Abstract
Background
Clinical trials of acupuncture in chronic pain have largely failed to demonstrate efficacy of traditional over sham acupuncture. However, it should be noted that sham acupuncture is not inert.
Objective
To determine if experimental-pressure pain assessment and chemical neuroimaging can identify differential responsiveness to sham as opposed to traditional acupuncture.
Patients and Intervention
Fifty patients with fibromyalgia were randomized to either 9 traditional (TA) or sham (SA) acupuncture treatments over a period of 4 weeks. Both participants and assessors were blinded.
Main Outcome Measures
The main outcome measures were pressure-pain sensitivity at the thumbnail, insular glutamate+glutamine (Glx), and clinical pain.
Results
Patients with low pain sensitivity (LPS), but not with high pain sensitivity (HPS), had a significantly reduced clinical pain response to SA (change in mean [standard deviation (SD)]: HPS −8.65 [7.91]; LPS −2.14 [6.68]; p=0.03). This relationship was not the case for TA (HPS −6.90 [4.51]; LPS −6.41 [9.25]; p=0.88). SA-treated patients who were more sensitive also had greater baseline levels of insular Glx than patients who were less sensitive (Glx mean [SD]: HPS 11.3 [1.18]; LPS 10.2 [0.54]; p=0.04).
Conclusions
Pressure-pain testing may identify patients who are less likely to respond to SA. This effect may relate to the levels of brain excitatory neurotransmitters.
Key Words: Acupuncture, Fibromyalgia, Sham, Pain, Sensitivity, Glutamate, Glx
Introduction
Randomized sham-controlled clinical trials of acupuncture in chronic pain conditions have struggled to show efficacy—because both traditional (TA) and sham (SA) acupuncture reduce clinical pain.1–4 In the most-recent, large-scale, individual-patient–data meta-analysis, combining treatment results from >5000 patients in sham-controlled trials, the effect sizes of TA and SA were of similar magnitude, albeit with a statistically significant benefit for TA.5 At a minimum, these data indicate that SA is not an inert intervention. While many approaches have been taken to improve the analgesic effects of TA, such as using individualized acupuncture needling locations6,7 and varying the methods of needle stimulation (manual, electrical, laser, and thermal stimulation), less attention has been paid to exploring or identifying specific analgesic factors that are operative in SA.
Fibromyalgia (FM) is a chronic, widespread pain condition with a history of inconclusive clinical trials regarding acupuncture efficacy.8,9 However, in these trials an increased analgesic response to acupuncture has been shown for more-invasive interventions, such as electroacupuncture (EA): Acupuncture trials that compared EA versus SA were efficacious, whereas trials using manual acupuncture versus SA were not.10 As EA induces more-robust somatosensory afference than manual acupuncture, some researchers have speculated that the degree of sensation that the patient with pain experiences during needling may play a factor in treatment response.11
One hallmark of central pain conditions such as FM is a global reduction in evoked pain thresholds, or increase in pain sensitivity, at sites throughout the body.12,13 Interestingly, the current authors' group demonstrated that the degree of enhanced pain sensitivity in FM is associated with elevated levels of combined glutamate (Glu), an excitatory neurotransmitter, and glutamine (Gln; combination, Glx) within the insula, a brain region involved in higher-order sensory processing.14,15 These data have led the current authors and other researchers to speculate that elevated excitatory neurotransmission may play a role in enhanced pain sensitivity in FM. However, the role of this enhanced sensitivity in treatment response has largely been unexplored.
Here it is proposed that the degree of enhanced sensitivity to experimental-pressure pain (hyperalgesia) may actually be a critical factor in determining whether a given patient responds or does not respond to SA treatment. The current authors reasoned that, because interventions that generate greater sensory sensations (somatosensory afference) engender greater clinical response in FM, patients who are less sensitive to experimental-pain stimuli might actually be poor clinical pain responders to SA, an intervention that is likely to generate less sensation than TA. Moreover, those individuals who are less sensitive to pressure pain may also have lower levels of brain Glx within pain-processing areas in the brain, such as the insula.
