Abstract
Introduction
The treatment of spinal deformities has rapidly changed during the past decade. The advent of new surgical techniques, particularly thoracic pedicle screws and spinal osteotomies, allow more aggressive deformity correction, and require an increased focus on safety.
Materials and methods
Review of the navigation systems and neuromonitoring techniques currently available.
Conclusion
Navigation systems today are where intraoperative neuromonitoring was 20 years ago: new, under investigation, not widely accepted, with concerns for cost, safety and efficiency. Navigation enhances the accuracy of pedicle screws placement in deformed spines, reducing the rate of misplaced screws and potential complications. With further use and investigation, navigation, like neuromonitoring, will soon become standard at major spine centers throughout the world.
Keywords: Scoliosis, Spine surgery, Navigation, O-arm, Neuromonitoring
Introduction
Over the past two decades, rapid advances in instrumentation and techniques have given spine surgeons the tools to achieve correction of spinal deformities to a far greater extent than was possible in the past. Pedicle screw fixation, vertebral column resection, direct vertebral rotation, and a host of sophisticated implants and correction tools have been mastered at many centers. Increasingly, attention is now turned towards making these powerful techniques and implants are safer. Multimodal neurologic monitoring was in its infancy 20 years ago, but has now been standardized, and been shown to be reliable, safe, and effective. Image guided navigation holds similar great promise for improving accuracy of pedicle screw placement, but navigation technology is in its infancy, where neurologic monitoring was in 1990. This manuscript will highlight recent advances in image guided navigation and neurologic monitoring.
Pedicle screw fixation
Thoracic pedicle screws (TPS) have been used for the treatment of idiopathic scoliosis for more than 15 years. The first report by Suk et al. started a new era in the treatment of spinal deformities. The advantages of TPS were widely reported in the literature and include: better multidimensional correction compared to hooks, shorter operative time, less blood loss and implant dislodgement rates, improved postoperative pulmonary function, and shorter fusions. The techniques also obviate the need for a combined anterior approach or bracing after surgery, including curves measuring up to 100° [1–9].
As the evidence accumulated that pedicle screws aided a powerful spine deformity correction, an increase in complications was reported, with neurologic deficits (0.69 to 0.84 %) [2, 10, 11], dural tears (0.1 to 12.1 %) [2, 12, 13], pleural effusion [12], pedicle fractures (0.24 to 13 %) [2, 12] and vascular injury [14–17], all related to misplaced pedicle screws.
Studies published to date suggest that pedicle screws are more commonly misplaced in adults compared to children, [7, 18]. This may seem counterintuitive: however, immature pedicles have more plasticity, supporting pedicle screws of 115 % of its diameter without decreasing the pullout strength or increasing the risk of neurologic injuries, unlike adult pedicles, for which the best screw diameter is estimated to be about 65 to 80 % of the pedicle diameter [19, 20]. Studies show that even experienced surgeons have misplaced screws and that the rate of medial wall breaches decrease over time [21–25]. Other intraoperative methods to assess pedicle screw position were attempted such as plain radiography, pedicle wall palpation, intraoperative fluoroscopy, laminotomy with direct visualization of the pedicle, but have not shown any benefit. Instead, intraoperative fluoroscopy has shown to increase surgical time, the risk of contamination and radiation exposure for the surgeon and the patient [12].
Navigation system
Over the last two decades, the advent of pedicle screw fixation and corrective osteotomies have given pediatric spinal surgeons the tools necessary to attain far superior correction of spinal deformity. With these advances have also come many increased risks. Several studies have shown a relatively high rate of misplaced pedicle screws. Lehman and Lenke’s study [25] showed that 107 of 1,023 screws (10.5 %) had “significant medial or lateral pedicle wall violations”. Smorgick et al. [26] found 12.5 % of screws were misplaced, and two were on the aorta. Sarlak et al. [27] showed a 10 % rate of thoracic screws misplaced. In their presentation at the Scoliosis Research Society in 2011, Amaral et al. [28] presented even more concerning findings. They used postoperative CT (considered the gold standard in identifying pedicle screw breeches) to evaluate the postoperative position of the 2,229 pedicle screws they placed in 106 patients. They found that 25 % of their patients had pedicle screws that put viscera at risk. They found 13 screws in 6 patients were impinging or distorting the aortic wall, and screws were also impinging on the trachea and esophagus and diaphragm. This study is the latest to show that although many surgeons have completed the learning curve for placing pedicle screws in the deformed pediatric spine, misplaced screws are still putting patients at risk. It remains unclear whether these screws impinging viscera will cause a problem in the future.
