Abstract
Introduction
The surgical approach in the treatment of idiopathic thoracic scoliosis depends on the type of curve involved. In anterior correction, the rib hump is corrected by derotating the thoracic spine. In posterior scoliosis surgery, additional rib hump resection is sometimes necessary to achieve an optimal cosmetic result. The aim of this study was to compare pulmonary function in these two patient groups.
Materials and methods
Forty patients in the anterior group (A) were treated with standard double thoracotomy, with an anterior derotation spondylodesis and a primary stable dual-rod system. The posterior group (P) included 29 patients who were treated with a pedicle screw-based posterior instrumentation spondylodesis, with additional rib hump resection. Pulmonary function was evaluated preoperatively, on the 12th postoperative day, and at 3, 6, 12 and 24 months during the follow-up.
Results
The patients’ mean age was 15 years in group A and 19 in group P with a standard deviation 8.7 years and a significant difference. With regard to body height or weight there were no significant differences between the two groups. In group A, the deterioration in pulmonary function immediately after the operation (from  75.3 %/71.3 % preoperatively to 38.5 %/36.1 % postoperatively) was clearer than in group P (
75.3 %/71.3 % preoperatively to 38.5 %/36.1 % postoperatively) was clearer than in group P ( 71.6 %/65.7 % preoperatively to 47.7 %/48.4 % postoperatively). During a follow-up period of 3 months, the values improved in both groups in comparison with the values immediately after the operation. Up to the 2 year follow-up, pulmonary function in the posterior and anterior groups corresponded to the preoperative values, with no significant differences. There was a trend toward moderately increased values in the posterior group and moderately decreased values in the anterior group at the 2-year follow-up examination, in comparison with the preoperative baseline, but without a statistically significant difference. Two major complications occurred in the anterior group, with reintubation and several bronchoscopy examinations due to atelectasis.
71.6 %/65.7 % preoperatively to 47.7 %/48.4 % postoperatively). During a follow-up period of 3 months, the values improved in both groups in comparison with the values immediately after the operation. Up to the 2 year follow-up, pulmonary function in the posterior and anterior groups corresponded to the preoperative values, with no significant differences. There was a trend toward moderately increased values in the posterior group and moderately decreased values in the anterior group at the 2-year follow-up examination, in comparison with the preoperative baseline, but without a statistically significant difference. Two major complications occurred in the anterior group, with reintubation and several bronchoscopy examinations due to atelectasis.
Conclusion
The severe deterioration in group A is caused by the substantial trauma with double thoracotomy in contrast to rib hump resection. For patients with severe restrictive pulmonary distress, posterior instrumentation in combination with rib hump resection would be preferable to an anterior procedure involving double thoracotomy. Respiratory physiotherapy exercise should be administered in order to minimise postoperative pulmonary distress. In conclusion opening of the chest wall leads to deterioration of pulmonary function with improvement to the preoperative values after 6 months in the posterior and after 24 months in the anterior group.
Keywords: Idiopathic thoracic scoliosis, Pulmonary function, Anterior open surgery with double thoracotomy approach, Posterior surgery with costectomy, Pulmonary complication
Introduction
Idiopathic thoracic scoliosis can be corrected using either an anterior or a posterior approach. The decision as to which procedure to carry out depends on the curvature type, the surgeon’s experience and the patient’s preoperative pulmonary function.
In anterior scoliosis surgery, dual-rod and dual-screw systems have been developed in order to prevent intraoperative screw loosening, postoperative rod breakage and pseudarthrosis and to achieve better curve correction in stiff curves. Biomechanical tests have shown that dual-rod techniques provide stiffer constructs than single-rod techniques [16]. Several authors have reported good results with dual-rod systems [1, 7, 14, 18]. Dual-rod instrumentation in the thoracic spine requires a double thoracotomy for accurate implant positioning. Single-rod instrumentation is feasible with a single thoracotomy, and in mild curves, it can even be achieved with an endoscopic procedure. As it is well known, open thoracotomy leads to decreased pulmonary function for up to 2 years after surgery [4, 10, 13, 20].
Posterior thoracic scoliosis surgery with pedicle screw constructs allows satisfactory correction. There are several viewpoints on whether posterior or anterior procedures should be carried out. The main argument involves the curvature type (for example, Lenke type 2 or 4), but reduced preoperative pulmonary function is a relative contraindication against anterior open thoracic scoliosis surgery [19]. A good Cobb angle correction can be achieved with posterior instrumentation, although segmental derotation has not yet been confirmed. However, particularly with stiff curves, derotation is only possible with the anterior procedure, with extensive disc release. In patients with a very large rib hump, rib hump resection or thoracoplasty is therefore recommended to allow an optimal cosmetic result. Correction of the rib hump with satisfactory cosmesis is essential for the patient. Patients are dissatisfied with their cosmesis if they have good Cobb angle correction without improvement of the rib hump deformity [2, 17].
