Abstract
Purpose
This review summarizes the experience with the vertical expandable prosthetic titanium rib (VEPTR) device, which enables five new procedures to treat complex spine and chest wall abnormalities in pediatric patients, which cause thoracic insufficiency syndrome, the inability of the thorax to support normal respiration or lung growth.
Methods
The literature on VEPTR was reviewed and discussed by the author, the inventor of the VEPTR.
Results
The central VEPTR treatment principle is to correct volume depletion deformity of the thorax, and maintain the correction until skeletal maturity, at which time procedures such as spinal fusion can be considered. For individual cases of complex deformity, VEPTR strategies can differ remarkably. The goal of VEPTR surgery is to pursue the surgical strategy that provides the largest, most symmetrical, most functional thorax that can grow as normally as possible. Assessment of these results is difficult, since natural history of VEPTR-treated diseases are not clearly known and no current imaging test can measure thoracic insufficiency syndrome, but dynamic lung MRI have promise for the future in better defining this potentially lethal condition.
Conclusion
VEPTR and its principles of use have become an important first step toward improving the quality of life and longevity of children with thoracic insufficiency syndrome, but much work remains to advance both its design and its use.
Keyword: VEPTR
The vertical expandable prosthetic titanium rib (VEPTR) was based on a crude Steinmann pin chest wall prosthesis that was placed in an 8-month-old ventilator dependent infant in 1987 who was dying from respiratory insufficiency due to severe rib agenesis and scoliosis. The pins were placed longitudinally to provide a stable perpendicular load orientation for the attachment of the pins to the vestigial proximal and distal ribs, but also in the hope of stabilizing the curve in case the infant did manage to survive. To everyone’s surprise, post-operatively the infant was weaned off the ventilator in days, and then oxygen weeks later, going on to grow and thrive. Even the scoliosis improved, likely through rib distraction correcting it indirectly. Once the euphoria wore off, though, the reality of the new long-term problems for the child became apparent. As he became older, his chest and spine would grow, but the non-expandable Steinmann Pin construct would soon became a tether to both spinal growth and the underlying concave lung. Two apparent choices were obvious to continue the clinical success of the chest wall construct. The Steinmann pin prosthesis could be completely changed out periodically through a full thoracotomy with probable skin slough, infection, and eventual failure, or a true chest wall prosthesis could be developed that was easy to implant, providing a stable chest wall and curve control, and would be expandable through periodic out-patient surgery through a limited incisions, which would likely decrease risk of infection and skin slough. The latter course was chosen, and at that time it was thought that at best, a practical rib prosthesis could be developed that would be useful for the extremely rare cases of congenital agenesis of ribs with, at most, two to three cases a year performed. That estimate proved to be inaccurate.
In 1988, the search began for a manufacturer capable and willing to make such a device. Because of the rarity of their use, no surgical manufacturer had a fixed dimension chest wall prosthesis that could be modified to become expandable in a practical fashion, so the inventor, an orthopedist, decided to approach orthopedist manufacturers. Several manufacturers were approached, but the huge technical challenges and the small market (N = 1) made the project impractical for them. Finally, a custom orthopedic device manufacturer, Techmedica Corporation, of Camarillo, California, agreed to make custom expandable rib prosthesis devices of titanium alloy and two were used to replace the Steinmann pin construct on April 19th, 1989, at Christus Santa Rosa Children’s Hospital in San Antonio, Texas. The procedure was a success, and news spread through the lay press about an alternative surgical approach to chest and spine abnormalities. This was the beginning of what would soon be called “The Titanium Rib Project”.
Referrals of pediatric patients with many distinct types of chest and spine abnormalities to the Titanium Rib Project mounted, with congenital rib absence in a small minority of cases. It soon became clear that the classic syndromic diagnosis for each child seldom was adequate to explain why they were on nasal oxygen or ventilator dependent. With increasing experience, it became clear that there were two principal factors that were common to all these children: the chest and spine (the thorax) had considerable deformity that not only reduced lung volume, but also affected the rib cage’s ability to expand the lungs with respiration, and because of the deformity, the rib cage and thoracic spine grew poorly, limiting the thoracic space available for lung growth. These two factors could result in life threatening extrinsic restrictive lung disease, and by 1992 [1] the term thoracic insufficiency syndrome (TIS) was introduced to orthopedics, and it was defined as the inability of the thorax to support normal respiration or lung growth [2].
The concept of thoracic insufficiency syndrome soon became the central guiding VEPTR principle, with restoration of thoracic volume through thoracic reconstruction and prosthetic stabilization now a practical objective, but the bewildering complexity of deformities of both the spine and ribcage of the referral patients made standardization of a single “VEPTR procedure” almost impossible. Over time, however, surgical intuition led to establishment of basic techniques that could address recurring themes of deformity, and a classification system for thoracic deformity evolved to guide surgical strategy, emphasizing the effect of volume reduction of the thorax due to deformity.
Volume depletion deformities (VDD) of the thorax [3]
- Type I
Rib absence and scoliosis
- Type II
Fused ribs and scoliosis
- Type III
Hypoplastic thorax
- IIIa
Foreshortened (Jarcho–Levin syndrome)
- IIIb
Narrowed (Jeune syndrome)
Type I and II are unilateral volume depletion deformities best addressed by unilateral VEPTR expansion thoracoplasty. Type III global volume restriction of the thorax usually is associated with severe restrictive lung disease and a high rate of mortality from respiratory failure. Staged bilateral VEPTR expansion thoracoplasty can be considered. Windswept deformity of the thorax due to the spine twisting into the convex hemi-thorax from rotation and lordosis in early onset scoliosis effectively narrows the thorax, a Type IIIb VVD.
Five new surgical procedures made possible by the VEPTR device were then developed over a period of 3 years by the surgical partnership of Campbell, the pediatric orthopedist, and the inventor of the device, and Melvin Smith, a pediatric general surgeon. These core VEPTR procedures were refined over the years and can now address almost any complex chest and spine deformity of the young child. The common goal of these procedures is to achieve the largest, most symmetrical, most functional thorax by skeletal maturity. This is accomplished through open thoracic reconstruction to enlarge and stabilize the malformed chest with indirect correction of the scoliosis, then VEPTR implants are used to stabilize the completed reconstruction. The devices are then periodically lengthening to “take the slack out of the system” as the child grows. The many types of deformities that needed treatment beyond just rib absence forced the development of adaptations of the VEPTR to address the new needs. A hybrid VEPTR device, originally termed a “thoracodorsal distractor”, was designed to attach to ribs, and then extend down to the spine, to provide greater distraction for rigid scoliosis associated with fused ribs. This “hybrid VEPTR” now serves as the workhorse for most VEPTR surgery. Also an extremely curved rib-to-rib VEPTR was designed to provide more lateral chest expansion for a 14-month-old infant referred from Birmingham, England, with Jeune Syndrome. The infant died before she could be transferred to the United States, but the device was successfully used on a Texas infant with Jeune syndrome in 1990.
