Abstract
Study design and objective
The purpose of this prospective clinical study is to evaluate the clinical and radiographic outcomes using a silicate-substituted calcium phosphate (Si-CaP) as a bone graft substitute in surgery for adolescent idiopathic scoliosis (AIS).
Summary of background data
In posterior corrective surgery for AIS, harvesting autologous bone from the iliac crest still represents the gold standard to augment the local bone graft though it is comparatively invasive and associated with donor site morbidity. Si-CaP enriched with bone marrow aspirate (BMA) might be an appropriate bone graft extender to overcome these difficulties.
Methods
Eighteen female and three male patients with AIS who underwent corrective posterior instrumentation were observed clinically and radiographically for a minimum of 24 months. In all cases, 20–40 ml Si-CaP granules (ACTIFUSE) mixed with BMA from vertebral bodies was used to extend the local bone graft. Fusion was assessed by standardized conventional radiographs regarding loss of correction and implant failure. Clinical outcome was evaluated with use of the Scoliosis Research Society-22 patient Questionnaire (SRS-22) and a Visual Analog Scale (VAS) for back pain.
Results
Cobb angle of major curves averaged 63° preoperatively, 22° after surgery, and 24° at final follow-up, with a maximum loss of correction of 7° recorded after 4 months. No adverse effects related to the study material had been observed. In all patients, there was no evidence of implant failure, and formation of an increasingly densifying ‘fusion mass’ was visible, as assessed by conventional radiography. VAS score for back pain averaged 1.7 before surgery, 2.3 at discharge, and 1.5 at final follow-up. Outcome assessment using the SRS-22 revealed a significantly enhanced overall health-related quality of life (84 vs. 74 % before surgery; P = 0.0005) due to a significant improvement of the domains ‘self image’ (77 vs. 59 %; P = 0.0002) and ‘pain’ (88 vs. 80 %; P = 0.02). Patients’ management satisfaction averaged 93 %.
Conclusions
Si-CaP augmented with BMA from vertebral bodies seems to prove an effective, safe, and easy to handle bone graft extender in scoliosis surgery and thus a suitable alternative to bone harvesting procedures.
Keywords: Spinal fusion, Bone graft substitute, Bone marrow aspirate, Donor site morbidity, Silicate-substituted calcium phosphate
Introduction
In the posterior correction of AIS, the local bone procured from the resection of spinous processes and facet joints presents the basic graft material. Nevertheless, additional grafting is frequently performed to obtain ample volume for long segment spinal fusion [1]. Autologous iliac crest bone harvesting still remains the accepted gold standard but the amount of bone that can be harvested safely is limited [2]. It implies extra surgery, affecting intact anatomy, with increase of operation time and blood loss and might involve substantial intra- and postoperative morbidity [3–6].
Alternatively, using allograft has been recommended by various authors reporting equivalent outcomes to autograft augmentation in scoliosis surgery [7–10]. However, the demand for allograft exceeds the supply. Regarding its biological properties, allograft is highly variable in strength and performance depending on its source and processing [11–13]. Using freeze-dried allograft alone, Price et al. [14] found a significantly higher failure rate (28 %) than after iliac crest bone grafting (13 %) in surgery for AIS. Further concerns exist regarding the potential immunogenicity and microbial contamination of allograft [15–20]. These factors have led to increasing interest in synthetic bone graft substitutes.
A multiplicity of mainly osteoconductive bone graft substitutes have been developed, composed of hydroxyapatite, tricalcium phosphate, or a mixture of these minerals (biphasic calcium phosphates) [21]. When combined with the local bone graft and BMA, derived from vertebral bodies or the iliac crest, the resulting composite possesses osteoinductive and osteogenic potential [21–26]. Several studies already reported a beneficial use of these materials as a graft extender in posterior spinal fusion procedures, presenting results comparable to those after iliac crest bone grafting [27–35].
Si-CaP represents a novel synthetic, porous bone graft substitute (ACTIFUSE; ApaTech Ltd., Elstree, Hertfordshire, UK). It is as phase pure as bone mineral, containing no secondary phases. Manufactured to a highly controlled specification, it provides a trabecular structure similar to that of cancellous bone. The struts are macroporous and microporous with high levels of interconnectivity, accelerating osseointegration [36]. The silicate-substitution triggers the material’s negative surface charge and significantly improves bone formation performance compared to existing materials by its effect on the activity of bone forming and resorbing cells [37–41]. This allows vascularization of the Si-CaP matrix, supporting cell differentiation, facilitating fast bone ingrowth, greater volume of bone growth, and promoting nutrient transfer to host bone, with subsequent remodelling of the graft to mature bone.
The purpose of this prospective clinical study is to evaluate the clinical and radiographic outcomes of the use of Si-CaP as a bone graft substitute in posterior correction and fusion for AIS.
Materials and methods
Study design
From August 2007, 21 consecutive patients with AIS who were scheduled for surgery at our institution were enrolled in this prospective study. All deformities were treated by means of posterior corrective instrumentation with fusion applying the local bone graft, which was augmented with Si-CaP granules enriched with BMA from vertebral bodies. Written informed consent was obtained from all patients. All patients satisfied the following inclusion and exclusion criteria.
