Abstract
Background
Lung disease (LD) is the leading cause of death in systemic sclerosis (SSc). The diagnosis of SSc-related LD (SSc-LD) is often a contraindication to lung transplantation (LT) due to concerns that extra-pulmonary involvement will yield worse outcomes. We sought to evaluate post-transplant outcomes in persons with SSc-LD with esophageal involvement compared to persons with non-connective tissue disease related interstitial lung disease (nCTD-ILD).
Methods
From 1998-2012, persons undergoing LT for SSc-LD were age and gender matched in a 2:1 fashion to controls undergoing LT for nCTD-ILD. Esophageal function was assessed by pH testing and manometry. We defined esophageal dysfunction as the presence of a DeMeester score >14 or dysmotility more severe than “mild non-specific disorder”. The primary outcome was post-transplant survival. Secondary outcomes included freedom from bronchiolitis obliterans syndrome (fBOS) and rates of acute rejection. Survival and fBOS were estimated with Kaplan-Meier methods. Acute rejection was compared with Students t-test.
Results
Survival was similar in 23 persons with SSc-LD and 46 controls who underwent LT (p=0.47). For the SSc-LD group, 1- and 5-year survival was 83% and 76% compared to 91% and 64% in the nCTD-ILD group. There were no differences in fBOS (p=0.83). Rates of acute rejection were less in SSc-ILD (p=0.05). Esophageal dysfunction was not associated with worse outcomes (p>0.55).
Conclusions
Persons with SSc-LD appear to have similar survival and fBOS as persons transplanted for nCTD-ILD. The risk of acute rejection after transplant may be reduced in persons with SSc-LD. Esophageal involvement does not appear to impact outcomes.
Keywords: Lung Transplantation, Systemic Sclerosis, Interstitial Lung Disease, survival, esophageal dysmotility, bronchiolitis obliterans syndrome
INTRODUCTION
Pulmonary disease is the leading cause of death in persons with systemic sclerosis (SSc).1 SSc causes fibrosis and small vessel vasculopathy of multiple organ systems including the skin, lungs, heart, gastrointestinal tract and kidneys. Since the widespread use of angiotensin converting enzyme inhibitors, mortality from scleroderma renal crisis and its sequelae has fallen dramatically. As a result, SSc-related lung disease (SSc-LD), manifesting as either interstitial fibrosis (SSc-Interstitial Lung Disease [SSc-ILD]) or pulmonary hypertension (PH), is now the predominant cause of death. For patients with advanced SSc-LD, medical therapies are frequently ineffective and three year mortality exceeds 50%.2-5
The systemic involvement of SSc makes consideration of lung transplantation (LT) controversial. In 2006 the International Society of Heart and Lung Transplantation (ISHLT) endorsed LT as an option for persons with advanced lung disease secondary to connective tissue diseases.6 Many programs, however, consider SSc a contraindication to transplant based on the concern that extra-pulmonary organ involvement may negatively impact post-transplant allograft function and patient survival. Specifically, there is concern that esophageal dysfunction and gastroparesis may increase the risk of aspiration with resultant allograft injury.7,8 Additional concerns include corticosteroid precipitation of SSc-renal crisis and calcineurin inhibitor acceleration of renal dysfunction.9
A limited number of studies have demonstrated equivalent survival in carefully selected persons transplanted for SSc-LD compared to persons with idiopathic pulmonary fibrosis (IPF) or pulmonary arterial hypertension (PAH).10-14 In two of these studies, there were no differences in acute rejection and risk of bronchiolitis obliterans syndrome (BOS), either.10,13 Commonacross these studies was the exclusion of persons with evidence of more than mild proximal gastrointestinal tract pathology (e.g., esophageal stricture, dysmotility, achalasia, delayed gastric emptying, or symptoms not controlled by acid-suppressive medications). Thus, the impact of more severe proximal gastrointestinal involvement on outcomes following LT remains unknown.
The lung transplant program at UC San Francisco (UCSF) does not exclude persons with SSc-LD with proximal gastrointestinal tract disease, regardless of severity. Therefore, we sought to evaluate the risks of mortality, BOS, and acute cellular rejection in persons undergoing LT for SSc-LD, including those with moderate to severe gastrointestinal involvement.
RESULTS
Between 1998 and 2010, 328 persons underwent LT. Of these, 23 (7%) underwent LT for SSc-LD. All 23 subjects and the 46 nCTD-ILD matched controls underwent bilateral LT. The SSc-LD group was 53% female; mean age was 49.3 years (SD±8.8) (Table 1). No differences in age, gender, ethnicity, smoking history, serum creatinine, pulmonary function, or lung allocation scores were identified between the SSc-LD and the controls (IPF n=44, nCTD-non-specific interstitial pneumonitis n=2) (all p-values>0.19; Table 1). Subjects with SSc-LD, however, had lower BMI (22.9 vs. 26.9, p<0.01). While the SSc-LD group had higher mean pulmonary artery pressure (41.2±16.4 vs. 29.0±12.8; p<0.01) and pulmonary vascular resistance (8.2±7.1 vs. 3.6±2.1; p=0.01) than the control group; pulmonary capillary wedge pressures were similar (8.2±3.7 vs. 8.8±5.8; p=0.93).
