Abstract
Dysplastic nevi (DN) are described as being on a continuum between common acquired nevi and melanoma because they are morphologically and biologically intermediate between these two entities. Since initially being reported as histologic lesions observed in melanoma-prone families, there has been considerable debate about the definition of dysplastic nevi, the histologic and clinical criteria used to define them, and their biological importance. Their role as precursor lesions for melanoma is not their primary role in their relationship to melanoma because of the rarity of transformation of any individual nevus to a melanoma. Although there is still no single universally agreed upon histologic or clinical definition or even name for these nevi, dysplastic nevi should be considered important because of their association with an increased risk for melanoma.
Introduction
Since dysplastic nevi (DN) were first reported in 1978 by Clark and colleagues(1) and shortly thereafter by Lynch and colleagues(2, 3) as histologically defined lesions in melanoma-prone families, there has been acrimonious debate about the definition, classification, and biological importance of these lesions. The initial names used by Clark and colleagues were BK moles (and BK mole syndrome), named after two of the first melanoma-prone families seen with these lesions, and familial atypical multiple moles and melanoma syndrome (FAMMM) by Lynch and colleagues(1-4). Subsequently, the term ‘dysplastic nevus’ (and dysplastic nevus syndrome (DNS)) was proposed since these benign melanocytic nevi are characterized by architectural disorder and cytologic atypia, similar to dysplastic lesions in other organs, such as the cervix or esophagus(4-6).
There have been numerous debates about the name of these lesions and both the histologic and clinical criteria used to define them [recently reviewed in (4, 7, 8)]. In 1990, the International Agency for Research on Cancer (IARC) proposed a detailed protocol to clinically identify and record these nevi for epidemiologic studies(9). The protocol proposed the following requirements: the presence of a macular component of the lesion in at least one area plus the presence of at least three of the following features: i. not well defined border, ii. size greater than or equal to 5 mm, iii. variegated color, iv. uneven contour, v. erythema(9). Figure 1 shows examples of clinically defined dysplastic nevi. Lesions A and B were subsequently excised and classified histologically as DN based on pathologic review.
figure 1. Examples of dysplastic nevi defined based on clinical criteria.
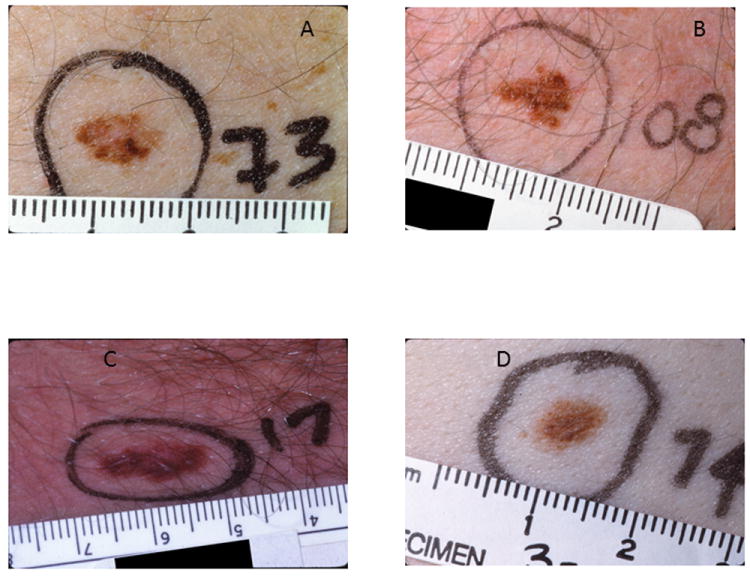
A. The lesion is 8 mm in greatest diameter. The lesion is partially flat with an irregular and indistinct outline and variable pigmentation. Because of changes to the lesion, it was excised and diagnosed as a dysplastic nevus with severe melanocytic dysplasia.
B. The lesion is 7 mm in diameter and is very irregular with indistinct borders, variable pigmentation, and an asymmetric configuration. Excision of the lesion resulted in a histologic diagnosis of dysplastic nevus with severe melanocytic dysplasia.