Core procedures for VEPTR surgery using expansion thoracoplasty
Fused ribs and scoliosis This condition is probably the most common deformity treated by VEPTR. Based on the original rib absence treatment strategy, the fused ribs are cut apart at the center of the rib fusion mass through one or two transverse rib osteotomies, then the constricted hemithorax is lengthened by distraction of the proximal ribs away from the distal ribs. Next, a hybrid VEPTR and rib-to-rib VEPTR are used to stabilize the thoracic reconstruction. This expansion thoracoplasty is termed an opening wedge thoracotomy.
Absence of ribs and scoliosis This is usually due from either congenital absence of ribs or defects from chest wall tumor resection, and extremely large defects have a high mortality rate. Stabilization of the flail chest is accomplished by lengthening the collapsed hemithorax and stabilizing it with multiple rib-to-rib VEPTRs, much like a “picket fence”. Sometimes, there are enough fused rib mass proximal or distal to the defect to transversely osteotomize a two rib section that can be rotated centrally to provide osseous stability to the chest wall. Complete absence of proximal ribs for VEPTR attachment can be addressed by the “clavicle augmentation” procedure. Through anterior and posterior thoracotomies, a rib autograft harvested from the contralateral side is inserted posteriorly under the brachial plexus and secured to the posterior elements of the proximal thoracic spine. Next, through a longitudinal osteotomy of the clavicle, the anterior portion of the clavicle is mobilized and brought down and into the chest for attachment to the free autograft. The anterior clavicle functions as a medially based vascularized pedicle graft, and the grafts hypertrophy over several months to form proximal rib mass that can serve as a VEPTR attachment in a later procedure.
Narrow chest For a hypoplastic thorax due to narrowing, such as Jeune asphyxiating thoracic dystrophy, the chest wall is mobilized by anterior and posterior osteotomies of ribs 3 through 8, creating a large flail chest wall segment that is brought out laterally and attached to a special curved 7 cm radius rib to rib VEPTR that serves as a support arch for the expanded chest. This expansion thoracoplasty technique is termed Dynamic Postero-Lateral Expansion Thoracoplasty. The technique is also useful for unilateral transverse volume depletion deformities often seen in VATER syndrome.
Symmetrically shortened chest Usually due to bilateral rib fusion and a shortened thoracic spine, such as seen in spondylothoracic dysplasia, a variant of Jarcho–Levin syndrome. The shortened rib cage can be lengthened by a modified opening wedge thoracostomy. A “V” osteotomy is cut through the rib mass, apex medial, the hemi-thorax lengthened, and a rib-to-rib VEPTR is placed. The contralateral side is treated 3 months later.
Syndromic and neuromuscular scoliosis Lateral thoracic contracture on the concave side of the curve is diagnosed by the presence of persistent intercostal space narrowing on bending radiographs. This is addressed by intercostal muscle releases by cautery, next the concave hemi-thorax is lengthened, then the VEPTRs are implanted. Thoracic curves are treated with a hybrid VEPTR from rib to spine, often adding a rib-to-rib VEPTR laterally for load-share. Thoracolumbar curves are treated with the “Eiffel Tower Construct”, a bilateral rib to pelvis hybrid VEPTR construct, with an intercostal muscle release added on the concave side just above the diaphragm between ribs eight and nine. This stabilizes or reverses the volume depletion deformity due to windswept chest and lengthens the concave hemi-thorax.
VEPTR was first use as a non-investigational device in Europe after it received a CE mark of approval. In 2002, the first VEPTR surgery in Europe was performed in Basil, Switzerland Kinderhaus by Professor Dr. Fritz Hefti and Dr. Anna Hell-Vocke, and it went on to be used throughout Europe. Campbell mentored many orthopedic surgeons about its usage by traveling to their institutions to assist in first cases. Results of VEPTR treatment from Europe [3, 4] were similar to those in the United States, and Hasler et al. from Basel [5] reported the first major series of VEPTR treatment of progressive spine deformities without rib fusion.
In the United States, the VEPTR was approved as a Humanitarian Use Device (HUD) in 2004 after one of the lengthiest device trials in US history. The reasons for this long device trial were complex, and related mainly to the difficulties in accruing a large number of patients with rare diseases. A sole site feasibility FDA VEPTR device trial had been conducted from 1992 to 1994 in San Antonio, Texas, then Synthes Spine Co., of West Chester, Pennsylvania, took over VEPTR development, and expanded it to a multi center trial to see if the San Antonio results could be duplicated by other surgeons. Participating institutions included: Pittsburgh Children’s Hospital, Boston Children’s Hospital, Children’s Hospital of Philadelphia, Shriner’s Hospital of Philadelphia, Christus Santa Rosa Children’s Hospital, San Antonio, Los Angeles Children’s Hospital, Inter-Mountain Children’s Hospital of Salt Lake City, and Children’s Regional Medical Center of Seattle. All the other institutions had similar clinical success. The VEPTR HUD was approved in the USA for treatment of Thoracic Insufficiency Syndrome in skeletally immature patients with an anatomic diagnosis of absent ribs, constrictive chest wall syndrome, including fused ribs and scoliosis, hypoplastic thorax syndrome, congenital scoliosis without rib anomaly, and neurogenic scoliosis. Immediately following its approval, Synthes Spine Company sponsored extensive training for its use and supported travel of senior surgeons who had participated in the FDA device trial to mentor surgeons wishing to use the device in their own institution.