Inclusion criteria
Patients with AIS who will be undergoing corrective posterior spinal fusion surgery
Patients aged 12–21 years at date of surgery
Cobb angle of thoracic major curves ≥45° and of lumbar major curves ≥40°
Patients who understand the conditions of the study and provide consent for clinical research
Exclusion criteria
Patients who as judged by the surgeon are mentally incompetent or reasonably unlikely to be compliant with the prescribed postoperative routine and follow-up evaluation schedule
Patients unable or unwilling to follow the surgeon recommendations regarding postoperative rehabilitation
Patients with active local or systemic infection
Patients with any known active malignancy
Surgical procedure
All patients underwent corrective instrumentation through a posterior procedure by means of a multisegmented titanium pedicle screw and rod system (Expedium Spine System, Depuy Spine Inc., Raynham, Massachusetts, USA). All operations were performed by one surgeon (U.L.) using a standard technique. After exposure of the spine and release of the facet joints, pedicle screws were placed at the strategic vertebrae. A pre-bent rod was then inserted, and correction was accomplished by a combination of the cantilever and rod rotation maneuver with slight concave distraction and convex compression. The spinous processes were resected and the laminae decorticated. Depending on fusion length, 20–40 ml Si-CaP was enriched with 10–20 ml BMA directly taken from vertebral bodies during instrumentation. After opening the pedicle with an awl and perforation of the vertebral body with a probe, the extruding bone marrow was aspirated with a syringe. The resulting composite was then mixed with the morselized local bone graft and applied on the bleeding bone surfaces (see Figs. 1, 2).
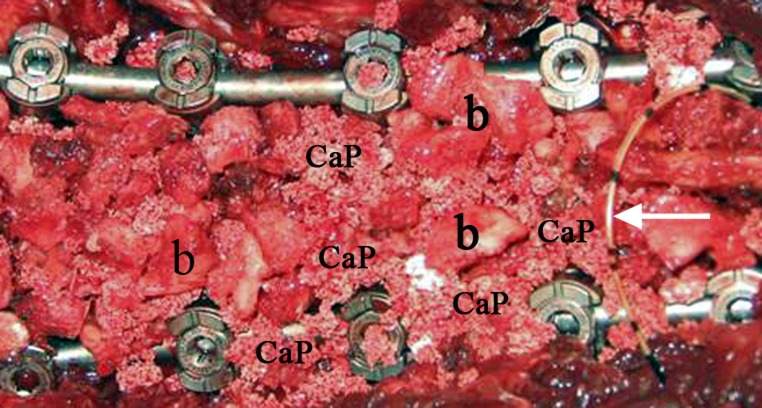
Fig. 1.
Intraoperative view showing the pedicle screw and rod instrumentation in place, the morselized local bone (b) augmented with Si-CaP granules (CaP) applied on the decorticated laminae, and a peridural catheter for postoperative analgesia (arrow)
Fig. 2.
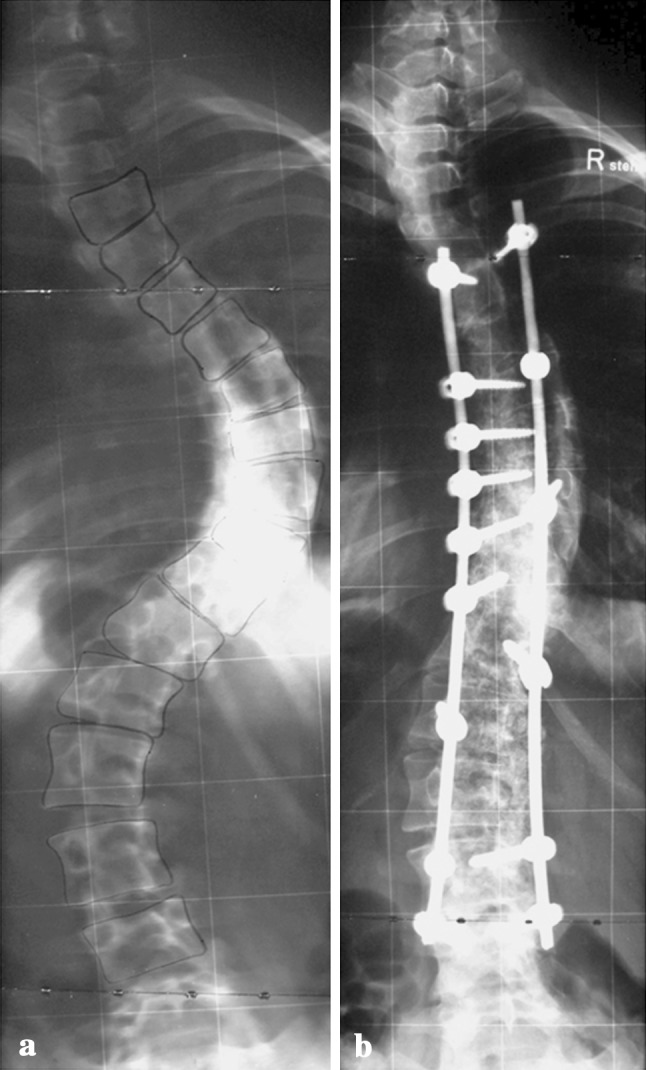
Posteroanterior radiographs of a female patient with a Lenke 3C scoliosis with a main thoracic curve of 84° (T5–T11) and a structural lumbar curve of 60° (T12–L4) (a) and the instrumentation from T5–L4 (b)
Patient data
The study group included 18 female and 3 male patients with an average age of 16 years (median 16 ± 2 years; 12–21 years) at date of surgery. Curve types were classified according to the Lenke classification system, which is displayed in Table 1 [42]. All patients were examined clinically and by means of conventional radiography before surgery, prior to discharge from hospital, and at follow-up visits (4-, 12-, and 24-months). Follow-up averaged 25 months (24 ± 2 months; 24–33 months).