Table 1.
Baseline Characteristics of Persons with SSc-LD and nCTD-ILD
| SSc – ILD | nCTD-ILD | p-value | |
|---|---|---|---|
| Values presented as mean ± standard deviation or n (%) | |||
| N | 23 | 46 | |
| Age | 49.3 ± 8.8 | 51.5 ± 7.9 | 0.25 |
| Male | 11 (47.8 % ) | 25 (54.4%) | 0.26 |
| White, Non-Hispanic | 18 (78.3 %) | 38 (82.6 %) | 0.19 |
| Non-Smokers | 13 (56.5%) | 18 (46.2%) | 0.49 |
| FEV1 | 1.48 ± 0.45 | 1.82 ± 1.32 | 0.57 |
| FEV1 % predicted | 50.0% ± 14.1 | 48.5% ± 17.2 | 0.43 |
| FVC | 1.75 ± 0.55 | 1.88 ± 0.72 | 0.87 |
| FVC % predicted | 46.7% ± 16.7 | 46.3 ± 17.2 | 0.76 |
| BMI | 22.9 ± 3.2 | 26.9 ± 4.9 | 0.0006 |
| Serum creatinine | 0.83 ± 0.21 | 0.90 ± 0.26 | 0.34 |
| Mean PAP | 41.2 ± 16.4 | 29.0 ± 12.8 | 0.0054 |
| PVR | 8.2 ± 7.1 | 3.6 ± 2.1 | 0.01 |
| PCWP | 8.2 ± 3.7 | 8.8 ± 5.8 | 0.934 |
| Lung Allocation Score | 50.8 ± 17.6 | 51.5 ± 21.8 | 0.43 |
| DeMeester Score | 65.2 ± 58.2 | 31.0 ± 26.8 | 0.13 |
| Esophageal Dysfunction | 12 (52.2%) | 19 (41.3%) | 0.47 |
SSc-LD: systemic sclerosis related lung disease, nCTD-ILD: non-connective tissue disease related interstitial lung disease, FEV1: forced expiratory volume at one second, FEV1%: percent predicated of forced expiratory volume at one second, FVC: forced vital capacity, FVC%: percent predicted of forced vital capacity, Esophageal Dysfunction: more than mild esophageal dysfunction via manometry or DeMeester score > 14, BMI: body mass index, PAP: pulmonary artery pressure, PVR: pulmonary vascular resistance (Woods units), PCWP: pulmonary capillary wedge pressure
Fourteen of 23 (61%) persons with SSc-LD, and 25 of 46 (54%) persons with nCTD-ILD underwent esophageal testing before LT. The SSc-LD group trended towards higher DeMeester scores than the nCTD-ILD group (65.2±58.2 vs. 31.0±26.8, p=0.13). Overall esophageal dysfunction was common in both the SSc-LD group (52%) and the nCTD-ILD group (41%) (p=0.47).
Subject Characteristics
Persons with SSc-LD were further classified as limited cutaneous SSc (lcSSc) (n=17;74%), diffuse cutaneous SSc (dcSSc) (n=4;17%), and SSc-sine-sclerosis (n=2;9%) (Table 2). Time from diagnosis of SSc to LT was 7.3±6.0 years.
Table 2.