Methods
Participants
As part of a larger study investigating the effects of acupuncture treatment in FM, 56 female patients were recruited and examined. Because of missing clinical data, 6 participants were excluded from the final analysis (N=50; mean [standard deviation (SD)] age: 46.0 [13.9] years). All participants were randomized to receive either nine TA (n=22) or nine noninsertive SA (n=28) treatments over a 4-week period. Demographics of the sample population are presented in Table 1. No significant differences were detected between participants in the TA and SA groups for age, race, duration of FM symptoms, or pretreatment clinical pain scores. Participants gave written informed consent and all study protocols were approved by the University of Michigan's institutional review board. All participants: (1) met the American College of Rheumatology (1990) criteria16 for the diagnosis of FM for at least 1 year; (2) had continued presence of pain more than 50% of days; (3) were willing to limit the introduction of any new medications or treatment modalities for control of FM symptoms during the study; (4) were over 18 and under 75 years of age; (5) were right handed; and (6) had no alcohol intake 48 hours prior to proton magnetic resonance spectroscopy (1H-MRS) studies; and (7) were capable of giving written informed consent. Participants were excluded if they: (1) had previous experience with acupuncture; (2) had current use or a history of use of opioid or narcotic analgesics; (3) had a history of substance abuse; (4) had the presence of a known coagulation abnormality, thrombocytopenia, or bleeding diathesis that could preclude the safe use of acupuncture; (5) had the presence of concurrent autoimmune or inflammatory disease, such as rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, etc., that causes pain; (6) had concurrent participation in other therapeutic trials; (7) were pregnant and nursing mothers; (8) had severe psychiatric illnesses (current schizophrenia, substance abuse within 2 years); (9) had current major depression; or (10) had contraindications to 1H-MRS.
Table 1.
Participant Demographics
| Variables | TA | SA | p-Value |
|---|---|---|---|
| Age yrs mean (SD) | 47.5 (12.8) | 44.8 (14.7) | 0.39* |
| Duration FM yrs mean (SD) | 7.33 (6.59) | 5.78 (6.23) | 0.41* |
| Clinical pain (SF MPQ Total) mean (SD) | 16.6 (6.48) | 17.9 (8.66) | 0.57* |
| Race | 0.39** | ||
| Caucasian | 20 | 24 | |
| African American | 1 | 2 | |
| Asian | 1 | 0 | |
| Uncertain | 0 | 2 |
Independent samples t-test; **Chi square.
TA, traditional acupuncture; SA, sham acupuncture; yrs, years; SD, standard deviation; FM, fibromyalgia; SF MPQ, Short Form of McGill Pain Questionnaire.
Treatment
An acupuncture treatment protocol was used based on Traditional Chinese Medicine that was previously utilized in a large clinical trial of TA versus SA in patients with FM.4 This protocol was used because: (1) participants could not determine whether they received TA or SA, and (2) acupuncture produced robust analgesic effects in both treatment groups. Participants were randomized via a random number generator to either TA or SA groups using a block of 4 participants. Patient allocation was kept in opaque envelopes that were numbered consecutively and opened by the practitioner following consent of each participant. For both TA and SA, the current authors used a fixed-needle formula wherein all patients, within a group, received needling in the same locations. During TA, 9 acupuncture needles (Seirin 0.25×50 mm) were inserted at GV 20, ear Shenmen, LI 4, LI 11, SP 6, LR 3, GB 34, and bilateral ST 36. Needle-insertion depth was approximately 2 cm for all TA points except for DU 20 and ear Shenmen, which had shallower insertion depths. All needles below the neck level were manually manipulated to elicit De Qi sensations. SA participants experienced a non–skin penetrating pricking sensation at 9 nonacupuncture point locations, which was evoked using a previously validated sham procedure.17 Given that this sham intervention did not penetrate the skin and was designed to not elicit De Qi, the current authors reasoned that the somatosensory component generated by this procedure would be likely to be less than the skin penetrating-TA protocol that elicited De Qi. The sham locations were within similar body locations as the TA points; however, the SA location were not on known acupuncture points or meridians. A diagram of the current authors' point locations was previously published.18 The length of time was similar for needle insertion and manipulation for TA and skin pricking for SA, as was the time of needle retention for TA and waiting for SA (approximately 30 minutes total time for both treatments). Participants in both groups received no more than 3 treatments per week. All participants were blindfolded during each treatment to prevent patient knowledge of treatment assignment. All TA and SA treatments were performed by a single practitioner who was certified by the National Certification Commission for Acupuncture and Oriental Medicine, with 6 years of previous experience in treating patients who have chronic pain.