Image guided navigation allows the most accurate possible placement of pedicle screws in the deformed pedicles and vertebral bodies of the young spine. The technology is in its infancy, providing several concerns and obstacles to use. First and foremost, the technology is expensive. Companies that have pioneered image guided navigation systems understandably are commanding a premium for the technology, putting these devices out of reach for many healthcare systems and hospitals. Secondly, surgeons who been placing implants using a freehand technique, or freehand with fluoroscopic or radiographic confirmation, report that they have not had problems with paralysis or aortic rupture, and are concerned that the technique will slow the pace of their surgery. Finally, any technique that employs a CT scan has radiation concerns. Surgeons and health systems are making every effort to reduce the radiation that their patients receive; checking screw placement with CT scans is one more source of radiation.
We have extensive experience using a CT-based image guided navigation system (O arm, Medtronic Corporation). After an initial learning curve of 5 to 10 cases, we have found that this image guided navigation system is extremely accurate, reproducible, and efficient to use. In 2011, Ughwanogho et al. compared navigated and non-navigated pedicle screws using the Oarm system. They found that 4.9 % of the non-navigated screws were removed intra-operatively whereas only 0.6 % of the navigated screws had to be removed (p = 0.003). They also reported that a significant medial breach, defined as >50 % of the screw diameter, was 7.6 times more likely to occur wthout navigation (95 % CI 2.3–25.1 %; p < 0.001) [29, 30]. Image guidance leads to more accurate placement of the pedicle screws, fewer dangerous screws, and less screw removal.
Navigation technique
After standard spine exposure, we drape the O arm and use the fluoroscopic feature to confirm levels. We then attach one or two of the arrays to the spinous processes (one mid-spine, at the apex the deformity, and one at the most proximal spinous process that is exposed), and obtain one or two CT scans (Fig. 1). The radiation from these scans can be markedly diminished by using the lowest possible settings, thus limiting to the radiation to a fraction of a normal diagnostic CT scan. Our preliminary data published in 2011 in spine showed that intra-operative thoracic CT scan with the O-arm exposes an adolescent patient to 2.86 mSv of radiation, which is approximately the equivalent of 20–40 fluoroscopy images. A posterior spinal fusion for idiopathic scoliosis with the freehand technique utilizes from 4 to 6 C-arm images per screw in average, resulting in a total of 40 to 70 images for all thoracic screws in each case. During the CT scan the only the O arm technician stays in the radiation zone—and he/she always wears protection gears. The entire staff stays protect behind a window shield. We then use anatomic landmarks to determine our starting point, confirming the axial trajectory with the registered probe (Figs. 2 and 3). Although most systems allow the surgeon to navigate with all tools, we do the awl and tapping and screw placement freehand (Fig. 4). We then use the registered probe in line with the shank of the screw to confirm anatomic screw placement. Image guided navigation allows a very precise selection of individual screw diameter and length. After using this technique to play screws in thousands of scoliotic vertebra, we have learned the dramatic differences in pedicle diameter, shape and length, as well is the vertebral body deformities that are commonly present. Because of the patient to patient and even vertebra to vertebral variation, image guidance offers a great margin of safety for pediatric spine deformity. After all the screws are placed, we obtain a few fluoroscopic images to confirm the cascade and accurate screw placement. Although some surgeons obtain a CT scan with the O arm after screw placement, or even again after correction, we do not recommend this approach. The additional CT scans with the screws in place are difficult to interpret (due to scatter) and add excessive radiation. Early in our experience, we found such great consistency between the registered probe and the post-implant CT, that we abandoned the latter.
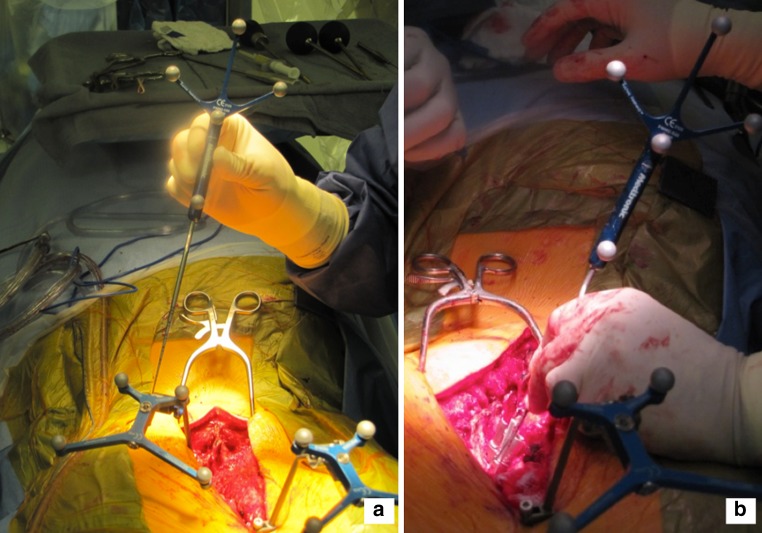
Fig. 1.