Baseline Characteristics of Systemic Sclerosis Cohort
| Age | Sex | Disease Duration (yrs) |
SSc Type | Sclerodactyly | Raynaud | Cr | Mean PAP |
ANA Titer |
Pattern | Scl70 Titer |
|---|---|---|---|---|---|---|---|---|---|---|
| 52 | F | 9 | lcSSc | + | + | 0.8 | 27 | 1:2560 | Nucleolar | ND |
| 35 | F | 1 | lcSSc | + | + | 0.9 | 35 | >1:640 | Nucleolar | ND |
| 58 | F | 10 | lcSSc | + | + | 0.7 | 44 | >1:640 | Nucleolar | Negative |
| 59 | F | 3 | SSSS | - | + | 0.6 | 60 | >1:640 | ND | ND |
| 47 | F | 6 | lcSSc | + | + | 0.7 | 31 | 1:1280 | ND | ND |
| 60 | M | 0.5 | lcSSc | + | + | 1.2 | 30 | 1:160 | Speckled | ND |
| 42 | M | 10 | lcSSc | + | + | 1.1 | 49 | 1:2560 | Nucleolar | ND |
| 44 | F | ND | lcSSc | + | + | 0.7 | 73 | 1:320 | Speckled | ND |
| 52 | M | 17 | lcSSc | + | ND | 0.7 | 27 | 1:320 | Speckled | Negative |
| 40 | M | 7 | dcSSc | + | + | 0.8 | 28 | ND | ND | ND |
| 56 | F | 11 | lcSSc | + | + | 0.8 | 28 | ND | ND | ND |
| 58 | M | 1 | lcSSc | + | + | 0.7 | 17 | ND | ND | 4.92 |
| 45 | M | ND | lcSSc | + | + | 0.7 | 29 | ND | ND | ND |
| 54 | F | 8 | lcSSc | + | + | 1.2 | 58 | >1:640 | Nucleolar | ND |
| 44 | F | 9 | lcSSc | + | + | 1.0 | 48 | >1:640 | Centromere | Negative |
| 66 | F | ND | lcSSc | + | + | 1.1 | 60 | ND | ND | ND |
| 52 | M | 2 | dcSSc | + | + | 1.2 | 63 | 1:640 | Speckled | Negative |
| 50 | M | 15 | dcSSc | + | + | 0.8 | ND | 1:320 | Speckled | Negative |
| 34 | M | 3 | dcSSc | + | + | 0.5 | 23 | ND | ND | >5 |
| 42 | F | 13 | lcSSc | + | ND | 0.5 | ND | ND | ND | ND |
| 34 | M | 1 | SSSS | - | + | 1.0 | ND | 1:320 | Speckled | Negative |
| 52 | F | 20 | lcSSc | + | ND | 0.6 | 53 | ND | ND | ND |
| 46 | M | 2 | lcSSc | + | + | 0.8 | ND | 1:80 | Homogeneous | ND |
PAP: pulmonary artery pressure, Cr: creatinine, ANA: anti-nuclear antibody, Scl70: anti-topoisomerase I, ND: not determined, lcSSc: limited cutaneous systemic sclerosis, dcSSc: diffuse cutaneous systemic sclerosis; SSSS: systemic sclerosis sine scleroderma
Twelve subjects (52%) underwent both pH testing and esophageal motility evaluation. Normal motility, “mild non-specific dysmotility”, and aperistalsis were each observed in 3 (25%) persons; “severe non-specific dysmotility” in 2 (17%), and nutcracker esophagus in 1 (8%). Two additional subjects underwent pH testing without manometry. Only 4 of 14 persons (27%) had normal DeMeester scores (≤14). Six (26%) subjects underwent Nissen fundoplication an average of 4.9 months post-LT for clinical evidence of aspiration (median-4.9 months; 25%:1.1, 75%:10.5). One subject did not have esophageal dysmotility pre-LT. This subject underwent fundoplication 5.7 years post-LT for severe reflux and recurrent aspiration. Five control subjects underwent Nissen fundoplication an average of 4.8 months post-LT (median-4.8 months; 25%:3.5, 75%:5.5). One underwent Nissen fundoplication 2.3 years pre-LT. In all cases, fundoplication occurred before the diagnosis of BOS.
Survival
Survival was similar between the SSc-LD and nCTD-ILD groups (p=0.47) (Figure 1). For the SSc-LD group, estimated survival was 83%, 83%, and 76% at 1-, 3-, and 5-years post-LT compared to 91%, 77%, and 64% for the nCTD-ILD group.
Figure 1. Post-Transplant Survival in Persons with SSc-LD compared to non-CTD-ILD.
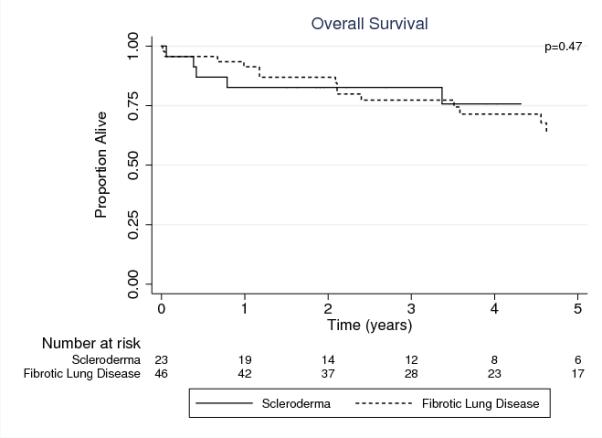
SSc-LD: systemic sclerosis related lung disease, non-CTD-ILD: non-connective tissue disease related interstitial lung disease
Analyses restricted to subjects with esophageal evaluation data showed that survival was similar between the two groups (p=0.99). For the SSc-LD group, 1-, 3-, and 5-year estimated survival was 86%, 86%, 76% compared to 96%, 83%, and 75% for patients transplanted for nCTD-ILD (Figure 2).