C. The lesion is 14 mm in greatest diameter. It has an irregular, asymmetric scalloped-shaped outline. The lesion is partially flat with indistinct borders and variably pigmented.
D. The lesion is 8 mm × 5 mm. It is partially flat with an irregular outline and indistinct borders.
However, even after multiple NIH consensus conferences and studies to examine reproducibility of dysplastic nevi by pathologists and/or clinicians studying these lesions(4, 10-18), no single definition or name for these melanocytic nevi has yet been accepted by all pathologists, dermatopathologists, dermatologists, oncologists, other clinicians, epidemiologists, or geneticists(4, 7, 8, 16). In addition, for some investigators, the use of the term dysplastic requires histologic evaluation and atypical is considered more appropriate for clinical classification. Again, there is not universal agreement for this differentiation in terminology. A problem with using “atypical” is that “atypical nevi” include a broad range of different types of unusual morphology(19). Since the criteria used to classify/define dysplastic nevi may vary (substantially), it is critical that all studies examining these lesions provide the clinical and/or histologic criteria used in the methods section. Even though no single definition is accepted by all, the most commonly used term in the literature is dysplastic nevi(16, 19). For purposes of this review, we will use dysplastic nevi (DN).
There has been extensive discussion about the histologic criteria for dysplastic nevi in the pathologic and dermatopathologic literature and this topic is not the focus of the current review. However, the major histologic criteria involve architectural disorder and cytologic atypia(4). The reader is referred to several recent reviews detailing more information about the histologic criteria of DN(4, 7, 8). The goals of this review are to examine the relationship between dysplastic nevi and melanoma, and the implications for screening, detection, and management.
Dysplastic nevi as a risk factor for melanoma
Melanoma results from the interplay of genetic, environmental, and host factors. The major environmental risk factor for melanoma is ultraviolet radiation. Increased number of nevi is one of the major host risk factors. Other host factors include increased freckling, poor tanning ability, fair complexion, light hair and eye color, and family history of melanoma(20-22). The major genetic risk factors for melanoma include the high-risk susceptibility genes CDKN2A and CDK4 as well as numerous low-risk susceptibility loci identified primarily through candidate gene or genomewide association studies of melanoma, pigmentation, or nevi(20, 23, 24).
Most epidemiologic studies that have evaluated dysplastic nevi as a risk factor for melanoma have used clinical criteria primarily or exclusively to classify these melanocytic lesions(9). Overall, dysplastic nevi are relatively common with a frequency of about 10% (range, 7%-24%) in populations of northern European descent(21, 22). In contrast to common acquired nevi that occur predominantly in both sun-exposed and intermittently sun-exposed areas of the body, dysplastic nevi not only occur in sun-exposed and intermittently sun-exposed areas of the body but also in areas with little or no sun exposure such as the scalp, breast, and buttocks(22). Even though the criteria for dysplastic nevi sometimes differ between studies, there is remarkable consistency in the risks identified in diverse high-risk and low-risk populations(21). However, since the criteria used to classify DN vary across epidemiologic studies, it may be challenging to conduct joint studies by directly combining data across studies. Even with these challenges, joint or meta-analyses have been conducted and have shown DN to be a consistent risk factor for melanoma (for example, see (9, 25).
The largest meta-analysis to date was conducted by Gandini and colleagues(9). This meta-analysis examined 47 datasets that contained 10,499 cases and 14,256 controls. Of these, 27 datasets published risk estimates for dysplastic nevi (denoted as atypical nevi by Gandini et al(9)). For all of the 27 datasets, the assessment of the nevi was performed by clinicians, although the diagnostic criteria were not identical across all studies. Overall, dysplastic nevi were confirmed to be a highly significant risk factor for melanoma. Thirteen studies provided dichotomous data for DN. Among these 13 studies, the presence of DN conferred a 10-fold increased risk for melanoma (RR=10.1, 95% CI 5.0-20.3). For the 15 studies with continuous data on number of DN, the relative risks ranged from 1.6 (95% CI 1.4-1.8) for subjects with one DN to 10.5 (95% CI 5.1-21.8) for subjects with 5 or more DN. There was, however, significant heterogeneity between studies with hospital-based controls showing lower risk estimates than other types of control subjects. Similarly, relative risks for one DN in case-control studies (n=20) were much lower and more precise than those in cohort studies (n=8). In fact, the RR for having 5 DN was reduced to 6.4 (95% CI 3.8-10.3) when only case-control studies were considered. Regardless, even with inconsistent definitions and different study designs, one fact is clear; DN are one of the strongest and most consistent risk factors for melanoma.