a A pair of 3D arrays are attached to the spinous process. The most caudal rays should be placed at or near the apical vertebra, so that the infrared signal from the head of the OR table is not blocked by the most cephalad array. The most cephalad array is placed on the most cephalad spinous process exposed. b After sterile draping the O-arm®, a low radiation CT scan of the intended instrumented vertebrae is performed. Typically, between five and seven vertebrae can be captured successfully on the CT, depending on the size of the patient. Since most spine fusions for scoliosis include between 5 to 14 vertebrae, 2 CT scans are typically required. The information is then fed into the StealthStation®. With experience, the scanning and image preparation typically takes 5–10 minutes
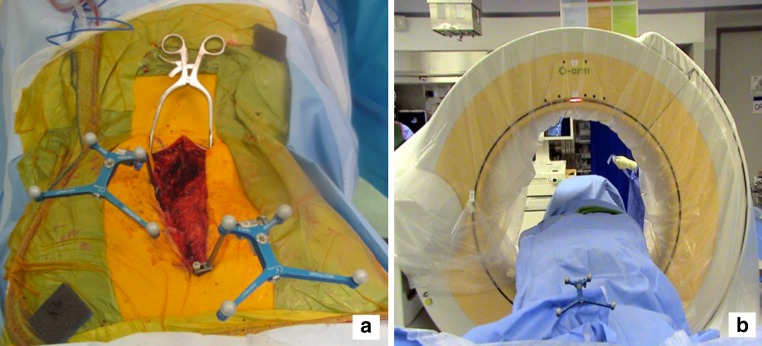
Fig. 2.
a Prior to navigating, the surgeon registers the probe by touching the previously located arrays. There is no need to register on anatomic landmarks as in older navigation systems. b The identification and preparation of the pedicle screw entry point is accomplished using a standard free hand technique. We place screws caudad to cephalad. After preparing the pedicle entry point, we confirm axial trajectory with the probe
Fig. 3.
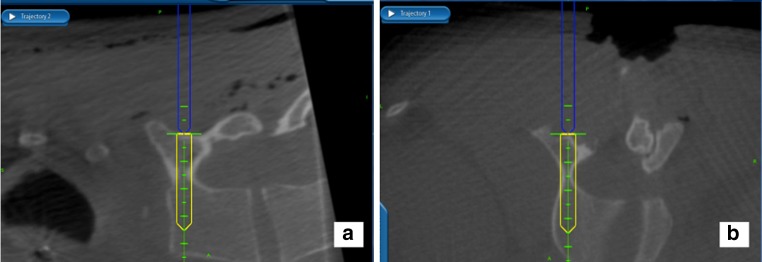
The system provides real-time information about the precise length and diameter of the screw that will best fill the pedicle—a crucial feature for instrumenting the variably deformed scoliotic vertebrae. a A sagittal plane image, confirming the optimal cephalad–caudad starting point and sagittal trajectory. b An axial image, confirming the optimal medial–lateral starting point and the largest diameter screw that fits in this pedicle
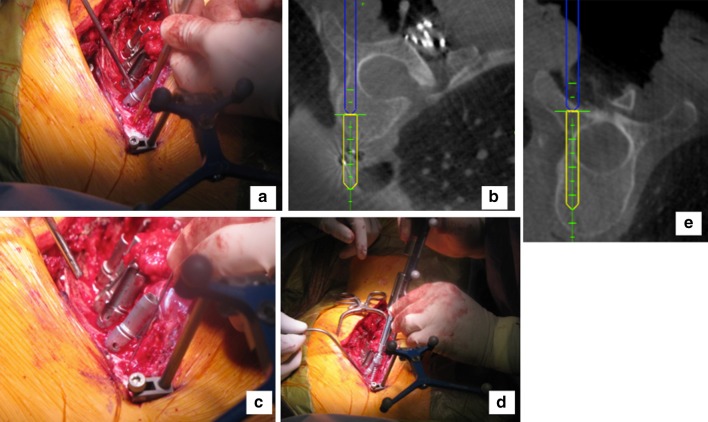
Fig. 4.