Figure 2. Post-Transplant Survival in Persons with SSc-LD compared to non-CTD-ILD with in Subjects with Esophageal Testing.
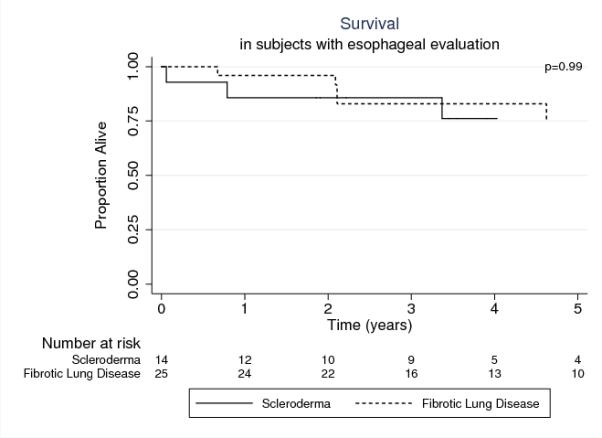
SSc-LD: systemic sclerosis related lung disease, non-CTD-ILD: non-connective tissue disease related interstitial lung disease, BOS: bronchiolitis obliterans syndrome
Neither the diagnosis of SSc-LD (odds ratio [OR] 1.5, 95% confidence interval [CI]: 0.27-8.65; p=0.64) ,esophageal dysfunction (OR 2.0, 95%CI 0.28-20.66; p=0.55), nor Nissen fundoplication were associated with death (p = 0.72).
Freedom from BOS
Freedom from BOS (fBOS) was similar between SSc-LD and nCTD-LD groups (p=0.83) (Figure 3). For the SSc-ILD group, 1-, 3-, and 5-year estimated fBOS was 100%, 74%, and 74% respectively compared to 98%, 77%, and 69% in the nCTD-ILD group.
Figure 3. Freedom from BOS in persons with SSc-LD compared to non-CTD-ILD.
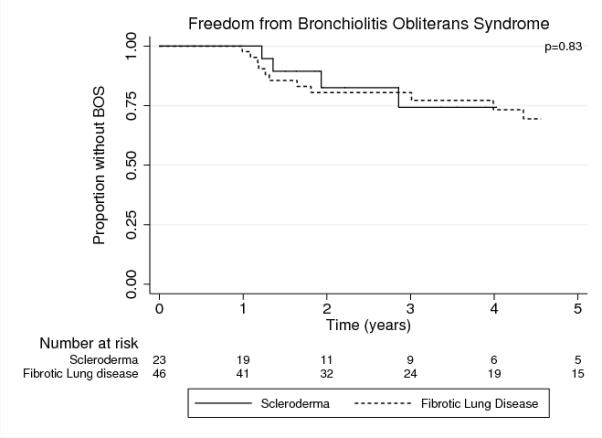
SSc-LD: systemic sclerosis related lung disease, non-CTD-ILD: non-connective tissue disease related interstitial lung disease, BOS: bronchiolitis obliterans syndrome
Analyses restricted to only those patients who underwent esophageal evaluation demonstrated that fBOS was similar between the two groups (p=0.87). For the SSc-LD group, 1- , 3-, and 5-years estimated fBOS for was 100%, 79%, and 79% respectively compared to 96%, 83%, and 75% for patients transplanted for nCTD-ILD.
Neither the diagnosis of SSc-LD (OR 1.0, 95% CI:0.16-6.69; p=0.98), esophageal dysfunction (OR 1.3, 95% CI:0.17-9.26; p=0.81), nor Nissen fundoplication were associated with fBOS (p = 0.46).
Acute Rejection
The SSc-LD and nCTD-ILD groups had similar rates of acute rejection (grade A≥2) (Figure 4). The SSc-LD group experienced 1.4 episodes per 10 patient-years compared to 2.4 episodes per 10 patient-years (p=0.05). In the SSc-LD group, there were 12 episodes of acute rejection amongst nine persons; within the nCTD-ILD group there were 43 episodes amongst 20 persons. This difference was not attributable to differences in the number of bronchoscopies with trans-bronchial biopsies performed per group. The SSc-LD group underwent 227 bronchoscopies with trans-bronchial biopsy (mean per patient-11.7±5.4) and the nCTD-ILD group underwent 447 bronchoscopies with trans-bronchial biopsy (12.0±4.9)(p=0.4).
Figure 4. Rate of Acute Rejection in persons with SSc-LD compared to nCTD-ILD.
