23.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im JS, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peggs KS, Allison JP. Costimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily. Br. J. Haematol. 2005;130:809–824. doi: 10.1111/j.1365-2141.2005.05627.x. [DOI] [PubMed] [Google Scholar]
- 26.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe AH. Mechanisms of costimulation. Immunol. Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavano R, et al. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat. Cell Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- 29.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starck L, et al. Costimulation by CD137/4-1BB inhibits T cell apoptosis and induces Bcl-xL and c-FLIP(short) via phosphatidylinositol 3-kinase and AKT/protein kinase B. Eur. J. Immunol. 2005;35:1257–1266. doi: 10.1002/eji.200425686. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Lockhart M, et al. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J. Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 32.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 33.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 34.Park SH, et al. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur. J. Immunol. 2000;30:620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.D'Andrea A, et al. Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur. J. Immunol. 2000;30:1544–1550. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Parekh VV, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, et al. Cutting edge: CD28 engagement releases antigen-activated invariant NKT cells from the inhibitory effects of PD-1. J. Immunol. 2009;182:6644–6647. doi: 10.4049/jimmunol.0804050. [DOI] [PubMed] [Google Scholar]
- 38.Williams JA, et al. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J. Immunol. 2008;181:907–917. doi: 10.4049/jimmunol.181.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung Y, et al. A critical role of costimulation during intrathymic development of invariant NK T cells. J. Immunol. 2008;180:2276–2283. doi: 10.4049/jimmunol.180.4.2276. [DOI] [PubMed] [Google Scholar]
- 40.Zheng X, et al. Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation. PLoS. One. 2008;3:e2703. doi: 10.1371/journal.pone.0002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayakawa Y, et al. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 2001;166:6012–6018. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 42.Kaneda H, et al. ICOS costimulates invariant NKT cell activation. Biochem. Biophys. Res. Commun. 2005;327:201–207. doi: 10.1016/j.bbrc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Pal E, et al. Costimulation-dependent modulation of experimental autoimmune encephalomyelitis by ligand stimulation of V alpha 14 NK T cells. J. Immunol. 2001;166:662–668. doi: 10.4049/jimmunol.166.1.662. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, et al. Control of NKT cell differentiation by tissue-specific microenvironments. J. Immunol. 2003;171:5913–5920. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- 45.Uldrich AP, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parekh VV, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J. Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbari O, et al. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J. Immunol. 2008;180:5448–5456. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe S, et al. Suppression of Con A-induced hepatitis induction in ICOS-deficient mice. Immunol. Lett. 2010;128:51–58. doi: 10.1016/j.imlet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Pentcheva-Hoang T, et al. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol. Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 50.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 51.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 52.Butte MJ, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butte MJ, et al. Interaction of human PD-L1 and B7-1. Mol. Immunol. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akbari O, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal. Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang WS, et al. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J. Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 56.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 57.Iwata A, et al. Protective roles of B and T lymphocyte attenuator in NKT cell-mediated experimental hepatitis. J. Immunol. 2010;184:127–133. doi: 10.4049/jimmunol.0900389. [DOI] [PubMed] [Google Scholar]
- 58.Miller ML, et al. Cutting edge: B and T lymphocyte attenuator signaling on NKT cells inhibits cytokine release and tissue injury in early immune responses. J. Immunol. 2009;183:32–36. doi: 10.4049/jimmunol.0900690. [DOI] [PubMed] [Google Scholar]
- 59.Tomura M, et al. A novel function of Valpha14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J. Immunol. 1999;163:93–101. [PubMed] [Google Scholar]
- 60.Tonti E, et al. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009;113:370–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 61.Kitamura H, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hermans IF, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 63.Diana J, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Zaini J, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J. Clin. Invest. 2007;117:3330–3338. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haeryfar SM. The importance of being a pDC in antiviral immunity: the IFN mission versus Ag presentation? Trends Immunol. 2005;26:311–317. doi: 10.1016/j.it.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Marschner A, et al. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur. J. Immunol. 2005;35:2347–2357. doi: 10.1002/eji.200425721. [DOI] [PubMed] [Google Scholar]