Table 1.
Curve classification
| Curve types according to the Lenke classification system [42] | Lumbar modifier | |||
|---|---|---|---|---|
| A | B | C | ||
| 1 (Main thoracic) | 10 | 5 | 1 | 4 |
| 2 (Double thoracic) | 3 | 2 | 1 | |
| 3 (Double major) | 4 | 4 | ||
| 4 (Triple major) | ||||
| 5 (Primary thoracolumbar/lumbar) | ||||
| 6 (Primary thoracolumbar/lumbar, structural thoracic) | 4 | 4 | ||
Perioperative parameters
The perioperative parameters collected and evaluated comprised surgery time, estimated intraoperative blood loss, number of segments fused, quantity of Si-CaP granules applied, blood retransfusion via cell saver, blood transfusion requirements, intra- and postoperative complications, as well as length of hospital stay.
Analysis of radiographs
In all patients, standardized long-cassette posteroanterior and lateral radiographs in standing posture inclusive preoperative side bending radiographs were performed. Postoperative radiographs were analyzed for implant failure in terms of breakage, dislocation, and loosening, as well as for defects in the fusion mass as indicators of pseudarthrosis. This analysis was conducted by a spine surgeon and an independent radiologist who both were not involved in the patients’ treatment. Cobb angle of major and instrumented minor curves, curve correction, and loss of correction (using the same vertebral segments as preoperative) were measured additionally [43]. With respect to the established criteria for possible and definite nonunion, a definite pseudarthrosis was proven by radiographically visible implant failure or by direct evidence of a defect in the fusion mass at the time of surgical exploration [14, 44]. A possible pseudarthrosis was considered in case of the following situations: (1) loss of correction >10°, (2) radiographically visible defect in the fusion mass, or (3) persistent midline back pain of moderate to severe intensity.
Evaluation of health-related quality of life, back pain, and analgesic use
The Scoliosis Research Society-22 patient Questionnaire (SRS-22) was applied to assess the patients’ health-related quality of life [45–47]. Back pain was evaluated by means of a Visual Analog Scale (VAS; 0–10). The use of any analgesic was also recorded.
Data analysis and statistics
Following summation of the presented data, mean values (together with median and standard deviation) were calculated for age at the date of surgery, perioperative parameters, follow-up time, preoperative, postoperative, and final visit Cobb angle of major and instrumented minor curves, postoperative and final visit proportional curve correction, loss of correction, SRS-22 and VAS scores. Preoperative and final visit SRS-22 values, loss of correction and VAS scores (preoperative, at discharge and final visit) were compared using the Wilcoxon test. Statistical significance was determined at the P < 0.05 level.
Postoperative treatment regime
No postoperative bracing was administered. Patients were restricted from bending or lifting greater than 5 kg for 6 months and from sport activities for 1 year, except for swimming and cycling, which were allowed after 3 and 6 months, respectively.
Results
Perioperative parameters
Surgery time was on average 154 min (159 ± 26 min; 105–196 min), with an average estimated blood loss of 814 ml (750 ± 442 ml; 300–1,585 ml). Blood retransfusion via cell saver of on average 543 ml (500 ± 238 ml; 150–1,023 ml) was conducted in 12 patients. During surgery, three patients received previously donated autologous blood transfusion of 250–500 ml. In the postoperative course, 11 patients received autologous and a further two patients, homologous blood transfusion. The number of motion segments fused averaged 10 (10 ± 2; 6–13). In 13 cases, 20 ml of Si-CaP granules was applied to extend the local bone graft, and 40 ml Si-CaP in the remaining eight patients. Hospital stay averaged 12 days (12 ± 1; 9–14).
Adverse events
There were no complications throughout surgery. Postoperative complications comprised a urinary tract infection in one case, pleural effusion with incipient pneumonia in one case, and initial sacral decubitus in another case. So far, no complications related to the study have been observed.
Curve correction and loss of correction
Cobb angle of major curves averaged 63° before surgery, 22° postoperatively (correction of 65 %), and 24° (correction of 62 %) at final follow-up. Cobb angle of instrumented minor curves (n = 14) measured on average 56° preoperatively, 21° postoperatively (correction of 62 %), and 23° at final follow-up (correction of 58 %). With respect to major curves, a statistically significant loss of correction of on average 2° was recorded at final follow-up (P = 0.0007), with a maximum loss of correction of 7° (8 %) in one patient observed at the 4-months follow-up. After the 4-months follow-up, there was no more noticeable loss of correction (P = 0.9). These results were comparable to those found for instrumented minor curves. The complete radiographic data are provided in Table 2.
Table 2.
Cobb angle of major and minor curves, curve correction, and loss of correction
| P | ||
|---|---|---|
| Cobb angle major curve | ||
| Preoperative mean (median; SD; range) | 63 (64 ± 10; 48–84) | |
| Postoperative | 22 (22 ± 9; 5–43) | |
| Final follow-up | 24 (24 ± 9; 8–50) | |
| Curve correction (%) | ||
| Postoperative | 65 (63 ± 12; 48–91) | |
| Final follow-up | 62 (60 ± 12; 40–85) | |
| Loss of correction (°) | 2 (2 ± 2; −1–7) | 0.0007* |
| Cobb angle minor curve if instrumented (°) (n = 14) | ||
| Preoperative | 56 (54 ± 13; 32–74) | |
| Postoperative | 21 (22 ± 5; 12–29) | |
| Final follow-up | 23 (23 ± 5; 12–30) | |
| Curve correction (%) | ||
| Postoperative | 62 (60 ± 9; 48–78) | |
| Final follow-up | 58 (59 ± 9; 44–71) | |
| Loss of correction (°) | 2 (2 ± 3; −4–6) | 0.02* |
* Statistically significant (P < 0.05)
Fusion assessment by conventional radiography
As assessed by conventional radiography, all patients were considered fused at final follow-up, presenting the formation of a continuous ‘fusion mass’ of increasing density, especially visible at the residual curves’ concavity, without any evidence of implant failure (see Figs. 3, 4, 5). The Si-CaP granules were still slightly circumscribable at the 12-month follow-up but were indistinguishable after 24 months. There were no patients with a loss of curve correction ≥10°, which was defined as a criterion for possible pseudarthrosis.