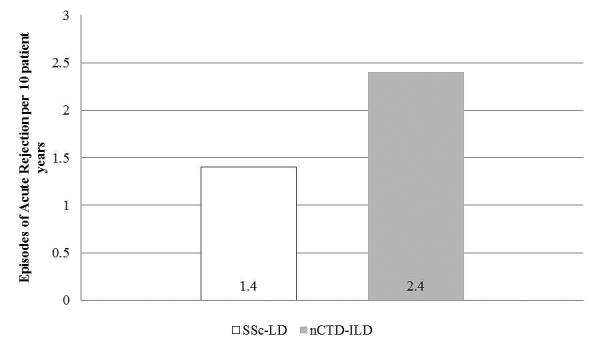
SSc-LD: systemic sclerosis related lung disease, nCTD-ILD: non-connective tissue disease related interstitial lung disease
DISCUSSION
We found that patients undergoing LT for SSc-LD who have esophageal dysfunction have similar five-year survival as patients transplanted for ILD unrelated to connective tissue diseases (nCTD-ILD). Notably, our five-year survival for persons with SSc-LD was similar to the international five-year survival for all LT recipients.15 Further, persons with SSc-LD do not appear to be at increased risk for bronchiolitis obliterans syndrome (BOS) or acute rejection compared to persons with nCTD-ILD.
Our study adds important additional information to the modest published literature evaluating LT for SSc-LD. Our experience with 23 subjects is one of the largest single-center studies of LT for SSc-LD representing 28% of the published experience in transplant for this condition.10-12,14 Moreover, our long-term follow up suggests that the previously reported short- and medium-term outcomes are durable. Notably, the 76% three-year survival in our cohort appears better than previously reported.10,13 An analysis of the US national registry identified a 67% three-year survival following lung transplant for SSc-LD.13 Further, our 74% fBOS at three-years is similar to or better than a report of 52%.10
An important finding with clinical implications is that patients with SSc-LD and severe esophageal dysfunction can undergo successful LT. One explanation may be our center’s comprehensive and proactive approach to esophageal dysfunction in persons with SSc-LD. Before and after LT, a multidisciplinary team of pulmonologists, rheumatologists, and gastroenterologists, thoracic and abdominal surgeons, and speech pathologists design individual treatment plans for each patient. Symptoms of GERD or evidence of aspiration are aggressively monitored and treated with a combination of behavior modification, medication, or surgery. It is notable that seven of the 14 persons with SSc-LD who underwent esophageal evaluation would have likely been declined for transplant in previous studies based solely on their manometry results.
Unlike a previous study, we identified modestly lower rates of acute rejection in those who underwent transplantation for SSc-LD compared to nCTD-ILD. Saggar, et al, found that 62% of persons transplanted for SSc-LD developed acute rejection (grade A≥2) at 1-year compared to 22% transplanted for IPF (p=0.007).10 Saggar, et al, postulated that esophageal dysfunction in the SSc-LD group may have explained this difference. Our findings in a cohort with more severe esophageal reflux and dysmotility than the Saggar cohort suggest that an alternative mechanism may be operational.10 Alternatively, our proactive approach to esophageal dysfunction in persons with SSc-LD attenuated any potentially attributable risk.
Our study has certain limitations. First, not all subjects underwent complete esophageal evaluation before LT (39% of SSc-LD and 46% of nCTD-ILD subjects did not). While this could have introduced selection bias, we did not observe worse (or a trend towards worse) outcomes in our sensitivity analysis. This suggests that either esophageal dysfunction in SSc-LD does not impact outcomes beyond that seen in persons without SSc-LD or that an aggressive multidisciplinary approach to screening and treatment can mitigate its effects.
Second, our modest sample size may limit our ability to detect small differences. In our analysis, neither SSc nor esophageal dysfunction were associated with an increased odds of death. The point estimates for these outcomes, however, were substantially greater than one. While the stability of these point estimates in larger sample sizes cannot be estimated, it is possible we were underpowered to detect true differences. Further, our modest sample size limited additional analyses investigating the impact of interventions such as Nissen fundoplication on outcomes. While our sensitivity analysis did not identify an association with fundoplication with either BOS or death, it would be speculative to interpret our results as demonstrating fundoplication is not effective in mitigating the possible risk of gastrointestinal reflux on allograft or patient outcomes. Additionally, the survival and fBOS in our cohort was similar to internationally reported outcomes.15 While modest from a statistical standpoint, however, our study represents one of the largest cohorts of patients undergoing LT for SSc-LD. Given the rarity of transplant for this condition, a multicenter study would be needed to detect small differences in outcomes.