After establishing optimal trajectory, we proceed with the free hand technique for pedicle screw placement. a Freehand awl, following the trajectory predicted by the navigation system. b The navigation probe is then placed in the awl track, confirming proper trajectory. c After tapping, manual palpation with the ball-tipped probe assures there is no breech. d The screw is then placed. e The final screw position can be confirmed by placing the navigation probe in line with the shank of the screw. The actual screw position that will be projected forward into the vertebral body, as shown on this image. A few fluoroscopic images are then taken of all the screws to confirm proper position and a smooth cascade through the deformity
Image guided navigation is an extremely valuable safety tool in pediatric spine deformity, but as a technology, it is in its infancy. Further advancement is necessary to make the machines easier to use and less expensive. Considerable attention must be directed towards minimizing the amount of radiation. Image guided navigation is currently where spinal cord monitoring was 15 years ago, and it is not surprising that the surgeon responses are the same. Skeptics are concerned that the safety afforded by this new technology is not worth the inconvenience, and that there may be safety concerns. Just as neural monitoring has advanced and become standard, image guided navigation will likely follow the same path over the next decade.
Intraoperative neuromonitoring
For many years, the wake-up test was a standard of care to assess spinal cord integrity following spine deformity surgery. The wake-up test was sensitive but not specific, as it did not allow the surgeon to determine the exact cause of the neurologic deficit. In addition, the wake-up test was performed sometime after potential neurologic injury. The wake-up test required great cooperation between the anesthesia and surgical teams, and even at its best, could be a harrowing intraoperative experience with a mobile patient and wildly fluctuating blood pressures. For these reasons and others, there is always been great interest in real time, accurate monitoring of the spinal cord and nerve roots.
Recording cerebral evoked potentials through the human scalp was first described by Dawson in 1947 [31] and increasing interest arouse since then for studying established spinal cord injuries [32]. Somatosensory evoked potentials (SEP) was introduced in the 1970’s for functional neurophysiological assessment during spine and spinal cord surgery have improved rapidly [32, 33]. The advent of corticospinal motor pathway monitoring through the use of motor evoked potentials (MEPs) elicited by transcranial electrical stimulation added more information for the surgeon. Such achievements during the last 15 years turned neuromonitoring into the standard of care to assess proper hardware position in the spine [34–40] and also making more aggressive corrections in spinal deformities possible [41–46].
Different protocols might combine evoked potentials (SEPs and MEPs) [33, 47–51], transcranial electric motor evoked potentials (tcMEP) [52] and triggered or free-running electromyography [35, 36, 53, 54] and it is crucial to understand their differences to prompt proper communication between surgeons, anesthetists and the neuromonitoring team.
Selective modalities include SEPs and MEPs that monitor respectively the dorsal somatosensory and the corticospinal motor systems [55]. Somatosensory evoked potentials assess the integrity of sensory pathways that traverse the spinal cord in areas at risk and can be recorded repeatedly and reproducibly. The stimulation of peripheral nerves (typically the posterior tibial nerve or the median nerve) is recorded at multiple sites depending on the level of surgery [56]. The importance of SEPs was highlighted by Dawson in 1991 who reported a decrease in the rate of intraoperative injury from 4 % pre-SEP to less than 0.55 % using SEPs [57].
Although the significant contribution to safety, a large multi-center survey by the Scoliosis Research Society showed a 0.127 % false-negative rate for SEPs [58] and its pathophysiology may be related to vascular injury to the spinal cord. Because SEP checks primarily the conduction of the dorsal columns and the blood supply differs from that of the anterior two-thirds of the spinal cord, which derives from the anterior spinal artery, loss of adequate blood flow through the anterior spinal artery would put the anterior portion of the spinal cord at risk but would not trigger SEP changes. Motor evoked potentials (MEPs) help identifying the SEPs false-negatives by assessing the descending motor pathways that run primarily in the lateral columns of the spinal cord. Stimulating these pathways intraoperatively supplements the SEPs by checking the blood supply in the anterior portion of the spinal cord [56]. Different techniques for MEPs eliciting exist but the most commonly used is the transcranial electrical stimulation with recordings made from subcutaneous or intramuscular needle electrodes placed in multiple muscles such as the tibialis anterior and the abductor hallucis longis for the lower extremities and the intrinsic muscles for the upper extremities [56, 59, 60]. MEPs require less than a minute to give a response allowing it to be used multiple times and without interrupting the surgical flow during critical parts of the procedure. MEPs disadvantages include avoidance of neuromuscular blockade, movements of the limbs and axial muscles during stimulations, forceful contractions of the masseter muscles with possible tongue laceration, mandible and tooth fractures. Although the very low complication rates, MEPs are contraindicated in patients with epilepsy, cortical lesions, skull defects, increased intracranial pressure, intracranial devices, cardiac pacemakers or other pumps [56].