Outcomes
Evoked pressure pain. Prior to TA and SA, discrete pressure stimuli were applied to each participant's right thumbnail using a custom-made stimulation device that eliminated any direct examiner–subject interaction. The apparatus induced pressure via a hydraulic system connected to a 1-cm2 hard rubber circular probe that was pressed against the right thumbnail. The thumbnail was chosen, as it has been shown to be highly representative of overall pressure sensitivity.19 The stimulator was positioned over the thumb by a plastic housing and the hydraulic system was activated by calibrated weights placed on a moveable platform. Valves incorporated into the hydraulic system controlled stimulus timing. The combination of valves and calibrated weights allowed for controlled and repeatable stimulation. Pain-intensity ratings for each pressure were recorded on a 21-box numerical descriptor scale.20 This scale had been constructed from previously quantified verbal descriptors21–23 and has been shown to be sensitive in other studies.24–27
Prior to the start of pressure-pain testing, the equipment was demonstrated and explained, using a scripted text and a few discrete pressure stimuli were applied to familiarize subjects with the procedure. Additional information and explanations were provided if required. To first determine the subject's pain-sensitivity range, stimuli of 5-second duration were applied to the right thumbnail in ascending order with an inter-stimulus interval of 20 seconds. Initial stimulation pressure was 0.25 kg/cm2, and the pressure was increased in 0.25- to 0.50-kg/cm2 increments up to either a subject's level of pain tolerance or to a maximum of 10 kg/cm2.
Custom software was then used to apply the data collected from this ascending series to compute starting stimulus intensities for another set of stimuli controlled by the method of multiple random staircases. This method is an interactive algorithm in which software logic continuously adjusts stimulus intensities to maintain pain ratings at several specific levels.28 Three independent staircases were titrated to produce pain sensations rated between 0 and 1 (faint pain), between 7 and 8 (mild–moderate pain), and between 13 and 14 (strong–slightly intense pain) on the 21-box numerical descriptor scale described above. On each trial, the method randomly selected a staircase and delivered the stimulus intensity associated with that staircase. The patient response determined the next stimulus intensity delivered by that staircase the next time it was selected. This determination was based on response history and used a dynamically changing step size to estimate the stimulus intensity required to produce the level of pain associated with each particular staircase. Each of the 3 staircases delivered 12, 5-second duration stimuli (36 total) at 20-second intervals. Stimulus intensities (in kg/cm2) obtained from the middle staircase (mild–moderate pain) were used to dichotomize participants into either high (HPS) or low (LPS) pain sensitivity groups for further analysis. Data from the other staircases were not analyzed in for the current report.
Clinical pain. Clinical pain was assessed—immediately prior to and following treatment—with the Short Form of the McGill Pain Questionnaire (SF MPQ)29 and a 10-cm visual analogue scale (VAS) for pain. The SF MPQ has two subscales that measure sensory and affective qualities of pain. The VAS was bounded by 0=“no pain” and 10=“worst pain imaginable.”
Proton Magnetic Resonance Spectroscopy
Sixteen subjects within the SA group underwent conventional magnetic resonance imaging of the brain on a General Electric 3.0 Tesla MR scanner (GE, Milwaukee) prior to treatment. Of these participants, 12 were analyzed in the current authors' previous work.14,15 Single-voxel spectroscopy (SVS) was performed using the following parameters: PRESS, TR 3000 ms/TE 30 ms, 90° flip angle; NEX 8, field of view 16, with a volume of interest (VOI) of 2×2×3cm. During each session, two separate SVS sequences were performed, once with the VOI placed in the right anterior insula and once in the right posterior insula as described previously.14,15 The approximate Montreal Neurological Institute (MNI) coordinates for the center of the anterior and posterior voxels were: 34, 19, and 0, and 38, −17, and 8 respectively. These coordinates include regions that have been shown previously to be activated during acute pain.30 In addition, fMRI trials in FM have shown augmented pain activity in these regions.27,31 Given the time constraints for a proton magnetic resonance spectroscopy (1H-MRS) session, the right insula was examined because it was contralateral to the pain stimuli previously used in the current authors' previous fMRI trials in FM.14,27 Participants were at rest during the 1H-MRS session. The raw data from each single-voxel magnetic resonance spectroscopy sequence underwent manual postprocessing using 1H-MRS software (LCModel; Oakville, Ontario, Canada). LCModel uses a linear combination of individual spectra obtained from pure molecular species to fit the experimental spectra. Values for Glu, Gln, and Glx were calculated as absolute concentrations using the water signal for normalization.32 Resulting metabolite absolute concentrations were reported in arbitrary institutional units (AIUs). Given that the voxels incorporated cerebrospinal fluid (CSF) and the volume of CSF dilutes 1H-MRS–derived metabolite values, the metabolite levels were corrected for CSF volume for each participant. For this voxel-based morphometry (VBM) was used; this is a “toolbox” that operates within the image-analysis program Statistical Parametric Mapping (SPM). High-resolution T1-weighted images were segmented into gray matter, white matter, and CSF and then regions of interest within the anterior and posterior insula were used to extract gray matter, white matter, and CSF volumes from these images using the SPM2 toolbox Marsbar. Metabolite values were corrected by dividing the observed concentration in AIU by the percentage of volume of the entire voxel that was not occupied by CSF (i.e., the percentage of voxel volume occupied by gray matter plus white matter). Corrected metabolite concentrations were used for calculation of differences between groups.