Third, we reported herein a single center retrospective experience. Therefore, the subclassification of SSc may have been subject to inaccuracies. We identified a high proportion of limited cutaneous SSc (lcSS) in our cohort. While possible, the proportion of lcSSc in our cohort is similar to an earlier study.10 We identified a substantial number of subjects with anti-nucleolar ANAs (which include anti-U3 RNP fibrillarin and anti-Th/To) that can be quite specific for SSc. Importantly, nucleolar-ANAs in SSc have been associated with a higher frequency of ILD and PAH which would be consistent with the need for LT.16 It is also possible that our cohort of SSc-LD may have included subjects with overlap syndromes with SSc (i.e.,mixed connective tissue disease [MCTD] or undifferentiated connective tissue disease [UCTD]). Indeed, approximately 20% of SSc patients may have overlap syndromes.17 We cannot speculate on the prevalence of these conditions in our cohort, however we do not consider MCTD or UCTD to be contraindications to LT. Finally, our findings may not be generalizable to other centers.
In conclusion, we show patients undergoing LT for SSc-LD have similar survival, freedom from BOS, and risk of acute rejection as patients with nCTD-ILD. These outcomes do not appear to be impacted by SSc involvement of the esophagus. At specialized transplant centers, SSc should not be a contraindication to LT.
METHODS AND MATERIALS
Study Population
We performed a retrospective cohort study of all persons undergoing LT for SSc-LD between January 1, 1998 and December 31, 2010. Follow-up data was abstracted through April 7, 2012. Controls with non-connective tissue disease related interstitial lung disease (nCTD-ILD) were randomly matched by age (±3 years) and gender in a 2:1 fashion to persons with SSc-LD. Persons with nCTD-ILD had either IPF or nCTD-non-specific interstitial pneumonitis. Diagnoses were identified by the listing diagnosis submitted to the United Network for Organ Sharing (UNOS) and were confirmed by explanted lung pathology. Institutional Review Board (IRB) approval was obtained (IRB#11-08211).
LT recipients with SSc-LD were initially identified by UNOS listing diagnosis. Two rheumatologists (T.K.,K.C.) with expertise in systemic sclerosis confirmed the diagnoses by applying the American College of Rheumatology (ACR) criteria for the diagnosis of SSc.18 Data were obtained from record review at our transplant center. and from referring physicians. Based on these data, patients were further classified as diffuse cutaneous SSc (dcSSc), limited cutaneous SSc (lcSSc), or systemic sclerosis sine scleroderma (SSSS) according to the classification of LeRoy, et al. and Poormoghim.19,20 Patients were classified as lcSSc by default if skin thickening proximal to the elbows/knees and/or trunk was not mentioned. Classification of SSSS required a clinical diagnosis of SSc without skin thickening and ≥1 of the following: distal esophageal hypomotility, small bowel hypomotility, pulmonary fibrosis, PH, cardiac involvement, or SSc-renal crisis.20
The most proximate data to the date of LT were collected from medical records including age, gender, ethnicity, body mass index (BMI), serum creatinine, anti-nuclear antibody (ANA) titer and pattern by immunofluorescense assay, anti-topoisomerase I (Scl-70) antibody titer, mean pulmonary artery pressure, pulmonary vascular resistance, and pulmonary capillary wedge pressure by right heart catheterization, FEV1, FEV1% predicted, FVC, FVC% predicted, lung allocation score (LAS), and duration of disease before LT. For persons transplanted prior to 2005, LAS was calculated from available medical records. If data needed for calculating the LAS were missing, assumptions included: a 2LPM oxygen requirement; functional status requiring “some assistance”; no mechanical ventilation; a partial pressure of carbon dioxide of 40millimeters of mercury; and six-minute walk distance of 500ft. These assumptions were consistent with clinical LAS scoring that assume the same values when missing.
As part of the LT evaluation, persons with SSc or nCTD-ILD usually undergo testing of esophageal function by pH testing (Bravo pH Monitoring, Given Imaging, Duluth, GA) and manometry (ManoScan-360, Sierra Scientific Instruments, Los Angeles, California). Patients referred for pH testing routinely stop proton-pump inhibitors fourteen days prior to testing and transition to H2 blockers. H2 blockers are stopped three days prior to testing. If studies were available, DeMeester scores (a composite pH monitoring score [normal≤14]) and esophageal motility data were collected. Esophageal motility reports included the descriptors: normal; mild non-specific motility disorder; severe non-specific motility disorder; nutcracker esophagus; or aperistalsis.
When evaluating patients with SSc-LD for LT, SSc-specific criteria are considered. We consider active digital ischemia and renal insufficiency (glomerular filtration rate <60 milliliters per minute calculated by Cockcroft and Gault or by nuclear medicine study21) an absolute contraindications to LT. Further, symptoms of gastroesophageal reflux disease (GERD) or frank aspiration must be controlled by lifestyle modifications and acid suppressive therapy. Persons with SSc-LD are not, however, excluded from LT based solely on abnormal pH monitoring or esophageal manometry studies.