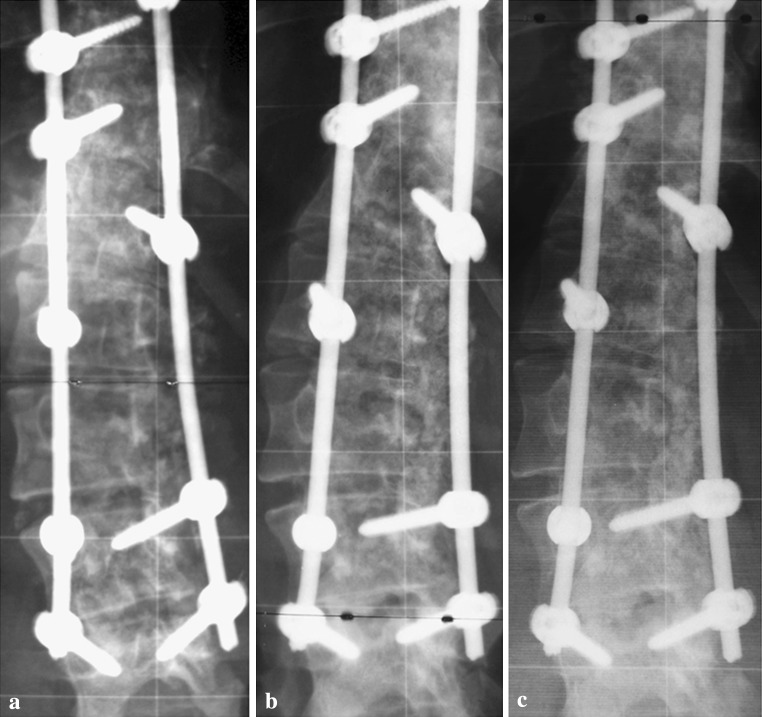
Fig. 3.
Postoperative (a) 12- and 24-months follow-up posteroanterior radiographs (b, c) of the patient presented in Fig. 2 with focus on the residual lumbar curve demonstrating the development of a continuous and increasingly densifying fusion mass
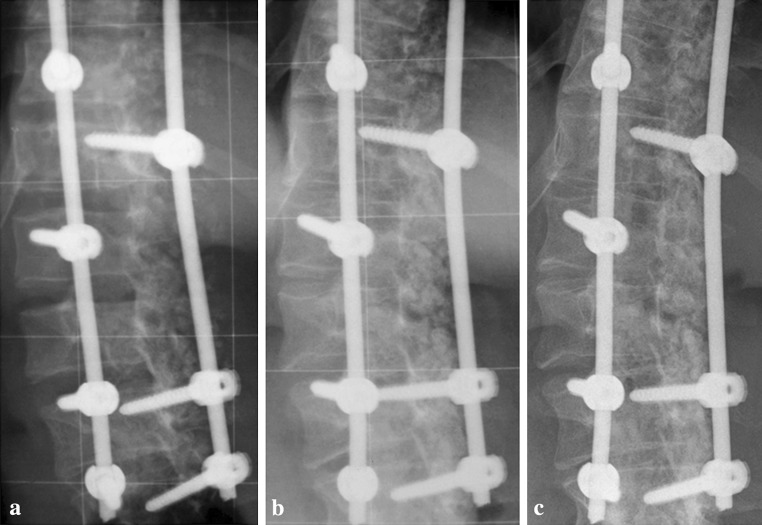
Fig. 4.
Postoperative (a) 12- and 24-months follow-up posteroanterior radiographs (b, c) of a male patient showing the formation of a fusion mass at lumbar levels and resorption of the Si-CaP granules right to the right rod
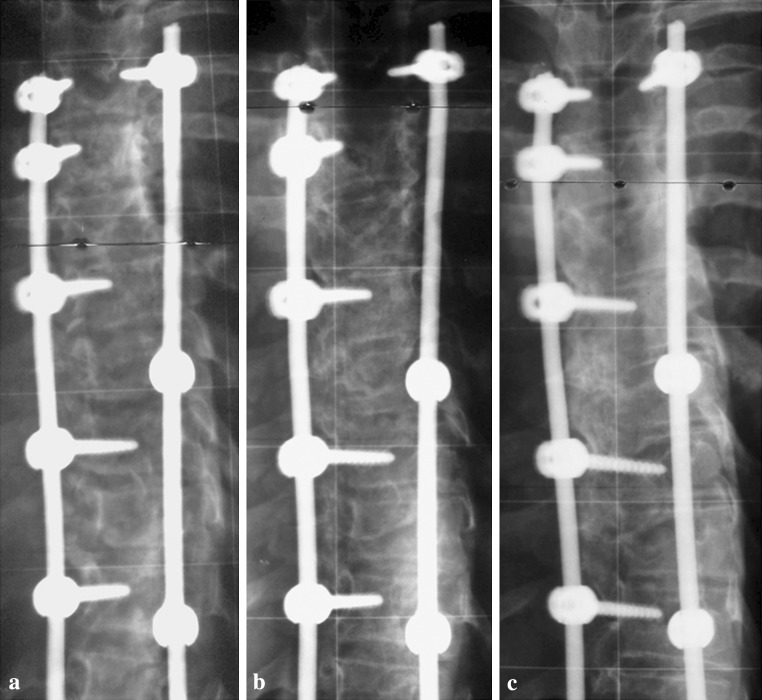
Fig. 5.