Etiology of dysplastic nevi
The etiology of dysplastic nevi is not well characterized. Similar to melanoma, DN appear to result from the interplay of genetic, host, and environmental factors. There is evidence for a genetic component for nevi in general with several loci including IRF4 (chromosome 6p25-p23), MTAP (9p21), and PLA2G6 (22q13) reported from genome-wide association studies of melanocytic nevus count(26, 27), but there is less information for DN. At present, molecular examination of DN lesions is challenging because of the small nests of melanocytes in these lesions. As technology improves and the ability to examine single dysplastic nevus cells advances, the opportunities for further molecular exploration of DN lesions will increase. In anticipation of technologic advances, new studies should collect tissue, if possible.
Although familial melanoma and dysplastic nevi (historically called FAMMM or DNS) were originally thought to be pleiotropic effects of a single gene, subsequent studies have shown that the genetics are more complex and that the genetic causes of familial melanoma and DN are not the same(20, 28-31). In particular, there is little evidence for DN being caused by mutations in the major melanoma susceptibility genes CDKN2A or CDK4(29-31). However, similar to DN in unselected melanoma patients, DN are risk factors for melanoma in melanoma-prone families independent of CDKN2A mutations(32-34). The discovery of additional high, intermediate, and low-risk susceptibility genes for both melanoma and DN will lead to further deciphering of the genetic similarities and differences between these entities.
Multiple linkage studies have attempted to identify the major genetic cause(s) for dysplastic nevi with limited success(29, 35). Zhu and colleagues(35) conducted a genome-wide linkage scan for mole counts, including atypical subtypes using 796 microsatellite markers in 424 families with 1024 twins and siblings plus genotypes for 690 parents. The analysis showed suggestive but unconfirmed evidence of linkage to chromosomes 1, 6, and X with lod scores ranging from 2.0-2.2 across the three regions(35). de Snoo et al(29) conducted a linkage analysis of dysplastic nevi (denoted atypical nevi in the study) in four Dutch p16-Leiden melanoma-prone families including subjects as affected if they had >=5 dysplastic nevi and were negative for the p16-Leiden CDKN2A founder mutation. The authors found suggestive but unconfirmed evidence for a DN susceptibility locus on chromosome 7q21.3(29). No replication of these preliminary findings has yet occurred and no susceptibility gene(s) for dysplastic nevi have yet been identified.
DN as precursors of melanoma
DN are clearly major risk factors for melanoma, but are they precursors of melanoma? And what does it mean to be a melanoma precursor? According to the online Medical dictionary, MedlinePlus (Merriam Webster)(36), a precursor is defined as: 1: One that precedes and indicates the onset of another <angina may be the precursor of a second infarction>; or 2: a substance, cell, or cellular component from which another substance, cell, or cellular component is formed especially by natural processes. Using these definitions, dysplastic nevi may be classified as a precursor of melanoma since DN are potential and occasionally actual nonobligate precursors of melanoma based on pathologic evaluation of melanoma tumors(4). Most studies have found that approximately 20% of melanomas arise out of a DN; the numbers arising out of other types of nevi have not been well quantified and the majority of melanoma tumors arise de novo(7).