Multimodal intraoperative monitoring (MIOM) combine different neuromonitoring techniques increasing its sensitivity and specificity [34, 57, 61–63]. In 2007, after analyzing 1.017 MIOM using total intravenous anesthesia (TIVA), Sutter et al. [64] reported 89 % of sensitivity (95 % CI 79.3–94.9 %) and 99 % of specificity (95 % CI 98.2–99.6 %). The development and research towards the safety enhancement is a major concern and is supported by several Societies such as the Spine Society of Europe (SSE), the Scoliosis Research Society (SRS) and the International Society of Intraoperative Neurophysiology (ISIN) and MIOM is strongly recommended as a routine procedure in spine centers dealing with severe spinal disorders [54, 55].
Summary
Powerful spine deformity techniques and implants require the surgeon to take every possible safety precaution. Neurologic monitoring has become a standard, allowing real-time alerts when the spinal cord or nerve roots are at risk. Although image guided navigation is relatively new and requires further enhancements and study, it offers very important safety features that will likely become standard at major spine centers throughout the world. Advances in image guided navigation should focus on reducing radiation and cost, and improving ease-of-use. The generation of pediatric spine surgeons who are being trained now will demand the widespread availability of image guided navigation, just as the previous generation demanded widespread availability of high-quality neurologic monitoring.
Acknowledgments
Conflict of interest
None.
Contributor Information
John M. Flynn, Phone: +1-215-5901533, FAX: +1-215-5901501, flynnj@email.chop.edu
Denis S. Sakai, Email: denis_saki@yahoo.com
References
- 1.Suk SI, Lee CK, Kim WJ, Chung YJ, Park YB. Segmental pedicle screw fixation in the treatment of thoracic idiopathic scoliosis. Spine. 1995;20(12):1399–4052. [PubMed] [Google Scholar]
- 2.Suk SI, Kim WJ, Lee SM, Kim JH, Chung ER. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine. 2001;26(18):2049–2057. doi: 10.1097/00007632-200109150-00022. [DOI] [PubMed] [Google Scholar]
- 3.Dobbs MB, Lenke LG, Kim YJ, Kamath G, Peelle MW, Bridwell KH. Selective posterior thoracic fusions for adolescent idiopathic scoliosis: comparison of hooks versus pedicle screws. Spine. 2006;31(20):2400–2404. doi: 10.1097/01.brs.0000240212.31241.8e. [DOI] [PubMed] [Google Scholar]
- 4.Lehman RA, Jr, Lenke LG, Keeler KA, Kim YJ, Buchowski JM, Cheh G, et al. Operative treatment of adolescent idiopathic scoliosis with posterior pedicle screw-only constructs: minimum three-year follow-up of one hundred fourteen cases. Spine. 2008;33(14):1598–1604. doi: 10.1097/BRS.0b013e318178872a. [DOI] [PubMed] [Google Scholar]
- 5.Liljenqvist U, Lepsien U, Hackenberg L, Niemeyer T, Halm H. Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur Spine J. 2002;11(4):336–343. doi: 10.1007/s00586-002-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenke LG, Kuklo TR, Ondra S, Polly DW., Jr Rationale behind the current state-of-the-art treatment of scoliosis (in the pedicle screw era) Spine. 2008;33(10):1051–1054. doi: 10.1097/BRS.0b013e31816f2865. [DOI] [PubMed] [Google Scholar]
- 7.Ledonio CG, Polly DW, Jr, Vitale MG, Wang Q, Richards BS. Pediatric pedicle screws: comparative effectiveness and safety: a systematic literature review from the Scoliosis Research Society and the Pediatric Orthopaedic Society of North America task force. J Bone Joint Surg Am. 2011;93(13):1227–1234. doi: 10.2106/JBJS.J.00678. [DOI] [PubMed] [Google Scholar]
- 8.Luhmann SJ, Lenke LG, Kim YJ, Bridwell KH, Schootman M. Thoracic adolescent idiopathic scoliosis curves between 70 degrees and 100 degrees: is anterior release necessary? Spine. 2005;30(18):2061–2067. doi: 10.1097/01.brs.0000179299.78791.96. [DOI] [PubMed] [Google Scholar]
- 9.Kuklo TR, Lenke LG, O’Brien MF, Lehman RA, Jr, Polly DW, Jr, Schroeder TM. Accuracy and efficacy of thoracic pedicle screws in curves more than 90 degrees. Spine. 2005;30(2):222–226. doi: 10.1097/01.brs.0000150482.26918.d8. [DOI] [PubMed] [Google Scholar]
- 10.Odgers CJt, Vaccaro AR, Pollack ME, Cotler JM (1996) Accuracy of pedicle screw placement with the assistance of lateral plain radiography. J Spinal Disord 9(4):334–338 [PubMed]