Postoperative (a) and follow-up posteroanterior radiographs after 12 (b) and 24 months (c) of a female patient displaying the development and maturation of an entire fusion mass at thoracic levels. At latest follow-up, the Si-CaP granules are no more indistinguishable
Outcome assessment via SRS-22, VAS for back pain, and analgesic use
Final follow-up outcome assessment using the SRS-22 showed a significantly increased overall health-related quality of life compared to the status before surgery (P = 0.0005). This resulted from a significant improvement of the domains ‘self image’ (P = 0.0002) and ‘pain’ (P = 0.02). As expected, the category ‘function/activity’ did not improve but also did not worsen. A similar result was found for the domain ‘mental health’. Patients’ management satisfaction was high, on average 93 %. The complete SRS-22 data are displayed in Table 3. VAS score for back pain averaged 1.7 preoperatively (0 ± 2.5; 0–7), 2.3 (2 ± 1.5; 0–6) at discharge, and 1.5 (1 ± 1.8; 0–7) at final follow-up. VAS score at final follow-up was significantly lower than at discharge (P = 0.04). VAS scores at final follow-up and before surgery were not significantly different (P = 0.8) neither were VAS scores before surgery and at discharge (P = 0.1). Preoperatively, two patients had declared use of pain killers. At discharge, 20 patients were adequately supplied with analgesics. At final follow-up, two patients reported still using an over the counter analgesic occasionally because of painful muscular tension. No patient complained persistent midline back pain, another criterion for possible pseudarthrosis.
Table 3.
SRS-22 outcome assessment
| P | ||
|---|---|---|
| Overall health-related quality of life (%) | ||
| Preoperative mean (median; SD; range) | 74 (76 ± 10; 51–89) | 0.0005* |
| Final follow-up | 84 (86 ± 8; 67–95) | |
| Pain (%) | ||
| Preoperative | 80 (80 ± 14; 52–100) | 0.02* |
| Final follow-up | 88 (88 ± 12; 53–100) | |
| Function/activity (%) | ||
| Preoperative | 79 (84 ± 13; 52–92) | 0.1 |
| Final follow-up | 83 (84 ± 7; 72–92) | |
| Mental health (%) | ||
| Preoperative | 79 (80 ± 13; 48–96) | 0.2 |
| Final follow-up | 83 (92 ± 18; 32–100) | |
| Self-image (%) | ||
| Preoperative | 59 (59 ± 10; 44–76) | 0.0002* |
| Final follow-up | 77 (80 ± 10; 60–95) | |
| (Treatment) satisfaction (%) | 93 (100 ± 10; 60–100) | – |
* Statistically significant (P < 0.05)
Discussion
This prospective clinical study evaluated the clinical and radiographic results with the use of a novel, synthetic, porous calcium phosphate with a silicate-substitution (Si-CaP), enriched with BMA from vertebral bodies, to augment the local bone graft in posterior corrective surgery for AIS.
So far, no adverse effects related to the study material have been observed.
As to the established criteria for possible and definite pseudarthrosis, all patients were considered fused at final follow-up, presenting the development of a continuous and increasingly densifying ‘fusion mass’, visible at the residual curve’s concavity. Radiographically, the Si-CaP granules were still slightly circumscribable at the 12-month follow-up but were indistinguishable after 24 months. Compared to a former study by the authors on the use of beta-tricalcium phosphate (β-TCP) granules as a graft extender in scoliosis surgery, the β-TCP granules had almost dissolved 6 months after surgery [35]. Muschik et al. [31] reported a complete resorption of β-TCP granules visible on radiographs 8 ± 2 months after posterior correction for AIS. In contrast to Si-CaP, β-TCP is much more soluble and thus degrades much faster. In this regard, Hing et al. [40] compared the performance of Si-CaP and β-TCP in a standard animal model and reported that both materials facilitated early bone apposition. However, β-TCP degradation products initiated an inflammatory response that affected and even reversed bone apposition, whereas Si-CaP provided a more stable scaffold, promoting faster angiogenesis and bone apposition, with formation of a functionally adaptive trabecular structure through resorption/remodelling of both scaffold and new bone. The silicate-substitution of porous calcium phosphate appears to considerably enhance bioactivity and trigger adaptive remodelling, leading to significantly higher amounts of bone ingrowth and bone/scaffold coverage compared to conventional hydroxyapatite [37–39, 41].
Furthermore, Si-CaP seems to prevent any severe inflammatory response of the host tissue thereby improving bioactivity. This can be supported by the authors on the basis of own histological findings [48]. During revision surgery for a dislocated set screw at the distal end of instrumentation, 1 year after posterior corrective surgery for Scheuermann kyphosis, a biopsy was taken from an entire fusion mass, without any macroscopic evidence of Si-CaP. Histological analysis of the specimen confirmed new bone formation next to Si-CaP particles and vascularization of the bone-scaffold composition but no relevant fibrosis [48].