Consideration for LT involves building a unique patient-specific risk profile. For patients with SSc-LD, the SSc-specific evaluation criteria are combined with our standard selection criteria. The same weighting of standard relative and absolute contraindications is applied to patients with SSc-LD. Indeed, from 2007-2012, reasons for transplant denial for patients with SSc-LD included multivessel coronary artery disease, illicit drug use, renal insufficiency, and poor medical adherence.
Post-Transplant Care
Our immunosuppression regimen includes prednisone, tacrolimus, and mycophenolate mofetil. Prophylaxis against opportunistic infections targets cytomegalovirus, pneumocystis, and fungus. Patients are prescribed proton pump inhibitors, calcium supplements, and bisphosphonates.
Persons with SSc-LD suspected of having oropharyngeal dysfunction undergo swallow evaluations immediately post-LT by clinical speech pathologists. Evaluations include fiberoptic study with liquids and solids coated in toluene blue to screen for macroaspiration. Patients are educated in swallow techniques to minimize aspiration. Feeding tubes or limitations on oral intake of food are not routinely instituted. If there is evidence of gross aspiration not attributable to oropharyngeal dysfunction, fundoplication is considered.
After LT, all patients undergo routine surveillance for occult infection or acute rejection that includes spirometry, high-resolution computed tomography (HRCT), fiberoptic-flexible bronchoscopy with bronchoalveolar lavage, and trans-bronchial biopsies. Surveillance is routinely performed on post-operative day 14 and months 1, 2, 3, 6, 12, 18, and 24. Annual surveillance HRCT and spirometry are continued thereafter.22 Additional testing is performed as clinically indicated. Trans-bronchial biopsies are performed during clinically indicated bronchoscopy if acute rejection is a diagnostic consideration. They are not routinely performed if there is clear evidence of infection (i.e., imaging consistent with infection and purulent secretions evident during bronchoscopy). Trans-bronchial biopsy specimens were evaluated by a single expert pulmonary pathologist and scored for acute rejection and bronchiolitis obliterans based on ISHLT criteria.23
Outcomes
Our primary outcome was overall survival. Secondary outcomes included freedom from bronchiolitis obliterans syndrome (BOS) Stage≥1 and the rate of acute cellular rejection. BOS was defined according to ISHLT criteria.24 Acute cellular rejection was defined as grade ≥A2 according to ISHLT criteria.23 Some of this data was published earlier in abstract form.25
Statistical Analysis
Baseline characteristics were compared by chi-squared or Wilcoxon rank-sum tests. Since differences in acute rejection might be attributable to indication bias, we collected the number of bronchoscopies with trans-bronchial biopsies. The number of bronchoscopies performed per patient were normally distributed. Therefore, the number of biopsies performed per patient in each group were tested by t-test. Survival and freedom from BOS (fBOS) at 1, 3, and 5 years was estimated using Kaplan-Meier methods. Equality of survivor functions was tested by log-rank. Acute rejection rates were compared by t-test.
Esophageal dysfunction was categorically defined as either an abnormal DeMeester score (>14) or manometry findings more severe than “mild non-specific disorder”. Multivariate logistic regression was used to test the relative risk of death and fBOS, controlling for gender, BMI, creatinine and esophageal dysfunction. Complete esophageal testing was not available for all subjects, introducing the potential for selection bias. We therefore performed a sensitivity analysis employing the same regression model and estimated survival for only those with complete data available. The associations between Nissen fundoplication and survival and between fundoplication and BOS were tested by Fisher exact test.
Analyses were performed using Stata 11.2 (StataCorp LP, College Station,Tx).
ACKNOWLEDGEMENTS
This work was supported by NIH/NHLBI Grant Number K23 HL111115-01 (JPS) and an American College of Rheumatology Research and Education Foundation Rheumatology Investigator Award (to TRK). The authors wish to thank Kerry Kumar, Jill Obata, Millie Camba, and Karen Breen, the nurse coordinators of the lung transplant program, for their assistance and perspective throughout the preparation of this manuscript. The authors wish to thank Joan Chen and Monica Dean for their help in acquiring data for this study.
Dr. Singer is a transplant pulmonologist involved in patient care who participated in study design, data analysis, and was the primary editor of the manuscript. He is the corresponding author and guarantor of the manuscript. He has no conflicts of interest to disclose. Support for this study was through NIH/NHLBI Grant Number F32 HL107003-01.