The device gradually gained acceptance throughout the pediatric orthopedic community for the treatment of scoliosis. Clinical studies [6], based on AP radiographs and pulmonary function studies, noted that the VEPTR treatment of congenital fused ribs and scoliosis resulted in curve correction, spinal and chest growth, increased height of the constricted hemithorax, and improved vital capacity in those children treated under age 2 years compared to those over 2 years of age when lung growth by alveolar cell multiplication is not as rapid. Results of Cobb angle correction on radiograph were confirmed by others [4]. Using Vitrea 2 software analysis of CT scans [7], significant growth of 7 %/year of both the concave side of the curve and the unilateral unsegmented bars of severe congenital scoliosis treated by VEPTR was seen, casting doubt on the long accepted orthopedic maxim that unilateral unsegmented bars cannot grow, the primary justification for using early spine fusion in infancy for treatment of congenital scoliosis despite the growth inhibition effects of the procedure. VEPTR in congenital scoliosis was also found to have a great corrective effect on truncal shift, shoulder imbalance, and cervical tilt [8]. In 2007, the concept of secondary thoracic insufficiency syndrome was introduced by Campbell and Smith [3], which describes the effect when the torso drops onto the pelvis from spine deformity, commonly seen in myelomeningocele patients with gibbus deformity of the lumbar spine, and also in pelvic obliquity, raising abdominal pressure, which obstructs diaphragmatic excursion, partially disabling the respiratory mechanism. They also coined the term “collapsing parasol deformity” of the thorax to describe the severe loss of thoracic volume from primary rib collapse in patients with spinal muscular atrophy that does not seem entirely due to spinal rotation.
A great interest in thoracic insufficiency syndrome began to build in the mid 2000s. Vitale and co-workers [9] noted a very poor quality of life questionnaire scores for untreated children with thoracic insufficiency syndrome. Skaggs et al. [10] found that in 76 patients with TIS, 79 % were under the 5th percentile for weight, and after VEPTR surgery 40 % improved with weight gain. VEPTR appears to make a positive difference for children with thoracic insufficiency syndrome clinically, but the lack of an objective lab test or imaging technique to measure thoracic insufficiency syndrome has made it difficult for orthopedists to fully understand the effect of VEPTR treatment on TIS. At this time, VEPTR outcomes are mostly assessed only by Cobb angle correction on the AP radiograph, so the assessment of the effect of VEPTR treatment on 3D thoracic deformity, function, and growth await further technological advances in imaging and other tests.
VEPTR is now used in over 26 countries across the world, and surgeons have gone on to modify its technique of use. John Smith of Salt Lake City has popularized a percutaneous bilateral rib to pelvis VEPTR technique using limited incisions without the release of inter-costal muscles for thoracic reconstruction. Because of its ease of implantation and low blood loss, this has gained wide acceptance worldwide with those surgeons performing VEPTR procedures just for scoliosis, but development of crouched gait postoperatively in some patients for unclear reasons makes it somewhat controversial. White et al. [11], emphasizing the versatility of the VEPTR expansion mechanism, consider it as a “growing rod” attached to the ribs for treatment of scoliosis. Skaggs advocates a “VEPTR approach”, but uses growing rod constructs attached proximally by spinal hooks in a claw around ribs. The effect of all these alternative approaches on correcting thoracic insufficiency syndrome is unknown, but all do appear to correct the Cobb angle of scoliosis.
Some surgeons have recently advocated for adoption of new terminology for growth-sparing instrumentation for spine deformity correction, based simply on attachment points, calling growing rods “spine-based” growth-sparing instrumentation, and VEPTR “rib based” growth-sparing instrumentation, suggesting that there is no difference between either approach other than their proximal attachment points. The AP radiograph appearance of these systems has some similarity, and both do share the common goal of correcting scoliosis, but VEPTR principles for correction of thoracic insufficiency syndrome demand much more of the surgeon than just Cobb angle correction on a radiograph.
Approaches
This case of a 12-month-old male infant with an 80 ° congenital curve (Fig. 1a) on AP radiograph, with an elevated respiratory rate and mild marionette sign, illustrates the important differences between the two approaches: Growing Rod Instrumentation versus VEPTR principles approach.
Fig. 1.
a AP Radiograph. The inferior vestigial ribs on the concave side appear too weak for VEPTR attachment. b 3D reconstruction of CT of chest and spine. The scan enables better visualization of the vestigial 11th and 12th ribs, which appear to be thick enough to carry the load of the VEPTR devices. c The coronal reconstruction of the CT scan. Note the anomalous insertion of the hemi-diaphragm proximally to the fused ribs on the concave side. This CT scan ruled out attachment of the hemi-diaphragm to soft tissue below the fused ribs, a flail hemi-diaphragm, which would have required a completely different operative strategy. d Supine lateral radiograph. Note kyphosis. It is not severe enough to warrant use of VEPTR II. VEPTR I is preferable for this age group, because it is more compact with greater expandability in the smaller sizes
The growing rod “spine-based” growth-sparing instrumentation approach
The goal would be to correct the curve while permitting spinal growth, but the infant is too small for the instrumentation, so surgery would be delayed until age 2 or 3 years with either observation or bracing in the interim. Growing rod treatment would continue until age 10 years at which time a definitive posterior spine fusion would be performed.
The VEPTR principles approach
The goal would be to arrive at an operative strategy that provided the largest, most symmetrical, most functional thorax by skeletal maturity. This would be the best chance to reverse the thoracic insufficiency syndrome. The first step is to define the 3D thoracic problems with regard to volume, function, and ability to grow.
Thoracic volume The height of the concave hemi-thorax on radiograph is 46 mm, compared to a height of 95 mm for the convex side, so the concave hemi-thorax longitudinally is constricted. The space available for lung (SAL) [2] ratio of concave lung height divided by convex lung height on the AP radiograph is, therefore, 48 %. The cause of the constricted hemi-thorax is absence of the inferior ribs 7–10, with anomalous proximal insertion of the hemi-diaphragm, and rib fusion of the remaining proximal ribs. The vestigial 11th and 12th ribs are diverted downwards into the abdomen in the area of an absent kidney (Fig. 1b). There is moderate volume depletion of the concave hemi-thorax (Fig. 1c). The lateral radiograph shows mild thoracic kyphosis (Fig. 1d). In severe kyphosis, VEPTR II is helpful because of the ability to conform to the proximal kyphosis through proximal rod bending.