- 67.Damayanti T, et al. Serial OX40 engagement on CD4+ T cells and natural killer T cells causes allergic airway inflammation. Am. J. Respir. Crit Care Med. 2010;181:688–698. doi: 10.1164/rccm.200910-1598OC. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, et al. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol. Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 69.Vinay DS, et al. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J. Immunol. 2004;173:4218–4229. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 70.Kim DH, et al. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J. Immunol. 2008;180:2062–2068. doi: 10.4049/jimmunol.180.4.2062. [DOI] [PubMed] [Google Scholar]
- 71.Teng MW, et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 72.Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv. Exp. Med. Biol. 2009;647:156–173. doi: 10.1007/978-0-387-89520-8_11. [DOI] [PubMed] [Google Scholar]
- 73.Chen S, et al. Co-inhibitory roles for glucocorticoid-induced TNF receptor in CD1d-dependent natural killer T cells. Eur. J. Immunol. 2008;38:2229–2240. doi: 10.1002/eji.200838167. [DOI] [PubMed] [Google Scholar]
- 74.Kim HJ, et al. Engagement of glucocorticoid-induced TNF receptor costimulates NKT cell activation in vitro and in vivo. J. Immunol. 2006;176:3507–3515. doi: 10.4049/jimmunol.176.6.3507. [DOI] [PubMed] [Google Scholar]
- 75.Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J. Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 76.Kane LP. T cell Ig and mucin domain proteins and immunity. J. Immunol. 2010;184:2743–2749. doi: 10.4049/jimmunol.0902937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HS, et al. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J. Immunol. 2010;184:4095–4106. doi: 10.4049/jimmunol.0901991. [DOI] [PubMed] [Google Scholar]
- 78.Morita M, et al. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J. Med. Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 79.Haeryfar SM. Invariant natural killer T cells in immune surveillance and tumor immunotherapy: perspectives and potentials. Arch. Iran Med. 2008;11:186–195. [PubMed] [Google Scholar]
- 80.Brossay L, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giaccone G, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 82.Nieda M, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 83.Ishikawa A, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 84.Chang DH, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Motohashi S, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 86.Uchida T, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol. Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kunii N, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veldt BJ, et al. Randomized placebo controlled phase I/II trial of alpha-galactosylceramide for the treatment of chronic hepatitis C. J. Hepatol. 2007;47:356–365. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Woltman AM, et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir. Ther. 2009;14:809–818. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]
- 90.Fourcade J, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J. Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 92.Song L, et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J. Clin. Invest. 2007;117:2702–2712. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujii S, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiba A, et al. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50:305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 95.Mizuno M, et al. Synthetic glycolipid OCH prevents insulitis and diabetes in NOD mice. J. Autoimmun. 2004;23:293–300. doi: 10.1016/j.jaut.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Haeryfar SM, et al. Prolongation of cardiac allograft survival by rapamycin and the invariant natural killer T cell glycolipid agonist OCH. Transplantation. 2008;86:460–468. doi: 10.1097/TP.0b013e3181806b72. [DOI] [PubMed] [Google Scholar]
- 97.Forestier C, et al. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J. Immunol. 2007;178:1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 98.Kim HY, et al. FcgammaRIII engagement provides activating signals to NKT cells in antibody-induced joint inflammation. J. Clin. Invest. 2006;116:2484–2492. doi: 10.1172/JCI27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Exley M, et al. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J. Exp. Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haeryfar SM, et al. Thy-1 signaling in the context of costimulation provided by dendritic cells provides signal 1 for T cell proliferation and cytotoxic effector molecule expression, but fails to trigger delivery of the lethal hit. J. Immunol. 2003;171:69–77. doi: 10.4049/jimmunol.171.1.69. [DOI] [PubMed] [Google Scholar]
- 101.Capasso M, et al. Costimulation via CD55 on human CD4+ T cells mediated by CD97. J. Immunol. 2006;177:1070–1077. doi: 10.4049/jimmunol.177.2.1070. [DOI] [PubMed] [Google Scholar]
- 102.La CA, et al. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 103.Zajonc DM, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J. Exp. Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]