A number of scientific reports already recommended the use of bone substitutes in combination with BMA to provide osteoinductive and osteogenic potential [21–26]. In this study, Si-CaP was enriched with BMA, which can be easily obtained from vertebral bodies during pedicle screw placement. According to McLain et al. [24, 26] aspirates of vertebral marrow showed equivalent or even greater concentrations of progenitor cells compared with matched controls from the iliac crest.
Nevertheless, it is essential to perform a thorough decortication of the laminae to allow linking between host bone and graft material, facilitating bone ingrowth. Referring to this, Korovessis et al. [49] reported that hydroxyapatite combined with local bone and BMA failed to work as a bone substitute for intertransverse posterolateral lumbar fusion, most likely due to the absence of a sufficient host bone area. Whereas, the application of hydroxyapatite on decorticated laminae, which represents a wide host area, was followed by solid fusion.
The SRS-22 outcome assessment revealed a significantly increased overall health-related quality of life because of significant improvements in the domains ‘self image’ and ‘pain’ as well, even though the majority of patients responded favorably upon questions in the ‘pain’ category before surgery. Moreover, patient management satisfaction was high (93 %).
With a mean value of 2.3, VAS score for back pain was already relatively low at discharge from hospital. However, it has to be considered that 20 patients were still supplied with analgesics. By comparison, average VAS score at final follow-up was significantly lower (1.5), with only two patients reporting the use of pain killers as an over the counter analgesic. In this regard, Skaggs et al. [5] observed that posterior iliac crest bone grafting in spine surgery in 87 children was associated with significant donor site pain in 24 % of patients, even after 4 years mean follow-up, with an average VAS score of 4. In 15 %, pain was severe enough to limit daily activity. Analgesics were taken by 9 % because of pain at the bone graft site. The authors concluded that the real dimension of pain after iliac crest bone grafting in children was severely underreported and might not be sufficiently recognized.
Betz et al. [44] generally questioned the need for supplemental bone graft in scoliosis surgery. In a prospective, randomized study, they compared the clinical results of posterior spinal fusion using a multisegmented hook-screw and rod system with allograft augmentation versus no graft for patients with AIS. One of 37 patients in the allograft group presented with definite pseudarthrosis 12 months postoperatively, versus 0 of 39 in the no graft group. However, two patients in each group showed a loss of correction >10° and thus met a criterion of possible pseudarthrosis. After a minimum follow-up of 24 months, loss of correction averaged 3.6° in both groups, which is comparable to patients with AIS and long segment spinal fusion with iliac crest bone grafting [35].
In the present study, loss of curve correction averaged 2° for both major and instrumented minor curves, with a maximum loss of correction of 7° in one patient found at the 4-months follow-up. Loss of correction was significant within the first 4 months. Afterwards, there was no further significant loss of correction to be observed.
Violas et al. [50] retrospectively analyzed 25 patients with AIS who had undergone posterior instrumentation and fusion with use of only the local bone graft. After a mean follow-up of 6 years, no definite or possible pseudarthrosis was observed. The authors concluded that using the local bone graft eliminates the need for any other graft.
Nevertheless, augmentation of the local bone graft to enhance fusion is mostly recommended. Using calcium phosphates as graft extenders instead of harvesting bone from the ilium permits reduction of surgery time and blood loss and avoids donor site morbidity. The same counts for allograft, with mostly satisfactory fusion rates [7–10], but inconsistent quality, donor-to-donor variation, potential immunogenicity, and risk of disease transmission remain incalculable shortcomings. A survey of spinal deformity surgeons identified pseudarthrosis and infection as their leading concerns with respect to efficacy and safety of allograft tissue [17].
Two retrospective studies deal with the use of demineralized bone matrix (DBM), an organic derivative of allograft bone, in surgery for AIS [14, 51]. Compared to conventional allograft, DBM is not only osteoconductive but also osteoinductive. Demineralization of pulverized allograft through mild acid extraction exposes the extracellular matrix, which contains small quantities of osteoinductive proteins. The osteoinductive potential strongly depends on the individual donor and skeletal portion. Betz et al. observed no definite or possible pseudarthrosis in 17 patients with a minimum follow-up of 24 months after instrumentation and fusion with DBM alone. As well Price et al. reported no definite pseudarthrosis in 36 patients with a minimum follow-up of 24 months after surgery for AIS with instrumentation and fusion applying DBM and BMA obtained from the iliac crest but four patients experienced a loss of correction >10°.
The use of recombinant human bone morphogenetic proteins (rhBMPs) provides pure osteoinduction and appears to prove beneficial in adult spinal deformity surgery to accomplish successful fusion, particularly in long segment spinal fusion to the sacrum [52, 53]. However, rhBMPs are contraindicated in skeletally immature patients. Anyway, there is no need for rhBMPs in adolescent patients regarding their excellent potential of bone regeneration and favorable results with the use of osteoconductive graft extenders.
Finally, it has to be considered that the absence of pseudarthrosis in this study may be the result of high fusion rates or late occurrence of implant breakage using a modern multisegmented screw and rod system. Thus, further follow-up is needed to prove the promising results with the use of Si-CaP as a bone graft substitute in scoliosis surgery.
Conclusions
Si-CaP augmented with BMA, obtained from vertebral bodies during pedicle screw placement, seems to prove an effective, safe, and easy to handle bone graft extender in scoliosis surgery and thus a suitable alternative to bone harvesting procedures.