The surgical strategy should lengthen and widen the constricted hemi-thorax
Options
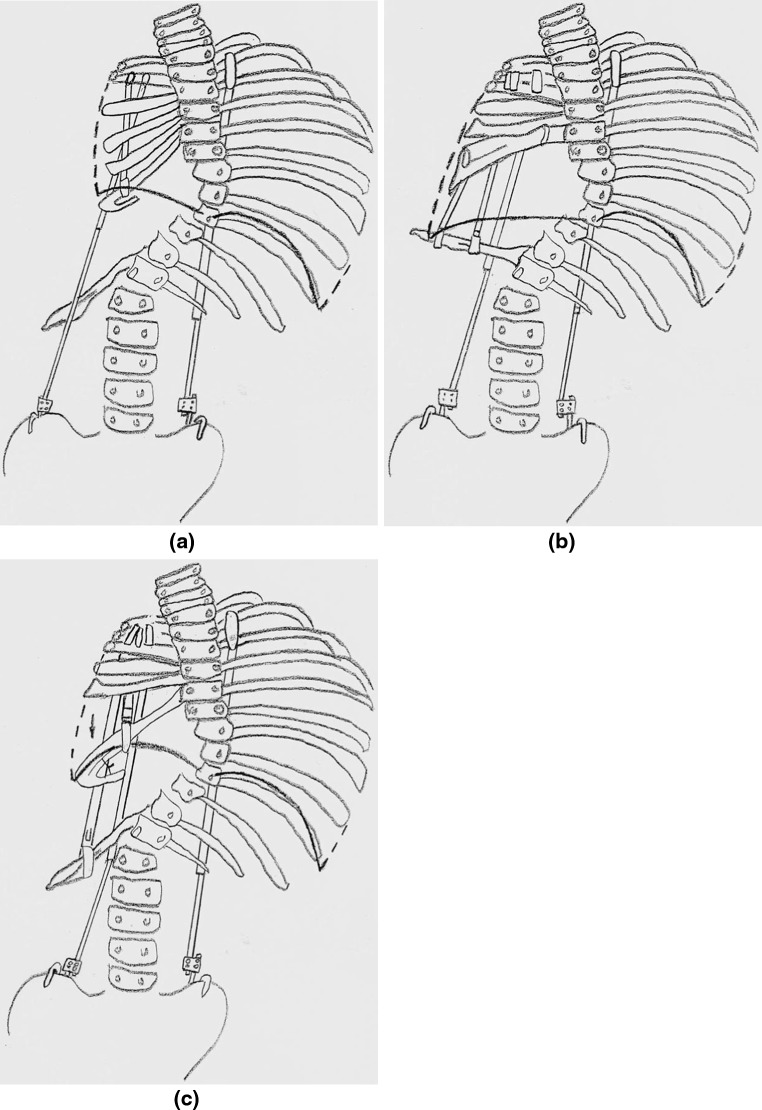
Option 1 Multiple transverse osteotomies of the fused ribs could be performed, spreading them apart to lengthen the hemi-thorax, with the multiple rib segments providing chest wall stability, with a rib-to-rib VEPTR stabilizing the expanded chest and a hybrid rib to pelvis VEPTR (the spine is too small for laminar hook attachment for the VEPTR) to stabilize the thoraco-lumbar curve (Fig. 2a). This is not practical for this infant, however, since the bone mass is so small that the osteotomies would be too close together, potentially devascularizing the osteotomized rib segments. The approach also would not widen the hemi-thorax.
Fig. 2.
a Option 1, b Option 2, c Option 3
Option 2 Another approach would be to mobilize the vestigial 11th and 12th ribs, pulling them upwards by osteoclasis so they become transverse in orientation at the level of T12, then the periphery of the elevated hemi-diaphragm is detached and transposed distally to attach to the mobilized 11th and 12th ribs, effectively lengthening the space available for lung with the new site of the hemi-diaphragm moved from T6 to the more normal level of T12. Scoliosis would be controlled by a hybrid rib to pelvis VEPTR. This would also widen the hemi-thorax (Fig. 2b). Chest wall stability in theory could be provided by multiple rib-to-rib VEPTRs, but the infant is so small that it is unclear whether there would be room enough for the devices. It is also not known if the mobilized ribs could support the pressure from the contracting hemi-diaphragm. A rib auto-graft from the contralateral side could be added to the mobilized ribs, but this would add more complexity to the procedure and months of healing would be needed to strengthen the bony diaphragm attachment with the possibility of resorption of the bone graft.
Option 3 A more simple approach would be to perform a single transverse osteotomy inferiorly in the bone mass, creating a two rib thick inferior rim of bone already attached to the diaphragm, rotating it downward 90 °, so it transports the diaphragm distally (Fig. 2c), providing more room for the lung above for growth, and perhaps the resultant increased curve of the hemi-diaphragm will provide better function. The transported rib segment with hemi-diaphragm would be stabilized in its new distal position with a rib-to-rib VEPTR I, with care to take the top of the rib cradle cap through a burred hole in the rib fusion mass to avoid impingement on the brachial plexus above. To widen the hemithorax, a lateral point of attachment is needed, so a second rib to rib VEPTR I is placed down to the distal vestigial ribs, slightly lateral to the transposed rib mass, and then no. 1 heavy Prolene suture is used to pull the rib mass attached to the hemi-diaphragm outward, securing it to the 2nd VEPTR. There would probably not be as much transverse volume addition as would be seen in Option 2, but the gain in diaphragm stability is considered a good trade-off. A hybrid rib to pelvis VEPTR would be added to control the scoliosis. It might not be possible to have 3 VEPTRs in the small proximal rib mass, so the rib to distal vestigial rib VEPTR might have to be sacrificed with some loss of transverse volume gain.
How do these options impact both thoracic function and growth?
Thoracic function Thoracic function is based on the four thoracic physiological determinants of vital capacity: the expansion of each lung by the rib cage on each side of the chest during inspiration, and the inferior expansion of each lung by the hemi-diaphragm. The poorly understood distortion of the rib cage and diaphragm by spine and chest wall deformity probably affects performance of each of the four determinants selectively and, to add to the complexity, the four determinants are probably interrelated normally.
In this patient, the chest wall is stiff to thumb excursion test, with probable loss of the two determinants of thoracic function dependent on chest wall expansion during respiration. Further loss of thoracic function is expected because of the small size of the right lung and the anomalous proximal insertion of the hemi-diaphragm, which probably degrades lung expansion. Secondary thoracic insufficiency syndrome also appears to be present with decreased movement of the left hemi-diaphragm on dynamic lung MRI (Fig. 3).
Fig. 3.