Statistical Analyses
Metabolite levels and pain ratings were entered into IBM SPSS, version 20 (Chicago IL). The current authors performed a median split of stimulus intensities from the mild–moderate pain staircase to classify participants as having either HPS or LPS. Participants with stimulus intensities below the median were classified as HPS (i.e., lower stimulus intensities were required to elicit mild–moderate pain), whereas those with stimulus intensities above the median were classified as LPS (i.e., higher stimulus intensities were required to elicit mild–moderate pain). Independent t-test analysis of response to treatment (post- minus pretreatment) for the HPS and LPS groups was performed separately for the SF MPQ (total and subscales) and the VAS for both TA and SA. Clinical response was also compared between TA and SA within HPS and LPS groups separately. Next, Glu, Gln, and Glx levels were examined from the posterior and anterior insula in the HPS and LPS groups who were treated with SA. As a final test, a median split of posterior insula Glx (high Glx versus low Glx), was performed for the SA participants with 1H-MRS data, to examine change in clinical pain following SA for high and low Glx participants. Significance was set at a p-value of 0.05, and no corrections were made for multiple comparisons.
Results
LPS Patients Had Reduced Clinical Response to SA
The mean and median pressure intensities that elicited ratings of mild–moderate pain in the 50 FM participants prior to treatment were 1.71 kg/cm2 and 1.63 kg/cm2, respectively. At baseline, patients in the LPS group had somewhat less clinical pain than those in the HPS group; however, this was not statistically significant (SF MPQ total score mean [SD]: HPS 18.7 [8.21]; LPS 15.8 [7.00]; p=0.18). There were also no differences in the TA and SA groups for baseline clinical pain levels (SF MPQ total score mean [SD]: TA 16.6 [6.48]; SA 17.9 [8.66]; p=0.57).
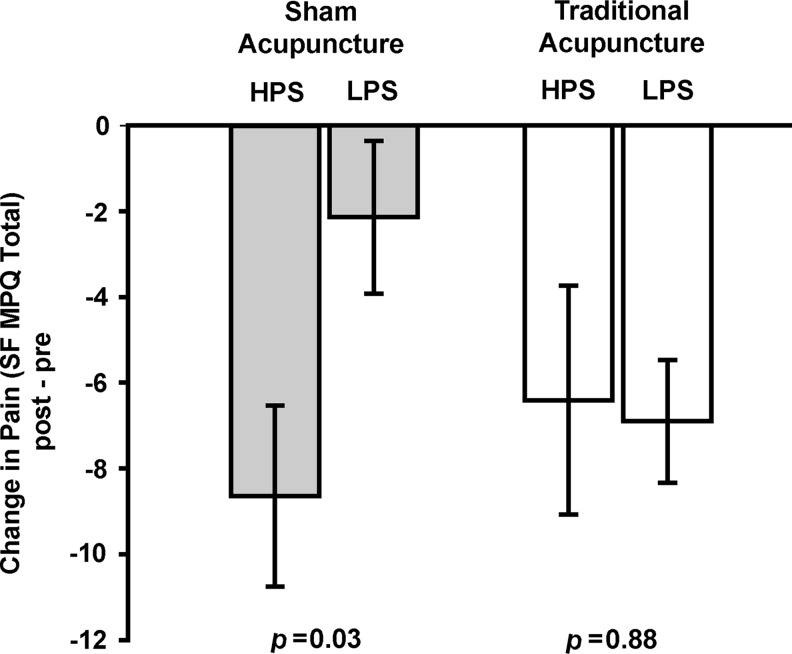
Interestingly, individuals who had lower evoked pressure-pain sensitivity at baseline, had less reduction in clinical pain following SA than those with higher evoked pressure-pain sensitivity (Figure 1; Table 2; post- minus pretreatment change in SF MPQ, total mean [SD]: HPS −8.65 [7.91]; LPS −2.14 [6.68]; p=0.03). This finding was largely the result of changes in the sensory as opposed to the affective dimension of pain (see