- 11.Coe JD, Arlet V, Donaldson W, Berven S, Hanson DS, Mudiyam R, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345–349. doi: 10.1097/01.brs.0000197188.76369.13. [DOI] [PubMed] [Google Scholar]
- 12.Di Silvestre M, Parisini P, Lolli F, Bakaloudis G. Complications of thoracic pedicle screws in scoliosis treatment. Spine. 2007;32(15):1655–1661. doi: 10.1097/BRS.0b013e318074d604. [DOI] [PubMed] [Google Scholar]
- 13.Lonstein JE, Denis F, Perra JH, Pinto MR, Smith MD, Winter RB. Complications associated with pedicle screws. J Bone Joint Surg Am. 1999;81(11):1519–1528. doi: 10.2106/00004623-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Minor ME, Morrissey NJ, Peress R, Carroccio A, Ellozy S, Agarwal G, et al. Endovascular treatment of an iatrogenic thoracic aortic injury after spinal instrumentation: case report. J Vasc Surg. 2004;39(4):893–896. doi: 10.1016/j.jvs.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Kakkos SK, Shepard AD. Delayed presentation of aortic injury by pedicle screws: report of two cases and review of the literature. J Vasc Surg. 2008;47(5):1074–1082. doi: 10.1016/j.jvs.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Choi JB, Han JO, Jeong JW. False aneurysm of the thoracic aorta associated with an aorto-chest wall fistula after spinal instrumentation. J Trauma. 2001;50(1):140–143. doi: 10.1097/00005373-200101000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Wegener B, Birkenmaier C, Fottner A, Jansson V, Durr HR. Delayed perforation of the aorta by a thoracic pedicle screw. Eur Spine J. 2008;17(Suppl 2):S351–S354. doi: 10.1007/s00586-008-0715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harimaya K, Lenke LG, Son-Hing JP, Bridwell KH, Schwend RM, Luhmann SJ, et al. Safety and accuracy of pedicle screws and constructs placed in infantile and juvenile patients. Spine. 2011;36(20):1645–1651. doi: 10.1097/BRS.0b013e318225b8f9. [DOI] [PubMed] [Google Scholar]
- 19.Misenhimer GR, Peek RD, Wiltse LL, Rothman SL, Widell EH., Jr Anatomic analysis of pedicle cortical and cancellous diameter as related to screw size. Spine. 1989;14(4):367–372. doi: 10.1097/00007632-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrom L, Jacobsson O, Karlstrom G, Pech P, Rauschning W. CT analysis of pedicles and screw tracts after implant removal in thoracolumbar fractures. J Spinal Disord. 1993;6(3):225–231. doi: 10.1097/00002517-199306030-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gang C, Haibo L, Fancai L, Weishan C, Qixin C (2011) Learning curve of thoracic pedicle screw placement using the free-hand technique in scoliosis: how many screws needed for an apprentice? Eur Spine J Epub 2011/11/15 [DOI] [PMC free article] [PubMed]
- 22.Gonzalvo A, Fitt G, Liew S, de la Harpe D, Turner P, Ton L, et al. The learning curve of pedicle screw placement: how many screws are enough? Spine. 2009;34(21):E761–E765. doi: 10.1097/BRS.0b013e3181b2f928. [DOI] [PubMed] [Google Scholar]
- 23.Lonner BS, Auerbach JD, Estreicher MB, Kean KE. Thoracic pedicle screw instrumentation: the learning curve and evolution in technique in the treatment of adolescent idiopathic scoliosis. Spine. 2009;34(20):2158–2164. doi: 10.1097/BRS.0b013e3181b4f7e8. [DOI] [PubMed] [Google Scholar]
- 24.Samdani AF, Ranade A, Saldanha V, Yondorf MZ (2010) Learning curve for placement of thoracic pedicle screws in the deformed spine. Neurosurgery 66(2):290–294 (discussion 4–5) [DOI] [PubMed]
- 25.Lehman RA, Jr, Lenke LG, Keeler KA, Kim YJ, Cheh G. Computed tomography evaluation of pedicle screws placed in the pediatric deformed spine over an 8-year period. Spine. 2007;32(24):2679–2684. doi: 10.1097/BRS.0b013e31815a7f13. [DOI] [PubMed] [Google Scholar]
- 26.Smorgick Y, Millgram MA, Anekstein Y, Floman Y, Mirovsky Y. Accuracy and safety of thoracic pedicle screw placement in spinal deformities. J Spinal Disord Tech. 2005;18(6):522–526. doi: 10.1097/01.bsd.0000154448.90707.a8. [DOI] [PubMed] [Google Scholar]
- 27.Sarlak AY, Tosun B, Atmaca H, Sarisoy HT, Buluc L. Evaluation of thoracic pedicle screw placement in adolescent idiopathic scoliosis. Eur Spine J. 2009;18(12):1892–1897. doi: 10.1007/s00586-009-1065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaral TD, Wollowick AL, Kulkarni PM, Thornhill B, Suggs W, Sugarman EP, et al (2011) How commonly are pedicle screws adjacent to the great vessels or viscera? A study of 2,295 pedicle screws. Presented at the 46th Annual Meeting of the Scoliosis Research Society