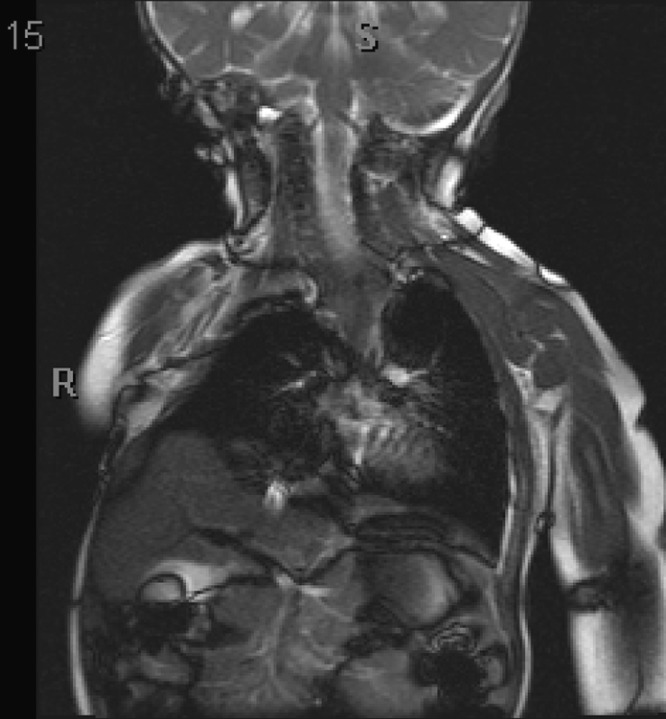
The coronal dynamic lung MRI view. Hemi-diaphragm motion is limited on the convex side
The surgical strategy should improve thoracic function
Options
All options already discussed would increase concave hemi-thoracic volume while moving the hemi-diaphragm distally, likely resulting in compensatory lung growth, so the end result could be a concave hemi-thorax with a larger lung which would add to the overall function of the thorax. Increasing the curvature of the hemi-diaphragm also might make it more effective. Adding a second hybrid VEPTR from ribs to pelvis on the convex side will help correct the truncal shift and, more importantly, elevate the chest away from the pelvis, probably relieving pressure on the compromised left hemi-diaphragm, decreasing secondary thoracic insufficiency syndrome. There is also a chance that with curve correction in time the chest wall motion on the convex side might increase, contributing to vital capacity. Although not normal by any means, these partial gains in volume, symmetry, and function of the thorax will likely improve the pulmonary prognosis for this child.
Thoracic growth Increased thoracic volume from growth is due to gains in height from growth of the thoracic spine, increase in width from rib growth, and increased chest depth from both rib growth and anterior inclination of the ribs. The congenital scoliosis is due to failure of formation of the concave side of the thoraco-lumbar spine. Some additional growth inhibition might be expected on the concave side of the central curve because of the magnitude of deformity. The proximal curve will also have poor growth because of the fused rib tether. The height of the thoracic spine is 11 cm, which is 76 % normal for age, so with 28 cm the normal height at skeletal maturity for males, the projected height of the child’s thoracic spine will likely be 21 cm. Karol’s [12] threshold for a thoracic spinal height at skeletal maturity that minimizes the risk of severe restrictive lung disease is 22 cm., so the risk of a mild pulmonary deficit could be seen even if the rest of the chest were normal. The gain in width by growth of the thorax on the concave side is compromised by the presence of fused ribs, and the increasing curve will also narrow the thorax on the convex side by formation of a rib hump. Continued deformity will probably limit the ability of the thorax to also increase in depth.
The surgical strategy should support growth of the spine and rib cage
Options
All options previously discussed do not seem to inhibit growth of the thorax and may actually stimulate it. The spine included in the thoraco-lumbar curve, placed in traction by the bilateral hybrid rib to pelvis VEPTRs, will grow in length, adding to thoracic volume and placing the thorax well above the pelvis which will treat the secondary thoracic insufficiency syndrome. There is a good chance that a transverse rib osteotomy will enable better growth of the proximal thoracic spine. The lateral traction on the concave ribcage in option 3 may provide increased growth in width. The growth of the convex rib cage will probably be enhanced by the VEPTR construct providing symmetry to the thorax, and this might also help with growth in depth of the chest.
Treatment
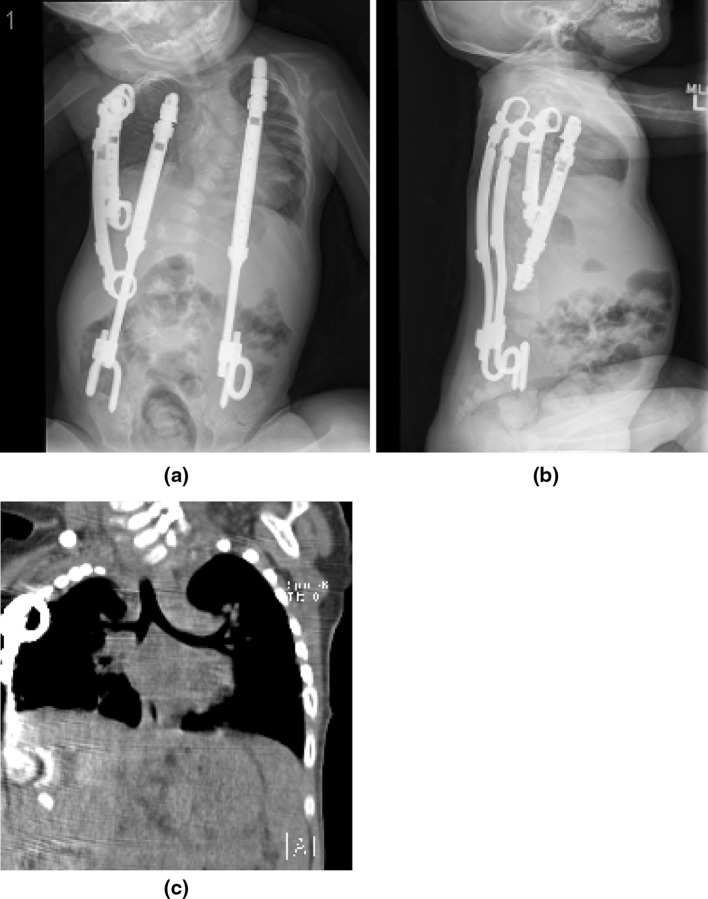
The child underwent the Option 3 VEPTR expansion thoracoplasty (Fig. 4a, b). There was adequate room to place three VEPTRs on the concave side for an optimal hemi-thorax reconstruction and the convex VEPTR was placed to provide an Eiffel Tower construct. Space available for lung improved to 64 %, providing new room for lung growth. Postoperative CT scan coronal reconstruction (Fig. 3c) show overall improved symmetry of the thorax. Because the small rib structure would not tolerate high distraction pressures, only partial curve correction to 49 ° is seen, but improved correction will be likely be achieved in the future with continued device lengthening and growth of the concave side. This patient will likely have improvement in his thoracic insufficiency syndrome. His thorax cannot provide support completely normal respiration, but there is improved volume of the concave hemithorax for lung growth, and possibly improved function of the hemi-diaphragm on that side, so the respiratory function of the thorax will probably be better. The spine, relieved of substantial deformity, will grow and contribute to thoracic volume, which supports lung growth, the other determinant of thoracic insufficiency syndrome to be corrected. The secondary thoracic insufficiency syndrome from collapse of the torso into the pelvis is also relieved by the bilateral rib to pelvis VEPTR distraction. The chosen constructs were the best compromise, given the limits of present technology and surgical technique, and will likely provide the best outcome expected for the correction of this child’s thoracic insufficiency syndrome. The problem is that there is currently no way to measure this outcome with a test that can easily document the gains in volume, rib cage and diaphragm excursion, and thoracic growth.