Conflict of interest
This study was supported financially by ApaTech LTD (Elstree, Herfordshire, UK).
References
- 1.Sengupta DK, Truumees E, Patel CK, Kazmierczak C, Hughes B, Elders G, Herkowitz HN. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine. 2006;31:985–991. doi: 10.1097/01.brs.0000215048.51237.3c. [DOI] [PubMed] [Google Scholar]
- 2.Kessler P, Thorwarth M, Bloch-Birkholz A, Nkenke E, Neukam FW. Harvesting of bone from the iliac crest—comparison of the anterior and posterior sites. Br J Oral Maxillofac Surg. 2005;43:51–56. doi: 10.1016/j.bjoms.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Skaggs DL, Samuelson MA, Hale JM, Kay RM, Tolo VT. Complications of posterior iliac crest bone grafting in spine surgery in children. Spine. 2000;25:2400–2402. doi: 10.1097/00007632-200009150-00021. [DOI] [PubMed] [Google Scholar]
- 6.Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine. 2001;26:1473–1476. doi: 10.1097/00007632-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 7.Bridwell KH, O’Brien MF, Lenke LG, Baldus C, Blanke K. Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis. Does it work? Spine (Phila Pa 1976) 1994;19:2658–2666. [PubMed] [Google Scholar]
- 8.Yazici M, Asher MA. Freeze-dried allograft for posterior spinal fusion in patients with neuromuscular spinal deformities. Spine (Phila Pa 1976) 1997;22:1467–1471. doi: 10.1097/00007632-199707010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Jones KC, Andrish J, Kuivila T, Gurd A. Radiographic outcomes using freeze-dried cancellous allograft bone for posterior spinal fusion in pediatric idiopathic scoliosis. J Pediatr Orthop. 2002;22:285–289. [PubMed] [Google Scholar]
- 10.Knapp DR, Jr, Jones ET, Blanco JS, Flynn JC, Price CT. Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech. 2005;18(Suppl):S73–S76. doi: 10.1097/01.bsd.0000128694.21405.80. [DOI] [PubMed] [Google Scholar]
- 11.An HS, Lynch K, Toth J. Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord. 1995;8:131–135. doi: 10.1097/00002517-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Jorgenson SS, Lowe TG, France J, Sabin J. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine. 1994;19:2048–2053. doi: 10.1097/00007632-199409150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ehrler DM, Vaccaro AR. The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res. 2000;371:38–45. doi: 10.1097/00003086-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Price CT, Connolly JF, Carantzas AC, Ilyas I. Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine. 2003;28:793–798. [PubMed] [Google Scholar]
- 15.Buck BE, Resnick L, Shah SM. Human immunodeficiency virus cultured from bone. Implications for transplantation. Clin Orthop Relat Res. 1990;251:249–253. [PubMed] [Google Scholar]
- 16.Journeaux SF, Johnson N, Bryce SL, Friedman SJ, Sommerville SM, Morgan DA. Bacterial contamination rates during bone allograft retrieval. J Arthroplasty. 1999;14:677–681. doi: 10.1016/S0883-5403(99)90222-X. [DOI] [PubMed] [Google Scholar]
- 17.Woolf SK, Gross RH. Perceptions of allograft safety and efficacy among spinal deformity surgeons. J Pediatr Orthop. 2001;21:767–771. [PubMed] [Google Scholar]
- 18.Barriga A, Diaz-de-Rada P, Barroso JL, Alfonso M, Lamata M, Hernaez S, Beguiristain JL, San-Julian M, Villas C. Frozen cancellous bone allografts: positive cultures of implanted grafts in posterior fusions of the spine. Eur Spine J. 2004;13:152–156. doi: 10.1007/s00586-003-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford MJ, Swenson CL, Arnoczky SP, O’Shea J, Ross H. Lyophilization does not inactivate infectious retrovirus in systemically infected bone and tendon allografts. Am J Sports Med. 2004;32:580–586. doi: 10.1177/0363546504263404. [DOI] [PubMed] [Google Scholar]
- 20.Eastlund T. Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank. 2006;7:147–166. doi: 10.1007/s10561-006-0003-z. [DOI] [PubMed] [Google Scholar]
- 21.Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;395:44–52. doi: 10.1097/00003086-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Erbe EM, Marx JG, Clineff TD, Bellincampi LD. Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur Spine J. 2001;10(Suppl 2):S141–S146. doi: 10.1007/s005860100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betz RR. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002;25:s561–s570. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 24.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, Davros WJ, Easley KA, Powell KA. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;432:242–251. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLain RF, Boehm CA, Rufo-Smith C, Muschler GF. Transpedicular aspiration of osteoprogenitor cells from the vertebral body: progenitor cell concentrations affected by serial aspiration. Spine J. 2009;9:995–1002. doi: 10.1016/j.spinee.2009.08.455. [DOI] [PubMed] [Google Scholar]
- 27.Le Huec JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A. Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg. 1997;63:202–211. [PubMed] [Google Scholar]
- 28.Ransford AO, Morley T, Edgar MA, Webb P, Passuti N, Chopin D, Morin C, Michel F, Garin C, Pries D. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80:13–18. doi: 10.1302/0301-620X.80B1.7276. [DOI] [PubMed] [Google Scholar]