Table 2). A trend for less reduction in clinical pain within the LPS group was also detected on the VAS scale (post- minus pretreatment change in VAS mean [SD]: HPS −1.71 [2.59]; LPS −0.19 [1.53]; p=0.07). The relationship between LPS and HPS following SA was not observed for TA (change in SF MPQ, total mean [SD]: HPS −6.90 [4.51]; LPS −6.41 [9.25]; p=0.88) in which both groups showed a similar clinically meaningful response to treatment.
FIG. 1.
Patients with LPS do not respond to SA. Change in the SF MPQ total score mean and SE for SA (gray bars) and TA (white bars) shows significantly less reduction of clinical pain during SA for LPS, compared to HPS participants. Similar reductions in clinical pain were seen for LPS and HPS during TA. LPS, low pain sensitivity; SA, sham acupuncture; SF MPQ, Short Form of McGill Pain Questionnaire; SE standard error; TA, traditional acupuncture.
Table 2.
Clinical Pain Response of HPS and LPS Groups to TA and SA
| |
|
|
|
95% CI |
|
|---|---|---|---|---|---|
| Change in clinical pain (post-treatment minus pretreatment) | LPS | HPS | p-Value | Lower | Upper |
| SA group | |||||
| SF MPQ Total | −2.14(6.68) | −8.65(7.91) | 0.027* | 0.81 | 12.19 |
| SF MPQ Sensory | −1.21(4.58) | −6.79(6.76) | 0.017* | 1.09 | 10.05 |
| SF MPQ Affective | −0.93(2.70) | −1.86(1.79) | 0.29 | −0.85 | 2.71 |
| VAS | −0.19(1.53) | −1.71(2.59) | 0.07 | −0.13 | 3.17 |
| TA group | |||||
| SF MPQ Total | −6.90(4.51) | −6.42(9.26) | 0.88 | −7.18 | 6.22 |
| SF MPQ Sensory | −5.10(3.63) | −5.17(8.13) | 0.98 | −5.74 | 5.88 |
| SF MPQ Affective | −1.80(1.23) | −1.25(1.71) | 0.41 | −1.90 | 0.80 |
| VAS | −0.67(1.71) | −0.13(2.02) | 0.52 | −2.22 | 1.15 |
p-Value<0.05 for t-test comparing change in clinical pain for HPS vs LPS.
HPS, high pain sensitivity; LPS, low pain sensitivity; TA, traditional acupuncture; SA, sham acupuncture; SF MPQ, Short Form of McGill Pain Questionnaire; VAS, visual analogue scale.
In a direct comparison between the SA and TA, there was a significant improvement for TA, compared to SA within the LPS group for the sensory score of the SF MPQ (post- minus pretreatment change mean [SD]: TA −5.10 [3.63]; SA −1.21 [4.58]; p=0.037) and a trend for the total score (post- minus pretreatment change mean [SD]: TA −6.90 [4.51]; SA −2.14 [6.68]; p=0.06). This was not observed for the HPS group, wherein both TA and SA engendered approximately equivalent analgesia (all p>0.50).