Fig. 4.
a Postoperative weight bearing AP radiograph. b Postoperative lateral radiograph. c AP reconstruction of postoperative CT scan. Increased thoracic symmetry is seen
What will be the long-term outcome of this child’s VEPTR treatment, and compared to what? Natural history, spine fusion, other existing growth sparing instrumentation such a dual growing rods, as well as the SHILLA technique, or magnetic powered self-expanding instrumentation currently under development, are the current choices, but prospective matched series comparing one technique to another do not currently exist, and natural history series are lacking since intervention is available. What is clear is that established growth sparing spine deformity treatment technologies are labor intensive and carry risk. The complication rate for VEPTR use, growing rods, and other forms of growth sparing instrumentation is considerable and is due to the nature of a repetitive surgery approach, the complexity of the combined spine and chest wall deformity, and the inherent poorly defined problems of the primary diagnosis with osteopenia, tendency for infection, and other confounding variables. In summary, the problems are infection, skin breakdown, dislodgement or slow migration of devices through their attachment to bone, device fatigue fracture, and neurologic injury such as brachial plexopathy or spinal cord injury. All growth sparing instrumentation clinical series are relatively small, extremely heterogeneous, and the authors are often comparing “apples to oranges” regarding the multiple core diagnoses, with treatment groups often based solely on a comparative degree of Cobb angle. Sankar et al. [13] in a retrospective series compared the complication rate of one surgeon’s experience using growing rods in 10 patients, a “hybrid growing rod system” using up-going spinal hooks proximally on the ribs in 7 patients, and 19 patients using the VEPTR system. The core anatomic diagnoses are unclear, with the authors stating they excluded multiple fused rib patients, but later stating that there were 20 patients with congenital scoliosis and/or fused ribs. Most of the analysis combined the three groups of congenital scoliosis, neuromuscular scoliosis, and syndromic scoliosis. The average follow-up was 4.3 years, but varied from 2 to nearly 10 years, with an increasing complication rate with passage of time until 36 months of follow-up, then a paradoxical decrease between 36 and 48 months without clear reason. Dual growing rods had an average of 0.52/year complications, “hybrid growing rods” had 0.36/year complications, and VEPTR patients had 0.52/year complications. Length of follow-up was not specified for each group, so it is difficult to determine whether “hybrid growing rods” had the same follow-up period as the other groups. Campbell et al. [6] reported 27 VEPTR patients with relatively homogeneous diagnosis of fused ribs and scoliosis at an average 5.7-year follow-up and found 32 % had a slow, asymptomatic migration of the upper rib cradle of the VEPTR into the rib of attachment with complete migration within 3–5 years, with reseatment usually done during scheduled surgery for expansion. Eighteen percent had skin slough, and the infection rate was an average of 1.9 % per procedure. In one of the largest series reported, Campbell and Smith [3] reported a 3.3 % infection rate per surgery in 1,412 surgeries of 201 heterogeneous VEPTR patients at an average follow-up of 6 years, with a skin slough rate of 8.5 %, and a rib cradle migration rate in 27 % of patients averaging complete cut-out in an average of 3 years. It is clear by these studies that complicated patients have complications, and VEPTR patients commonly have numerous comorbidities such as myelomeningocele, congenital heart disease, renal disease, and many other problems, which predisposes them to surgical complications, but attention to pre-operative nutrition, medical work-up [14], and meticulous soft tissue technique at surgery can help minimize these complications. To put this into perspective, the natural history of untreated thoracic insufficiency syndrome anecdotally is also complicated by severe morbidity and a high rate of lethal outcome.
In theory, the incidence of complications associated with growth sparing spine deformity instrumentation could be reduced by limiting duration of treatment. Recently, the term “law of diminishing returns” [15] has been popularized to describe the tendency of growing rods to lose the ability to lengthen surgically with time for unclear reasons, interpreted that the procedure has a limited time frame of effectiveness, so some advocate delaying growing rod treatment until the patients are older, which would shorten total surgical treatment time and possibly lower the incidence of complications. This finding, however, could also be explained by the early success of the rods in correcting the curve, with subsequent lengthening “stretching” a relatively straight spine with more reactive force to lengthening than would be needed for the early curve correction, in a way just “taking the slack out of the system”. The clinical goals of this series seemed satisfied, with T1–S1 growth in height of the spine normal, and curve correction remaining stable during treatment, so it is unclear why the authors consider their expected returns “diminished”. For VEPTR patients, the critical importance of lung growth that is dependent on correction of thoracic deformity argues that intervention should be as early as possible in life, ideally at age 6 months, when lung growth is most rapid.