- 29.Ughwanogho E, Patel NM, Baldwin KD, Sampson NR, Flynn J (2012) CT-guided navigation of thoracic pedicle screws for AIS results in more accurate placement and less screw removal. Spine [DOI] [PubMed]
- 30.Ughwanogho E, Patel NM, Baldwin KD, Sampson NR, Flynn JM. CT-guided navigation of thoracic pedicle screws for AIS results in more accurate placement and less screw removal. Presented at the 46th Annual Meeting of the Scoliosis Research Society [DOI] [PubMed]
- 31.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J. 2007;16(Suppl 2):S188–S196. doi: 10.1007/s00586-007-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perot PL., Jr Chapter 28. The clinical use of somatosensory evoked potentials in spinal cord injury. Clin Neurosurg. 1973;20:367–381. doi: 10.1093/neurosurgery/20.cn_suppl_1.367. [DOI] [PubMed] [Google Scholar]
- 33.Engler GL, Spielholz NJ, Bernhard WN, Danziger F, Merkin H, Wolff T (1978) Somatosensory evoked potentials during Harrington instrumentation for scoliosis. J Bone Joint Surg Am 60(4):528–532 [PubMed]
- 34.Gavaret M, Trebuchon A, Aubert S, Jacopin S, Blondel B, Glard Y, et al. Intraoperative monitoring in pediatric orthopedic spinal surgery: three hundred consecutive monitoring cases of which 10 % of patients were younger than 4 years of age. Spine. 2011;36(22):1855–1863. doi: 10.1097/BRS.0b013e3181f806d9. [DOI] [PubMed] [Google Scholar]
- 35.Calancie B, Lebwohl N, Madsen P, Klose KJ. Intraoperative evoked EMG monitoring in an animal model. A new technique for evaluating pedicle screw placement. Spine. 1992;17(10):1229–1235. doi: 10.1097/00007632-199210000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Calancie B, Madsen P, Lebwohl N. Stimulus-evoked EMG monitoring during transpedicular lumbosacral spine instrumentation. Initial clinical results. Spine. 1994;19(24):2780–2786. doi: 10.1097/00007632-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Maguire J, Wallace S, Madiga R, Leppanen R, Draper V. Evaluation of intrapedicular screw position using intraoperative evoked electromyography. Spine. 1995;20(9):1068–1074. doi: 10.1097/00007632-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Clements DH, Morledge DE, Martin WH, Betz RR. Evoked and spontaneous electromyography to evaluate lumbosacral pedicle screw placement. Spine. 1996;21(5):600–604. doi: 10.1097/00007632-199603010-00013. [DOI] [PubMed] [Google Scholar]
- 39.Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine. 1990;15(1):11–14. doi: 10.1097/00007632-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Shi YB, Binette M, Martin WH, Pearson JM, Hart RA. Electrical stimulation for intraoperative evaluation of thoracic pedicle screw placement. Spine. 2003;28(6):595–601. doi: 10.1097/01.BRS.0000049926.43292.93. [DOI] [PubMed] [Google Scholar]
- 41.Eggspuehler A, Sutter MA, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during surgical decompression of thoracic spinal stenosis in 36 patients. Eur Spine J. 2007;16(Suppl 2):S216–S220. doi: 10.1007/s00586-007-0425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenke LG, O’Leary PT, Bridwell KH, Sides BA, Koester LA, Blanke KM (2009) Posterior vertebral column resection for severe pediatric deformity: minimum two-year follow-up of thirty-five consecutive patients. Spine (Phila Pa 1976) 34(20):2213–2221 [DOI] [PubMed]
- 43.Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468(3):687–699. doi: 10.1007/s11999-009-1037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suk SI, Chung ER, Lee SM, Lee JH, Kim SS, Kim JH (2005) Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976) 30(23):E703–E710 [DOI] [PubMed]
- 45.Suk SI, Chung ER, Kim JH, Kim SS, Lee JS, Choi WK (2005) Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976) 30(14):1682–1687 [DOI] [PubMed]
- 46.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Porchet F, Dvorak J. Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J. 2007;16(Suppl 2):S209–S215. doi: 10.1007/s00586-007-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nash CL, Jr, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Relat Res. 1977;126:100–105. [PubMed] [Google Scholar]