LPS Patients Within the SA Group Had Reduced Insular Glx
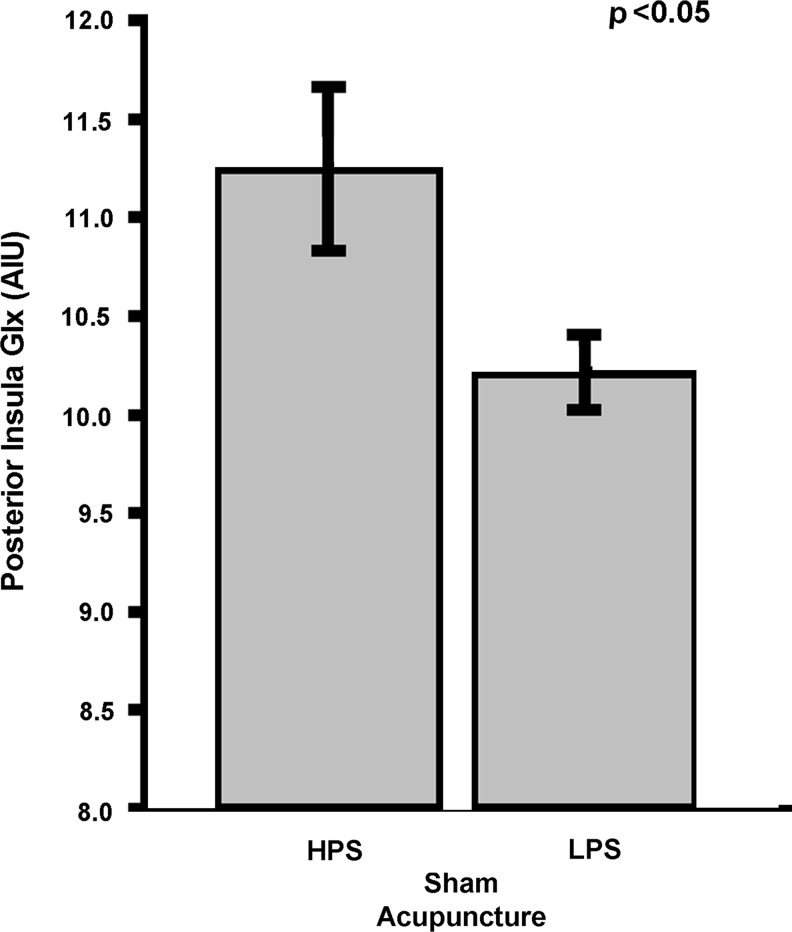
HPS patients in the SA group had elevated baseline levels of posterior insular Glx, compared to patients who were less sensitive (Figure 2; Glx mean [SD]: HPS 11.3 [1.18]; LPS 10.2 [0.54]; p=0.04). This effect was largely the result of differences in Glu (Glu mean [SD]: HPS 7.21 [0.49]; LPS 6.50 [0.56]; p=0.017) as Gln levels were similar across groups (Gln mean [SD]: HPS 4.05 [1.22]; LPS 3.72 [0.55]; p=0.50). Although this was not statistically significant, patients with higher posterior insula Glx levels had a numerically greater clinical pain reduction than patients with lower Glx levels (post- minus prechange in SF MPQ total score mean [SD]: High Glx −6.38 [9.65]; Low Glx −2.13 [6.22]; p=0.31). No significant differences between HPS and LPS for brain metabolite levels (Glx, Glu, and Gln) within the anterior insula were detected (all p>0.65).
FIG. 2.
Baseline Glx values in the posterior insula are lower for the LPS group, compared to the HPS group. Of the 16 patients in the SA group who underwent 1H-MRS, lower values for Glx were observed in the posterior insula for the LPS group, compared to the HPS group. No statistically significant differences were detected in the anterior insula. Glx, glutamate+glutamine; LPS, low pain sensitivity; HPS, high pain sensitivity; SA, sham acupuncture; 1H-MRS, proton magnetic resonance spectroscopy; AIU, arbitrary institutional unit.
Discussion
Previous, randomized sham-controlled trials of acupuncture in chronic pain have largely failed to show meaningful differences between SA and TA treatments. While this may, in part, be the result of a failure to use appropriate acupuncture methods, such as individualized treatments, this also may arise from an exaggerated response to SA of patients who have chronic pain. Specifically, the underlying pain mechanisms operative in a given patient may play a role in how well that patient responds to SA. Indeed the heterogeneity present in the symptom expression of complex, chronic pain disorders such as FM33 may be one such factor in sham responsiveness. Here the current study shows, for the first time, that an enhanced response to SA may be present in patients who are more sensitive to experimental-pressure stimuli and, moreover, this factor may result from an elevated concentration of excitatory neurotransmitters within the posterior insula, a multimodal sensory processing region of the brain.