Does VEPTR treatment improve pulmonary function? This is a very complex question. Most literature examining VEPTR pulmonary outcomes appear to have the unspoken hypothesis that VEPTR treatment should raise the percent-predicted forced vital capacity (FVC) back to normal, but is this a realistic assumption? Therapeutic intervention should always be measured against either natural history or another intervention. Assuming that natural history decreases the vital capacity in the diseases VEPTR surgery treats, VEPTR stabilization of the percent normal vital capacity, or even slowing the rate of decrease, could be considered a treatment success. Natural history studies are generally not available for the diseases causing thoracic insufficiency syndrome. Comparison of pulmonary outcome to another growth sparing techniques is also not feasible because pulmonary function tests are not available for these non-VEPTR techniques. Even for VEPTR, pulmonary function testing (PFT) is mostly limited to very small numbers of older children who could cooperate with standard spirometry. Campbell et al. [6] reported post-operative VEPTR treatment pulmonary results of a small but relatively standardized population of 27 patients with severe congenital scoliosis and fused ribs. The patients were unable to perform pre-operative PFTs because of their young age at time of first surgery, but as they got older in the follow-up period they were tested. There was an average percent normal FVC of 49 % normal at a mean follow-up of 5.9 years. Those patients under age 2 years at time of first surgery, during the time when lung growth is greatest, had a FVC of 58 % predicted at follow-up, while those older than 2 years at first surgery had a significantly less FVC of 44 % predicted. Mayer and Redding [16] reported PFT results of 40 VEPTR patients at an average age of 9.1 years, with many varied diagnoses, over only a 7.7-month follow-up period, the first interval between the initial implantation and the first VEPTR expansion, and found the percent normal vital capacity to be decreased significantly. This is difficult to interpret. It may represent the commonly seen acute decrease in FVC following posterior spine fusion [17] that is probably due to gradual respiratory muscle recovery from surgery. Motoyama et al. [18] reported an intra-operative deflation pulmonary function technique of pulmonary function that can be done without patient cooperation at both pre and post-VEPTR operative intervals in 10 patients having mostly congenital scoliosis, ranging in age between 1.5 and 9.8 years at surgery, and found that percent normal FVC remained stable at 70 percent. In a follow-up study, Motoyama et al. [19] reported results of 24 VEPTR patients, average age 4.6 years (1.8–10.8 years) at first surgery, and at average 3.2-year follow-up found that those younger than 6 years at time of first surgery had a 14 %/year increase in percent normal FVC, while those older than 6 years at time of surgery had only a 6.5 %/year increase. This suggests that earlier intervention with VEPTR seems to help pulmonary outcome, likely because of lung growth potential. Of the six children on mechanical ventilator support in this study, two were weaned off support and oxygen completely, three were weaned off oxygen, and one required less respiratory support. For the total group, the average vital capacity went from 71.9 % normal to 66.3 % normal at follow-up, a significant decrease, but probably of no clinical significance. There was a significant loss of chest wall compliance (increased chest wall stiffness). The increase in chest wall stiffness is difficult to interpret since many of the patients had fused ribs at presentation, and the natural history of chest wall compliance changes in such patients is unknown. Gadepalli et al. [20] recently reported the pulmonary outcomes of 23 heterogeneous VEPTR patients: one-half was evenly divided in diagnosis between congenital scoliosis and idiopathic scoliosis, the remainder was either neuromuscular scoliosis or undefined origin. The most compromised patients, ventilator dependent, were excluded. The average age at surgery was 7.6 years, but no range was given. Only patients able to cooperate with pulmonary function testing, probably age 6 years or above, were included, so the treatment population is probably older. The average percent normal FVC changed from 58 to 56 % after surgery, so was essentially stable. There was a 28 % improvement in residual volume, but it was not statistically significant. Pulmonary volumes by CT scan were unchanged by VEPTR treatment, but it is not clear whether volumes were at maximum inspiration or the less accurate CT scan lung volumes during spontaneous breathing. These volumes were also compared to the retrospective spontaneously breathing pediatric normative CT scan lung volumes reported by Gollogly et al. [21], and there was no increase in the age-corrected volumes. The authors also found nearly normal quality of life both before and after VEPTR treatment based on a modified SRS-22 instrument, although a validated Child Health Care Questionnaire study of children by Vitale et al. [10] in 2008 showed a marked decrease in quality of life for children with untreated thoracic insufficiency syndrome. The authors’ final conclusion was that “pulmonary function, lung volume, and patient subjective assessments did not increase dramatically after VEPTR placement”, which apparently was their working hypothesis, but the marked heterogeneity of their patient population and their suboptimal age of intervention may have influenced pulmonary outcomes in an unpredictable fashion, and their choice of a quality of life instrument may be unsuited for thoracic insufficiency syndrome. Another variable that is not considered in any of these reports is the incidence of repetitive pneumonias and obstructive lung disease in these patients, both having a great influence on pulmonary function. With virtually no natural history pulmonary outcome data available for the many diseases that are treated by VEPTR, it will be difficult to interpret the effect of intervention by pulmonary function testing.
The future
The long-time assessment of spine deformity correction by AP radiograph, the AP radiograph paradigm, remains the gold standard for treatment outcome assessment. This paradigm has served orthopedics well in the past, but this 2D static imaging technique is inadequate to characterize the 3D thoracic volume, symmetry, and function deficits that VEPTR must treat. VEPTR surgical strategies can expand the volume of a thorax, and it is assumed that the lung will grow to fill the expanded volume with the diaphragm “powering” the larger lung, but an AP radiograph cannot tell us much beyond the Cobb angle. For the twenty-first century, new metrics need to be developed to assess thoracic function. Lung ventilation–perfusion scans can provide tracer uptake ratios between right and left lungs, but clinical interpretation is unclear. CT scans can visualize the 3D correction of thoracic volume deficits by VEPTR techniques, but cannot tell us if the thorax functions better by improved rib cage motion or diaphragm excursion. Pulmonary function studies are a dynamic test, but are impractical at most institutions for children younger than age 6 years, and only summate the respiratory output of the four complex thoracic function biomechanical determinant mechanisms, so they cannot help us understand how each contribute to reduced vital capacity in scoliosis. Dynamic lung MRI imaging, however, has great promise as a means to actually visualize the functional deficits of both diaphragm and rib cage expansion of the lungs during respiration in early onset scoliosis patients [22], and efforts are underway to quantify these. Once validated, this approach may enable us to both define the full dynamic biomechanical deficits of spine and chest wall deformity and how treatment affects these deficits.
From a technological viewpoint, there is some encouraging progress in development of growth-sparing instrumentation. Magnetic expansion mechanisms are being developed for growing rods [23] that might enable lengthening of implanted devices without surgery, potentially decreasing the morbidity due to the repetitive surgeries currently used to manually lengthen devices to accommodate growth, but only long-term use will prove their safety and efficacy. VEPTR could probably take advantage of this technology. The biggest challenge ahead, however, is to develop successors to VEPTR that can not only correct the Cobb angle of scoliosis and lengthen a constricted hemi-thorax, but also to restore both normal volume and mobility to the scoliotic thorax, ensuring normal growth into skeletal maturity with preservation of pulmonary function throughout life. A much better understanding of thoracic insufficiency syndrome and its natural history is needed before this can be addressed, and dynamic lung MRI and other technology may enable this. A rabbit model of thoracic insufficiency syndrome is also being pioneered by Snyder and his colleagues [24], and this will provide valuable confirmatory knowledge about the degree of compensatory lung growth due to VEPTR expansion thoracoplasty as well as the effect of timing on its response.
VEPTR and its principles of use have become an important first step toward improving the quality of life and longevity of children with thoracic insufficiency syndrome, but much work remains to advance both its design and its use. Although presently used by many surgeons only to correct the angular deformity of scoliosis, its potential to address so many aspects of thoracic insufficiency syndrome will become more widely appreciated by its users once research clearly defines the biomechanical basis of the disease and metrics are available to gauge functional improvement in thoracic performance after VEPTR treatment. The challenge is large, but the rewards are great.
Conflict of interest
None.