ABBREVIATION LIST
- ACR
American College of Rheumatology
- ANA
anti-nuclear antibody
- BMI
body mass index
- BOS
bronchiolitis obliterans syndrome
- dcSSc
diffuse cutaneous systemic sclerosis
- FEV1
forced expiratory volume at 1 second
- FVC
forced vital capacity
- fBOS
freedom from bronchiolitis obliterans syndrome fBOS
- GERD
gastroesophageal reflux disease
- HRCT
high-resolution computed tomography
- IPF
idiopathic pulmonary fibrosis
- ISHLT
International Society of Heart and Lung Transplantation
- LAS
lung allocation score
- lcSSc
limited cutaneous systemic sclerosis
- LD
lung disease
- LT
lung transplantation
- nCTD-ILD
non-connective tissue disease related interstitial lung disease
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PH
pulmonary hypertension
- SSc
systemic sclerosis
- SSc-LD
systemic sclerosis related lung disease
- SSc-ILD
systemic sclerosis related interstitial lung disease
- SSSS
systemic sclerosis sine scleroderma
- UNOS
United Network for Organ Sharing
- UCSF
University of California San Francisco
Footnotes
Dr. Sottile participated in data collection and analysis in addition to being primary author of the manuscript. He has no conflicts of interest to disclose.
Dr. Itrube participated in data collection and study design in addition to providing critical review of the manuscript. He has no conflicts of interest to disclose.
Dr. Katsumoto is a rheumatologist who reviewed patient charts and classified scleroderma subtypes as well as provided critical review of the manuscript. She has no conflicts of interest to disclose.
Dr. Connolly is a dermatologist who reviewed patient charts and classified scleroderma subtypes as well as provided critical review of the manuscript. She has no conflicts of interest to disclose.
Dr. Collard is an interstitial lung disease pulmonologist who participated in study design and provided critical review of the manuscript. He has no conflicts of interest to disclose.
Dr. Leard is a transplant pulmonologist involved in patient care and provided critical review of the manuscript. She has no conflicts of interest to disclose.
Dr. Hays is a transplant pulmonologist involved in patient care who provided critical review of the manuscript. He has no conflicts of interest to disclose.
Dr. Golden is a transplant pulmonologist involved in patient care who participated in study design and provided critical review of the manuscript. He has no conflicts of interest to disclose.
Dr. Hoopes is a transplant cardiothoracic surgeon who provided critical review of the manuscript. He has no conflicts of interest to disclose.
Dr. Kukreja is transplant cardiothoracic surgeon who provided critical review of the manuscript. She has no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009;179:151–7. doi: 10.1164/rccm.200806-953OC. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 5.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford) 2006;45:1005–8. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]
- 6.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–5. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Gasper WJ, Sweet MP, Golden JA, et al. Lung transplantation in patients with connective tissue disorders and esophageal dysmotility. Dis Esophagus. 2008;21:650–5. doi: 10.1111/j.1442-2050.2008.00828.x. [DOI] [PubMed] [Google Scholar]
- 8.D’Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80:1254–60. doi: 10.1016/j.athoracsur.2005.03.106. [DOI] [PubMed] [Google Scholar]
- 9.Denton CP, Lapadula G, Mouthon L, Muller-Ladner U. Renal complications and scleroderma renal crisis. Rheumatology (Oxford) 2009;48(Suppl 3):iii32–5. doi: 10.1093/rheumatology/ken483. [DOI] [PubMed] [Google Scholar]
- 10.Saggar R, Khanna D, Furst DE, et al. Systemic sclerosis and bilateral lung transplantation: a single centre experience. Eur Respir J. 2010;36:893–900. doi: 10.1183/09031936.00139809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shitrit D, Amital A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant. 2009;23:178–83. doi: 10.1111/j.1399-0012.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 12.Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 13.Massad MG, Powell CR, Kpodonu J, et al. Outcomes of lung transplantation in patients with scleroderma. World J Surg. 2005;29:1510–5. doi: 10.1007/s00268-005-0017-x. [DOI] [PubMed] [Google Scholar]
- 14.Rosas V, Conte JV, Yang SC, et al. Lung transplantation and systemic sclerosis. Ann Transplant. 2000;5:38–43. [PubMed] [Google Scholar]
- 15.Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–71. doi: 10.1164/rccm.201101-0134CI. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol. 2010;37:42–53. doi: 10.1111/j.1346-8138.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- 17.Pakozdi A, Nihtyanova S, Moinzadeh P, Ong VH, Black CM, Denton CP. Clinical and serological hallmarks of systemic sclerosis overlap syndromes. J Rheumatol. 2011;38:2406–9. doi: 10.3899/jrheum.101248. [DOI] [PubMed] [Google Scholar]
- 18.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 19.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 20.Poormoghim H, Lucas M, Fertig N, Medsger TA., Jr. Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444–51. doi: 10.1002/1529-0131(200002)43:2<444::AID-ANR27>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–4. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61:744–6. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 25.Sottile PD, Iturbe D, Golden JA. Outcomes in Scleroderma-Related Interstitial Lung Disease Following Lung Transplant [abstract] Journal of Heart and Lung Transplanation. 2011:S204. [Google Scholar]