Although DN may be designated as precursors, the dysplastic nevus itself rarely progresses to melanoma. Tucker and colleagues(37) prospectively followed 33 melanoma-prone families for up to 25 years and found that most DN remained stable over time or regressed. During this follow-up study, few DN progressed to become suspicious for melanoma. Similar results have been observed for unselected individuals with DN; the vast majority of DN remained stable or regressed(38). In addition, Tsao and colleagues(39) evaluated the risk of nevi transforming into cutaneous melanoma. The authors estimated that the annual transformation rate of any single nevus into melanoma ranged from ≤1 in 200,000 for both men and women younger than 40 years to about 1 in 33,000 for men older than 60 years. In addition, the lifetime risk of any selected nevus transforming into melanoma by age 80 years (for a 20-year old individual) was about 0.03% for men and 0.009% for women. Although Tsao et al(39) did not assess the lifetime risk for a dysplastic nevus transforming into melanoma, the authors estimated the annual dysplastic nevus transformation rate and showed that the rate was very low at about 1 in 30,089 moles for males and 1 in 39,809 moles for females. Thus, dysplastic nevi are important primarily as risk factors for melanoma; their role as precursors is less critical because of the rarity of progression of any individual nevus to become a melanoma(4, 39).
Screening, detection, and management
Since early diagnosis of thin melanoma tumors is essential to survival after melanoma, it is important to appropriately screen and manage individuals at increased risk for melanoma. The presence of dysplastic nevi may be used to clinically identify individuals at increased risk for developing melanoma and to be part of the basis for developing clinical guidelines. As previously mentioned, however, there are multiple host, environmental, and genetic risk factors for melanoma and therefore screening and management should incorporate all risk-related information. However, for purposes of this review, we will focus on screening and management of dysplastic nevi.
Given the potential challenges in clinically diagnosing dysplastic nevi for the general clinician, one question is whether evaluation of nevus counts might be a useful alternative marker for risk. Few studies have had total body nevus counts of dysplastic and common acquired nevi to directly examine this question. However, in 1997, Tucker at al. (40) reported that the risk of melanoma associated with increased numbers of small (≥ 50) and large nevi (≥ 5) without any evidence of dysplastic nevi was 4.6 (95% CI 2.2-9.6) adjusted for age and freckling (18 cases and 17 controls). The risks associated with multiple dysplastic nevi ranged from 4.9 (95% CI 2.5-9.8) to 12 (95% CI 4.4-31) mutually adjusted for other types of nevi and adjusted for age, gender, number of sunburns, freckles, solar damage, nevus excisions, number of scars, and family history of melanoma (221 cases and 54 controls). Further, increased numbers of small and large nevi are correlated with the presence of dysplastic nevi; approximately half of the controls with ≥50 small nevi had dysplastic nevi compared to approximately a quarter of those with ≤25 small nevi. Therefore, although increased numbers of small and large nevi are clearly risk markers for melanoma, identifying dysplastic nevi adds additional information about level of risk.
Clinical guidelines for subjects with DN include surveillance of the skin and particularly pigmented lesions, routine skilled clinical examinations, regular self-skin evaluation, and use of sun protective measures(7, 8, 37). The frequency of the clinical examinations will vary depending on the age and sex of the individual and the activity of the pigmented lesions themselves. If nevi are not changing, then less frequent clinical examinations may be undertaken. A lesion that is changing in a manner suspicious for melanoma should be removed by excisional biopsy. Adherence to these guidelines in melanoma-prone families appears to decrease the risk of developing new melanomas and changing nevi and aids in the detection of melanoma at an earlier stage(37, 41).
Once dysplastic nevi are identified, routine care should include the use of total body photography to track changes of nevi over time. These lesions will change over time(37), but most changes are not worrisome for melanoma. The majority of dysplastic nevi undergo involution over years. As previously mentioned, lesions should only be biopsied when changing in a manner suspicious for melanoma. Dermoscopy is an important adjunctive to the use of photography(42). Use of either total body photography or dermoscopy or both may lead to a reduction in the number of benign nevi removed in proportion to melanomas removed, but the skill of the examiner appears to be an important component of dermoscopy success(43-45).
Although some patients may be tempted to have all of their nevi removed, prophylactic removal of all nevi is not appropriate since very few nevi progress to melanoma and progression is unpredictable(19, 22, 39). Further, even if all nevi were removed, risk for melanoma would not be eliminated and the frequency of clinical follow-up would not be altered because of the development of new nevi and de novo melanoma.