- 29.Cavagna R, Daculsi G, Bouler JM. Macroporous calcium phosphate ceramic: a prospective study of 106 cases in lumbar spinal fusion. J Long Term Eff Med Implants. 1999;9:403–412. [PubMed] [Google Scholar]
- 30.Delecrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine. 2000;25:563–569. doi: 10.1097/00007632-200003010-00006. [DOI] [PubMed] [Google Scholar]
- 31.Muschik M, Ludwig R, Halbhubner S, Bursche K, Stoll T. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(Suppl 2):S178–S184. doi: 10.1007/s005860100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadows GR. Adjunctive use of ultraporous beta-tricalcium phosphate bone void filler in spinal arthrodesis. Orthopedics. 2002;25:s579–s584. doi: 10.3928/0147-7447-20020502-06. [DOI] [PubMed] [Google Scholar]
- 33.Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424–429. doi: 10.1097/00024720-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Moro-Barrero L, Acebal-Cortina G, Suarez–Suarez M, Perez-Redondo J, Murcia-Mazon A, Lopez-Muniz A. Radiographic analysis of fusion mass using fresh autologous bone marrow with ceramic composites as an alternative to autologous bone graft. J Spinal Disord Tech. 2007;20:409–415. doi: 10.1097/BSD.0b013e318030ca1e. [DOI] [PubMed] [Google Scholar]
- 35.Lerner T, Bullmann V, Schulte TL, Schneider M, Liljenqvist U. A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J. 2009;18:170–179. doi: 10.1007/s00586-008-0844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hing KA, Annaz B, Saeed S, Revell PA, Buckland T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J Mater Sci Mater Med. 2005;16:467–475. doi: 10.1007/s10856-005-6988-1. [DOI] [PubMed] [Google Scholar]
- 37.Patel N, Best SM, Bonfield W, Gibson IR, Hing KA, Damien E, Revell PA. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med. 2002;13:1199–1206. doi: 10.1023/A:1021114710076. [DOI] [PubMed] [Google Scholar]
- 38.Hing KA, Revell PA, Smith N, Buckland T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials. 2006;27:5014–5026. doi: 10.1016/j.biomaterials.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Porter AE, Buckland T, Hing K, Best SM, Bonfield W. The structure of the bond between bone and porous silicon-substituted hydroxyapatite bioceramic implants. J Biomed Mater Res A. 2006;78:25–33. doi: 10.1002/jbm.a.30690. [DOI] [PubMed] [Google Scholar]
- 40.Hing KA, Wilson LF, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475–490. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler DL, Jenis LG, Kovach ME, Marini J, Turner AS. Efficacy of silicated calcium phosphate graft in posterolateral lumbar fusion in sheep. Spine J. 2007;7:308–317. doi: 10.1016/j.spinee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83-A:1169–1181. [PubMed] [Google Scholar]
- 43.Cobb J (1948) Outline for the study of scoliosis. In: Instructional course letters, vol 5. American Academy of Orthopaedic Surgeons, Ann Arbor
- 44.Betz RR, Petrizzo AM, Kerner PJ, Falatyn SP, Clements DH, Huss GK. Allograft versus no graft with a posterior multisegmented hook system for the treatment of idiopathic scoliosis. Spine. 2006;31:121–127. doi: 10.1097/01.brs.0000194771.49774.77. [DOI] [PubMed] [Google Scholar]
- 45.Haher TR, Gorup JM, Shin TM, Homel P, Merola AA, Grogan DP, Pugh L, Lowe TG, Murray M. Results of the Scoliosis Research Society instrument for evaluation of surgical outcome in adolescent idiopathic scoliosis. A multicenter study of 244 patients. Spine (Phila Pa 1976) 1999;24:1435–1440. doi: 10.1097/00007632-199907150-00008. [DOI] [PubMed] [Google Scholar]
- 46.Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the scoliosis research society-22 patient questionnaire for idiopathic scoliosis. Spine (Phila Pa 1976) 2003;28:63–69. doi: 10.1097/00007632-200301010-00015. [DOI] [PubMed] [Google Scholar]
- 47.Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted german version of scoliosis research society-22 questionnaire. Spine (Phila Pa 1976) 2009;34:818–821. doi: 10.1097/BRS.0b013e31819b33be. [DOI] [PubMed] [Google Scholar]
- 48.Lerner T, Griefingholt H, Liljenqvist U. Bone substitutes in scoliosis surgery. Orthopade. 2009;38:181–188. doi: 10.1007/s00132-008-1369-3. [DOI] [PubMed] [Google Scholar]
- 49.Korovessis P, Koureas G, Zacharatos S, Papazisis Z, Lambiris E. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur Spine J. 2005;14:630–638. doi: 10.1007/s00586-004-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Violas P, Chapuis M, Bracq H. Local autograft bone in the surgical management of adolescent idiopathic scoliosis. Spine. 2004;29:189–192. doi: 10.1097/01.BRS.0000105536.65164.B1. [DOI] [PubMed] [Google Scholar]
- 51.Betz RR, Lavelle WF, Samdani AF. Bone grafting options in children. Spine (Phila Pa 1976) 2010;35:1648–1654. doi: 10.1097/BRS.0b013e3181ce8f4b. [DOI] [PubMed] [Google Scholar]
- 52.Maeda T, Buchowski JM, Kim YJ, Mishiro T, Bridwell KH. Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine (Phila Pa 1976) 2009;34:2205–2212. doi: 10.1097/BRS.0b013e3181b0485c. [DOI] [PubMed] [Google Scholar]
- 53.Mulconrey DS, Bridwell KH, Flynn J, Cronen GA, Rose PS. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine (Phila Pa 1976) 2008;33:2153–2159. doi: 10.1097/BRS.0b013e31817bd91e. [DOI] [PubMed] [Google Scholar]