The current authors believe that there are differing amounts of sensation generated during TA and SA interventions. While, in the current trial, both groups experienced the sensation of needle pricking, there was no needle insertion or De Qi sought in the SA group. Therefore, it was reasoned that the SA group experienced none of the more-intense sensations that occur following needle insertion in TA. The current authors and other researchers11 hypothesize that there may be a threshold of sensation that has to be exceeded for an acupuncture intervention to induce analgesia. The current authors posit that this threshold was not reached for the group with LPS who received SA treatments, whereas the patients with FM who had HPS and who received this same SA treatment did exceed this threshold. This hypothesis is supported by the fact that that both LPS and HPS groups responded equally to TA.
Another interesting possibility is that TA is composed of both specific effects of needling plus other nonspecific placebo effects. HPS subjects may be more sensitive to placebo interventions and were, thus, responsive to SA, whereas TA was able to reduce pain in both LPS and HPS subgroups because of recruitment of both specific and nonspecific mechanisms underlying analgesia. As such, the current authors recommend further investigation into the relative amounts of specific and nonspecific effects within TA and SA interventions.
This trial has many limitations that need to be recognized before drawing conclusions. First these data originated from a fairly small number of patients with FM, and, thus, these findings may not be obtained in larger, more-definitive trials. Second all participants in this trial were women. It is unknown if these results would also be found in men with FM. Third, this trial was only performed in a single chronic-pain condition, and it is uncertain if these findings would be found in other chronic pain disorders. Further work is required to explore the generalizability of these results to other chronic pain states. Finally, although it was reasoned that the TA intervention resulted in enhanced sensory stimulation caused by the De Qi elicited, the current authors did not specifically assess if the participants responded with greater sensory sensations following TA, compared to SA. This information would have improved the significance of this overarching model.
Conclusions
Interindividual differences in pain sensitivity predict the subsequent analgesic response to SA as opposed to TA. This, in part, may originate from differences in the concentration of specific excitatory neurotransmitters within the brain. These data may be helpful in the design of future clinical trials of acupuncture in FM and potentially other chronic-pain disorders that have individual variations in evoked pain sensitivity.
Acknowledgments
The authors thank Craig Urwin, Laura Mayo Bond, and Greta Naylor for expert handling of our participants and their study data. We also thank Keith Newnham for expert assistance in acquiring 1H-MRS data. This work was funded, in part, by Department of Army grants DAMD (17/002-0018 to D.J.C. and W81XWH-07-2-0050 to R.E.H.), National Institutes of Health grants (M01-RR000042 and K01 AT01111-01 to R.E.H., and K01-AT002166 and P01-AT002048 to V.N.) and a Brain and Immuno-Imaging grant from the Dana Foundation to R.E.H.
Disclosure Statement
No competing financial interests exist for the authors.
References
- 1.Brinkhaus B. Witt CM. Jena S, et al. Acupuncture in patients with chronic low back pain: A randomized controlled trial. Arch Intern Med. 2006;166(4):450–457. doi: 10.1001/archinte.166.4.450. [DOI] [PubMed] [Google Scholar]
- 2.Linde K. Streng A. Jurgens S, et al. Acupuncture for patients with migraine: A randomized controlled trial. JAMA. 2005;293(17):2118–2125. doi: 10.1001/jama.293.17.2118. [DOI] [PubMed] [Google Scholar]
- 3.Melchart D. Streng A. Hoppe A, et al. Acupuncture in patients with tension-type headache: Randomised controlled trial. Br Med J. 2005;331(7513):376–382. doi: 10.1136/bmj.38512.405440.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris RE. Tian X. Williams DA, et al. Treatment of fibromyalgia with formula acupuncture: Investigation of needle placement, needle stimulation, and treatment frequency. J Altern Complement Med. 2005;11(4):663–671. doi: 10.1089/acm.2005.11.663. [DOI] [PubMed] [Google Scholar]