Dysplastic nevi are described as being on a continuum between common acquired nevi and melanoma because they are morphologically and biologically intermediate between these entities(4, 7). A subset of melanoma tumors have been found to arise out of dysplastic nevi based on histologic evaluation of the tumors. DN are, therefore, classified as potential and actual precursor lesions of melanoma, although they are nonobligate precursors since most melanomas do not develop from dysplastic nevi(4). Their role as precursors, however, is not their primary role in their relationship to melanoma because of the rarity of transformation of any individual nevus to a melanoma. In conclusion, although no unified definition or classification (or even name) for dysplastic nevi exists and although DN are precursor lesions for melanoma, dysplastic nevi should be considered important primarily because of their association with an increased risk for melanoma.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NCI, DCEG. The authors thank Mary Fraser and Geoff Tobias for their assistance.
Footnotes
The authors declare that they have no conflicts of interest related to this manuscript.
References
- 1.Clark WH, Jr, Reimer RR, Greene M, Ainsworth AM, Mastrangelo MJ. Origin of familial malignant melanomas from heritable melanocytic lesions. ‘The B-K mole syndrome’. Archives of dermatology. 1978;114:732–8. [PubMed] [Google Scholar]
- 2.Frichot BC, 3rd, Lynch HT, Guirgis HA, Harris RE, Lynch JF. New cutaneous phenotype in familial malignant melanoma. Lancet. 1977;1:864–5. doi: 10.1016/s0140-6736(77)92822-7. [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Frichot BC, 3rd, Lynch JF. Familial atypical multiple mole-melanoma syndrome. Journal of medical genetics. 1978;15:352–6. doi: 10.1136/jmg.15.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder DE. Dysplastic naevi: an update. Histopathology. 2010;56:112–20. doi: 10.1111/j.1365-2559.2009.03450.x. [DOI] [PubMed] [Google Scholar]
- 5.Elder DE, Goldman LI, Goldman SC, Greene MH, Clark WH., Jr Dysplastic nevus syndrome: a phenotypic association of sporadic cutaneous melanoma. Cancer. 1980;46:1787–94. doi: 10.1002/1097-0142(19801015)46:8<1787::aid-cncr2820460816>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Greene MH, Clark WH, Jr, Tucker MA, Elder DE, Kraemer KH, Fraser MC, et al. Precursor naevi in cutaneous malignant melanoma: a proposed nomenclature. Lancet. 1980;2:1024. doi: 10.1016/s0140-6736(80)92176-5. [DOI] [PubMed] [Google Scholar]
- 7.Duffy K, Grossman D. The dysplastic nevus: from historical perspective to management in the modern era: part I. Historical, histologic, and clinical aspects. Journal of the American Academy of Dermatology. 2012;67:1–e-16. doi: 10.1016/j.jaad.2012.02.047. quiz 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farber MJ, Heilman ER, Friedman RJ. Dysplastic nevi. Dermatologic clinics. 2012;30:389–404. doi: 10.1016/j.det.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 10.NIH Consensus conference. Diagnosis and treatment of early melanoma. JAMA : the journal of the American Medical Association. 1992;268:1314–9. doi: 10.1001/jama.1992.03490100112037. [DOI] [PubMed] [Google Scholar]
- 11.Diagnosis and treatment of early melanoma. NIH Consensus Development Conference. January 27-29, 1992; Consensus statement / NIH Consensus Development Conference National Institutes of Health Consensus Development Conference; 1992. pp. 1–25. [PubMed] [Google Scholar]
- 12.de Wit PE, van’t Hof-Grootenboer B, Ruiter DJ, Bondi R, Brocker EB, Cesarini JP, et al. Validity of the histopathological criteria used for diagnosing dysplastic naevi. An interobserver study by the pathology subgroup of the EORTC Malignant Melanoma Cooperative Group. Eur J Cancer. 1993;29A:831–9. doi: 10.1016/s0959-8049(05)80419-8. [DOI] [PubMed] [Google Scholar]