- 48.Spielholz NI, Benjamin MV, Engler GL, Ransohoff J. Somatosensory evoked potentials during decompression and stabilization of the spine. Methods and findings. Spine. 1979;4(6):500–505. doi: 10.1097/00007632-197911000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Brown RH, Nash CL., Jr Cortical evoked potential monitoring. A system for intraoperative monitoring of spinal cord function. Spine. 1984;9(3):256–261. doi: 10.1097/00007632-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Mostegl A, Bauer R. The application of somatosensory-evoked potentials in orthopedic spine surgery. Arch Orthop Trauma Surg. 1984;103(3):179–184. doi: 10.1007/BF00435551. [DOI] [PubMed] [Google Scholar]
- 51.Keim HA, Hajdu M, Gonzalez EG, Brand L, Balasubramanian E. Somatosensory evoked potentials as an aid in the diagnosis and intraoperative management of spinal stenosis. Spine. 1985;10(4):338–344. doi: 10.1097/00007632-198505000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Huang Z, Shu H, Chen Y, Sun X, Liu W, et al. Improving successful rate of transcranial electrical motor-evoked potentials monitoring during spinal surgery in young children. Eur Spine J. 2012;21(5):980–984. doi: 10.1007/s00586-011-1995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lenke LG, Padberg AM, Russo MH, Bridwell KH, Gelb DE. Triggered electromyographic threshold for accuracy of pedicle screw placement. An animal model and clinical correlation. Spine (Phila Pa 1976) 1995;20(14):1585–1591. doi: 10.1097/00007632-199507150-00006. [DOI] [PubMed] [Google Scholar]
- 54.Dvorak J, Sutter M, Eggspuehler A, Szpalski M, Aebi M. Multimodal intraoperative monitoring: towards a routine use in surgical treatment of severe spinal disorders. Eur Spine J. 2007;16(Suppl 2):S113–S114. doi: 10.1007/s00586-007-0415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutter M, Deletis V, Dvorak J, Eggspuehler A, Grob D, Macdonald D, et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007;16(Suppl 2):S232–S237. doi: 10.1007/s00586-007-0421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pajewski TN, Arlet V, Phillips LH. Current approach on spinal cord monitoring: the point of view of the neurologist, the anesthesiologist and the spine surgeon. Eur Spine J. 2007;16(Suppl 2):S115–S129. doi: 10.1007/s00586-007-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawson EG, Sherman JE, Kanim LE, Nuwer MR (1991) Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine 16(8 Suppl):S361–S364 [PubMed]
- 58.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96(1):6–11. doi: 10.1016/0013-4694(94)00235-D. [DOI] [PubMed] [Google Scholar]
- 59.Tabaraud F, Boulesteix JM, Moulies D, Longis B, Lansade A, Terrier G, et al. Monitoring of the motor pathway during spinal surgery. Spine. 1993;18(5):546–550. doi: 10.1097/00007632-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, et al. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatry. 1986;49(3):251–257. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deletis V. Basic methodological principles of multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007;16(Suppl 2):S147–S152. doi: 10.1007/s00586-007-0429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pastorelli F, Di Silvestre M, Plasmati R, Michelucci R, Greggi T, Morigi A, et al. The prevention of neural complications in the surgical treatment of scoliosis: the role of the neurophysiological intraoperative monitoring. Eur Spine J. 2011;20(Suppl 1):S105–S114. doi: 10.1007/s00586-011-1756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macdonald DB. Four-limb muscle motor evoked potential and optimized somatosensory evoked potential monitoring with decussation assessment: results in 206 thoracolumbar spine surgeries. Eur Spine J. 2007;16(Suppl 2):S171–S187. doi: 10.1007/s00586-007-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutter M, Eggspuehler A, Grob D, Jeszenszky D, Benini A, Porchet F, et al. The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective study of 1,017 patients. Eur Spine J. 2007;16(Suppl 2):S162–S170. doi: 10.1007/s00586-007-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]