References
- 1.Campbell RM, Smith MD, Mayes T, Pinero R (1992) The treatment of thoracic insufficiency syndrome scientific exhibit. In: American academy of orthopaedic surgeons annual meeting 1992
- 2.Campbell RM, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Adler ME, Duong HL, Surber J. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg. 2003;85-A:409–420. doi: 10.2106/00004623-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Campbell R, Smith MD. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg. 2007;89A(Suppl):108–220. doi: 10.2106/JBJS.F.00270. [DOI] [PubMed] [Google Scholar]
- 4.Hell AK, Campbell RM, Hefti F. New treatment concept for children with thoracic insufficiency syndrome due to congenital spine deformity. Klin Padiatr. 2005;217(5):268–273. doi: 10.1055/s-2004-832483. [DOI] [PubMed] [Google Scholar]
- 5.Hasler CC, Mehrkens A, Hefti F. Efficacy and safety of VEPTR instrumentation for progressive spine deformities in young children without rib fusions. Eur Spine J. 2010;19(3):400–408. doi: 10.1007/s00586-009-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86-A(8):1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Campbell RM, Hell-Vocke AK. Growth of the Thoracic Spine in Congenital Scoliosis after Expansion Thoracoplasty. J Bone Joint Surg. 2003;85-A:409–420. doi: 10.2106/00004623-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RM, Jr, Adcox BM, Smith MD, Simmons JW, 3rd, Cofer BR, Inscore SC, Grohman C. The effect of mid-thoracic VEPTR opening wedge thoracostomy on cervical tilt associated with congenital thoracic scoliosis in patients with thoracic insufficiency syndrome. Spine (Phila Pa 1976) 2007;32(20):2171–2177. doi: 10.1097/BRS.0b013e31814b2d6c. [DOI] [PubMed] [Google Scholar]
- 9.Vitale MG, Matsumoto H, Roye DP, Jr, Gomez JA, Betz RR, Emans JB, Skaggs DL, Smith JT, Song KM, Campbell RM., Jr Health-related quality of life in children with thoracic insufficiency syndrome. J Pediatr Orthop. 2008;28(2):239–243. doi: 10.1097/BPO.0b013e31816521bb. [DOI] [PubMed] [Google Scholar]
- 10.Skaggs DL, Sankar WN, Albrektson J, Wren TA, Campbell RM. Weight gain following vertical expandable prosthetic titanium ribs surgery in children with thoracic insufficiency syndrome. Spine (Phila Pa 1976) 2009;34(23):2530–2533. doi: 10.1097/BRS.0b013e3181bd09f5. [DOI] [PubMed] [Google Scholar]
- 11.White KK, Song KM, Frost N, Daines BK. VEPTR growing rods for early-onset neuromuscular scoliosis. Clin Orthop Relat Res. 2011;469:1335–1341. doi: 10.1007/s11999-010-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272–1281. doi: 10.2106/JBJS.G.00184. [DOI] [PubMed] [Google Scholar]
- 13.Sankar WN, Acevedo DC, Skaggs DL. Comparison of complications among growing spinal implants. Spine (Phila Pa 1976) 2010;35(23):2091–2096. doi: 10.1097/BRS.0b013e3181c6edd7. [DOI] [PubMed] [Google Scholar]
- 14.Campbell RM., Jr Spine deformities in rare congenital syndromes: clinical issues. Spine (Phila Pa 1976) 2009;34(17):1815–1827. doi: 10.1097/BRS.0b013e3181ab64e9. [DOI] [PubMed] [Google Scholar]
- 15.Sankar WM, Skaggs DL, Yazici M, Johnston CE, II, Shah SA, Javidan P, Kadakia RV, Day TF, Akbarnia BA. Lengthening of dual growing rods and the law of diminishing returns. Spine. 2011;36:806–809. doi: 10.1097/BRS.0b013e318214d78f. [DOI] [PubMed] [Google Scholar]
- 16.Mayer OH, Redding G. Early changes in pulmonary function after vertical expandable prosthetic titanium rib insertion in children with thoracic insufficiency syndrome. J Pediatr Orthop. 2009;29:35–38. doi: 10.1097/BPO.0b013e3181929c8b. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Lenke LG, Bridwell KH, Kim KL, Steger-May K. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J Bone Joint Surg Am. 2005;87:1534–1541. doi: 10.2106/JBJS.C.00978. [DOI] [PubMed] [Google Scholar]
- 18.Motoyama E, Deeney V, Fine G, Yang C, Mutich R, Walczak S, Moreland M. Effects on lung function of multiple expansion thoracoplasty in children with thoracic insufficiency syndrome: a longitudinal study. Spine. 2006;31:284–290. doi: 10.1097/01.brs.0000197203.76653.d0. [DOI] [PubMed] [Google Scholar]
- 19.Motoyama E, Yang C, Deeney V. Thoracic malformation with early-onset scoliosis: effect of serial VEPTR expansion thoracoplasty on lung growth and function in children. Paediatr Respir Rev. 2009;10:12–17. doi: 10.1016/j.prrv.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Gadepalli SK, Hirschl RB, Tsai WC, Caird MS, Vanderhave KL, Strouse PJ, Drongowski RA, Farley FA. Vertical expandable prosthetic titanium rib device insertion: does it improve pulmonary function? J Pediatr Surg. 2011;46(1):77–80. doi: 10.1016/j.jpedsurg.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Gollogly S, Smith J, White S, et al. The volume of lung parenchyma as a function of age: a review of 1050 normal CT scans of the chest with three-dimensional volumetric reconstruction of the pulmonary system. Spine (Phila Pa 1976) 2004;29:2061–2066. doi: 10.1097/01.brs.0000140779.22741.33. [DOI] [PubMed] [Google Scholar]
- 22.Campbell RM, Auber A, Simmons III J, Joshi A, Inscore S, Cofer B, Doski J (2008) The characterization of the thoracic biomechanics of respiration in thoracic insufficiency syndrome by dynamic lung MRI: a preliminary report. In: The 2nd international congress on early onset scoliosis and growing spine, Montreal, Quebec, 2008
- 23.Cheung K, Cheung J, Samartzis D, Mak K, Wong Y, Cheung W, Akbarnia B, Luk K. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet. 2012;379:1967–1974. doi: 10.1016/S0140-6736(12)60112-3. [DOI] [PubMed] [Google Scholar]
- 24.Olson JC, Kurek KC, Mehta HP, Warman ML, Snyder BD. Expansion thoracoplasty affects lung growth and morphology in a rabbit model: a pilot study. Clin Orthop Relat Res. 2011;469(5):1375–1382. doi: 10.1007/s11999-011-1807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]