- 13.Hartge P, Holly EA, Halpern A, Sagebiel R, Guerry D, Elder D, et al. Recognition and classification of clinically dysplastic nevi from photographs: a study of interobserver variation. Cancer Epidemiol Biomarkers Prev. 1995;4:37–40. [PubMed] [Google Scholar]
- 14.Piepkorn MW, Barnhill RL, Cannon-Albright LA, Elder DE, Goldgar DE, Lewis CM, et al. A multiobserver, population-based analysis of histologic dysplasia in melanocytic nevi. Journal of the American Academy of Dermatology. 1994;30:707–14. doi: 10.1016/s0190-9622(08)81499-5. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes AR, Mihm MC, Jr, Weinstock MA. Dysplastic melanocytic nevi: a reproducible histologic definition emphasizing cellular morphology. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1989;2:306–19. [PubMed] [Google Scholar]
- 16.Shapiro M, Chren MM, Levy RM, Elder DE, LeBoit PE, Mihm MC, Jr, et al. Variability in nomenclature used for nevi with architectural disorder and cytologic atypia (microscopically dysplastic nevi) by dermatologists and dermatopathologists. Journal of cutaneous pathology. 2004;31:523–30. doi: 10.1111/j.0303-6987.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Steijlen PM, Bergman W, Hermans J, Scheffer E, Van Vloten WA, Ruiter DJ. The efficacy of histopathological criteria required for diagnosing dysplastic naevi. Histopathology. 1988;12:289–300. doi: 10.1111/j.1365-2559.1988.tb01943.x. [DOI] [PubMed] [Google Scholar]
- 18.Weinstock MA, Barnhill RL, Rhodes AR, Brodsky GL. Reliability of the histopathologic diagnosis of melanocytic dysplasia. The Dysplastic Nevus Panel. Archives of dermatology. 1997;133:953–8. [PubMed] [Google Scholar]
- 19.Tucker MA. Atypical melanocytic nevi. Chapter 123. New York, NY: McGraw-Hill Companies; 2007. [Google Scholar]
- 20.Goldstein AM, Tucker MA. Genetic epidemiology of cutaneous melanoma: a global perspective. Archives of dermatology. 2001;137:1493–6. doi: 10.1001/archderm.137.11.1493. [DOI] [PubMed] [Google Scholar]
- 21.Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042–52. doi: 10.1038/sj.onc.1206444. [DOI] [PubMed] [Google Scholar]
- 22.Tucker MA. Melanoma epidemiology. Hematology/oncology clinics of North America. 2009;23:383–95. vii. doi: 10.1016/j.hoc.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law MH, Macgregor S, Hayward NK. Melanoma genetics: recent findings take us beyond well-traveled pathways. The Journal of investigative dermatology. 2012;132:1763–74. doi: 10.1038/jid.2012.75. [DOI] [PubMed] [Google Scholar]
- 24.Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053–62. doi: 10.1038/sj.onc.1206445. [DOI] [PubMed] [Google Scholar]
- 25.Chang YM, Newton-Bishop JA, Bishop DT, Armstrong BK, Bataille V, Bergman W, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. International journal of cancer Journal international du cancer. 2009;124:420–8. doi: 10.1002/ijc.23869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. The Journal of investigative dermatology. 2010;130:520–8. doi: 10.1038/jid.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falchi M, Bataille V, Hayward NK, Duffy DL, Bishop JA, Pastinen T, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nature genetics. 2009;41:915–9. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop JA, Wachsmuth RC, Harland M, Bataille V, Pinney E, Mac KP, et al. Genotype/phenotype and penetrance studies in melanoma families with germline CDKN2A mutations. The Journal of investigative dermatology. 2000;114:28–33. doi: 10.1046/j.1523-1747.2000.00823.x. [DOI] [PubMed] [Google Scholar]
- 29.de Snoo FA, Hottenga JJ, Gillanders EM, Sandkuijl LA, Jones MP, Bergman W, et al. Genome-wide linkage scan for atypical nevi in p16-Leiden melanoma families. European journal of human genetics : EJHG. 2008;16:1135–41. doi: 10.1038/ejhg.2008.72. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen K, Harbst K, Masback A, Jonsson G, Borg A, Olsson H, et al. Swedish CDKN2A mutation carriers do not present the atypical mole syndrome phenotype. Melanoma research. 2010;20:266–72. doi: 10.1097/CMR.0b013e3283341339. [DOI] [PubMed] [Google Scholar]