- 5.Vickers AJ. Cronin AM. Maschino AC, et al. Acupuncture for chronic pain: Individual patient data meta-analysis. Arch Intern Med. 2012;172(19):1444–1453. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manber R. Schnyer RN. Allen JJ. Rush AJ. Blasey CM. Acupuncture: A promising treatment for depression during pregnancy. J Affect Disord. 2004;83(1):89–95. doi: 10.1016/j.jad.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Wayne PM. Krebs DE. Macklin EA, et al. Acupuncture for upper-extremity rehabilitation in chronic stroke: A randomized sham-controlled study. Arch Phys Med Rehabil. 2005;86(12):2248–2255. doi: 10.1016/j.apmr.2005.07.287. [DOI] [PubMed] [Google Scholar]
- 8.Mayhew E. Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxf). 2007;46(5):801–804. doi: 10.1093/rheumatology/kel406. [DOI] [PubMed] [Google Scholar]
- 9.Cao H. Liu J. Lewith GT. Traditional Chinese Medicine for treatment of fibromyalgia: A systematic review of randomized controlled trials. J Altern Complement Med. 2010;16(4):397–409. doi: 10.1089/acm.2009.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langhorst J. Klose P. Musial F. Irnich D. Hauser W. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxf). 2010;49(4):778–788. doi: 10.1093/rheumatology/kep439. [DOI] [PubMed] [Google Scholar]
- 11.Lundeberg T. Lund I. Are reviews based on sham acupuncture procedures in fibromyalgia syndrome (FMS) valid? Acupunct Med. 2007;25(3):100–106. doi: 10.1136/aim.25.3.100. [DOI] [PubMed] [Google Scholar]
- 12.Petzke F. Clauw DJ. Ambrose K. Khine A. Gracely RH. Increased pain sensitivity in fibromyalgia: Effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 13.Clauw DJ. Fibromyalgia: An overview. Am J Med. 2009;122(12suppl):S3–S13. doi: 10.1016/j.amjmed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Harris RE. Sundgren PC. Pang Y, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58(3):903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 15.Harris RE. Sundgren PC. Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe F. Smythe HA. Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 17.Sherman KJ. Hogeboom CJ. Cherkin DC. Deyo RA. Description and validation of a noninvasive placebo acupuncture procedure. J Altern Complement Med. 2002;8(1):11–19. doi: 10.1089/107555302753507140. [DOI] [PubMed] [Google Scholar]
- 18.Harris RE. Zubieta JK. Scott DJ. Napadow V. Gracely RH. Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009;47(3):1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petzke F. Khine A. Williams D. Groner K. Clauw DJ. Gracely RH. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J Rheumatol. 2001;28(11):2568–2569. [PubMed] [Google Scholar]
- 20.Petzke F. Harris RE. Williams DA. Clauw DJ. Gracely RH. Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain. 2005;9(3):325–335. doi: 10.1016/j.ejpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Gracely RH. McGrath F. Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5(1):5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 22.Gracely RH. McGrath P. Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: Manipulation of affect by diazepam. Pain. 1978;5(1):19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- 23.Gracely RH. Dubner R. McGrath PA. Narcotic analgesia: Fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203(4386):1261–1263. doi: 10.1126/science.424753. [DOI] [PubMed] [Google Scholar]
- 24.Eliav E. Gracely RH. Sensory changes in the territory of the lingual and inferior alveolar nerves following lower molar extraction. Pain. 1998;77(2):191–199. doi: 10.1016/S0304-3959(98)00100-6. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg WF. Bailin D. Grant M. Gracely RH. Competition alters the perception of noxious stimuli in male and female athletes. Pain. 1998;76(1–2):231–238. doi: 10.1016/s0304-3959(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 26.Lembo T. Naliboff BD. Matin K, et al. Irritable bowel syndrome patients show altered sensitivity to exogenous opioids. Pain. 2000;87(2):137–147. doi: 10.1016/S0304-3959(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 27.Gracely RH. Petzke F. Wolf JM. Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 28.Gracely RH. Lota L. Walter DJ. Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32(1):55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 29.Melzack R. The Short-Form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 30.Brooks JC. Nurmikko TJ. Bimson WE. Singh KD. Roberts N. fMRI of thermal pain: Effects of stimulus laterality and attention. Neuroimage. 2002;15(2):293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 31.Cook DB. Lange G. Ciccone DS. Liu WC. Steffener J. Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31(2):364–378. [PubMed] [Google Scholar]
- 32.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 33.Giesecke T. Williams DA. Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48(10):2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]