- 31.Puig S, Ruiz A, Castel T, Volpini V, Malvehy J, Cardellach F, et al. Inherited susceptibility to several cancers but absence of linkage between dysplastic nevus syndrome and CDKN2A in a melanoma family with a mutation in the CDKN2A (P16INK4A) gene. Human genetics. 1997;101:359–64. doi: 10.1007/s004390050642. [DOI] [PubMed] [Google Scholar]
- 32.Chaudru V, Chompret A, Bressac-de Paillerets B, Spatz A, Avril MF, Demenais F. Influence of genes, nevi, and sun sensitivity on melanoma risk in a family sample unselected by family history and in melanoma-prone families. Journal of the National Cancer Institute. 2004;96:785–95. doi: 10.1093/jnci/djh136. [DOI] [PubMed] [Google Scholar]
- 33.Chaudru V, Laud K, Avril MF, Miniere A, Chompret A, Bressac-de Paillerets B, et al. Melanocortin-1 receptor (MC1R) gene variants and dysplastic nevi modify penetrance of CDKN2A mutations in French melanoma-prone pedigrees. Cancer Epidemiol Biomarkers Prev. 2005;14:2384–90. doi: 10.1158/1055-9965.EPI-04-0777. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein AM, Falk RT, Fraser MC, Dracopoli NC, Sikorski RS, Clark WH, Jr, et al. Sun-related risk factors in melanoma-prone families with CDKN2A mutations. Journal of the National Cancer Institute. 1998;90:709–11. doi: 10.1093/jnci/90.9.709. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G, Montgomery GW, James MR, Trent JM, Hayward NK, Martin NG, et al. A genome-wide scan for naevus count: linkage to CDKN2A and to other chromosome regions. European journal of human genetics : EJHG. 2007;15:94–102. doi: 10.1038/sj.ejhg.5201729. [DOI] [PubMed] [Google Scholar]
- 36.MedlinePlus (Merriam Webster) www.merriam-webster.com/medlineplus/precursor.
- 37.Tucker MA, Fraser MC, Goldstein AM, Struewing JP, King MA, Crawford JT, et al. A natural history of melanomas and dysplastic nevi: an atlas of lesions in melanoma-prone families. Cancer. 2002;94:3192–209. doi: 10.1002/cncr.10605. [DOI] [PubMed] [Google Scholar]
- 38.Halpern AC, Guerry Dt, Elder DE, Trock B, Synnestvedt M, Humphreys T. Natural history of dysplastic nevi. Journal of the American Academy of Dermatology. 1993;29:51–7. doi: 10.1016/0190-9622(93)70151-i. [DOI] [PubMed] [Google Scholar]
- 39.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Archives of dermatology. 2003;139:282–8. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 40.Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA : the journal of the American Medical Association. 1997;277:1439–44. [PubMed] [Google Scholar]
- 41.Tucker MA, Fraser MC, Goldstein AM, Elder DE, Guerry Dt, Organic SM. Risk of melanoma and other cancers in melanoma-prone families. The Journal of investigative dermatology. 1993;100:350S–5S. doi: 10.1111/1523-1747.ep12470264. [DOI] [PubMed] [Google Scholar]
- 42.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. The British journal of dermatology. 2008;159:669–76. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 43.Kovalyshyn I, Dusza SW, Siamas K, Halpern AC, Argenziano G, Marghoob AA. The impact of physician screening on melanoma detection. Archives of dermatology. 2011;147:1269–75. doi: 10.1001/archdermatol.2011.181. [DOI] [PubMed] [Google Scholar]
- 44.Risser J, Pressley Z, Veledar E, Washington C, Chen SC. The impact of total body photography on biopsy rate in patients from a pigmented lesion clinic. Journal of the American Academy of Dermatology. 2007;57:428–34. doi: 10.1016/j.jaad.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Salerni G, Carrera C, Lovatto L, Puig-Butille JA, Badenas C, Plana E, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. Journal of the American Academy of Dermatology. 2012;67:e17–27. doi: 10.1016/j.jaad.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