Two groups of patients were included in the present study: an anterior group (A), with indirect rib hump correction via segmental vertebral derotation, and a posterior group (P) with direct rib hump correction using rib hump resection [14]. The aim of this prospective study was to compare pulmonary function preoperatively, 10 days immediately after the operation, and after 3, 6, 12 and 24 months follow-up after anterior dual-rod instrumentation via double thoracotomy or after posterior instrumentation combined with rib hump resection. Special attention was given to the postoperative period, in order to assess whether the reduction in pulmonary function is more pronounced and whether the respiratory complication rate is higher in the double thoracotomy group in comparison with the posterior thoracoplasty group.
Patients and methods
Patients
This prospective nonrandomized cohort study included patients with adolescent idiopathic thoracic scoliosis (AITS) who were scheduled for either anterior surgery with dual-rod instrumentation via double thoracotomy (group A, n = 40) or posterior instrumentation combined with rib hump resection (group P, n = 29). Exclusion criteria were non-idiopathic aetiology, heart or lung disease, and thoracolumbar or lumbar main curves.
Sixty-nine AITS patients (60 females and 9 males) with a mean age of 16.8 years (SD 6.2 years; range 11–45 years) were included in the study. The preoperative mean major thoracic Cobb angle was 62.8° (SD 10°; range 46°–85°), with no significant differences between the two groups (P = 0.28) (Tables 1, 2).
Table 1.
Raw data of the anterior group
| Patient | Age at surgery in (years) | Height in (cm) | Weight in (kg) | Lenke type | Cobb angle preop (°) | Cobb angle postop (°) | Fusion segments |
|---|---|---|---|---|---|---|---|
| A.J. | 16 | 170 | 55 | 1B− | 53 | 18 | 6 |
| A.Y. | 14 | 170 | 54 | 1C+ | 50 | 28 | 6 |
| B.J. | 12 | 146 | 41 | 1CN | 69 | 26 | 5 |
| C.A. | 15 | 160 | 44 | 3BN | 68 | 28 | 6 |
| E.D. | 14 | 168 | 50 | 1CN | 66 | 38 | 6 |
| E.G. | 16 | 180 | 70 | 1CN | 65 | 40 | 6 |
| dG.J. | 13 | 162 | 48 | 2AN | 84 | 24 | 9 |
| G.J. | 13 | 152 | 49 | 1C+ | 72 | 27 | 6 |
| G.C. | 14 | 172 | 64 | 1C+ | 78 | 30 | 6 |
| H.F. | 13 | 169 | 48 | 1CN | 70 | 28 | 6 |
| H.P. | 14 | 178 | 70 | 1C− | 64 | 25 | 6 |
| H.K. | 13 | 160 | 50 | 1CN | 55 | 22 | 6 |
| H.J. | 16 | 181 | 60 | 3C− | 76 | 42 | 6 |
| J.J. | 11 | 150 | 44 | 1CN | 70 | 31 | 7 |
| J.T. | 16 | 175 | 51 | 1AN | 75 | 19 | 7 |
| J.M. | 17 | 180 | 70 | 1BN | 54 | 27 | 5 |
| K.U. | 22 | 168 | 70 | 1C+ | 56 | 32 | 6 |
| K.S. | 12 | 165 | 62 | 1C+ | 79 | 29 | 6 |
| K.K. | 12 | 164 | 47 | 1CN | 53 | 27 | 5 |
| K.C. | 14 | 170 | 53 | 3C− | 62 | 30 | 6 |
| K.P. | 14 | 153 | 46 | 1CN | 75 | 26 | 7 |
| M.La. | 16 | 170 | 65 | 1BN | 50 | 21 | 5 |
| M.Li. | 15 | 168 | 50 | 1CN | 51 | 22 | 6 |
| M.Ann. | 14 | 161 | 58 | 1BN | 50 | 14 | 6 |
| M. Ang. | 12 | 162 | 39 | 3BN | 85 | 30 | 6 |
| M.S. | 18 | 160 | 58 | 1BN | 73 | 37 | 6 |
| M.A. | 15 | 175 | 50 | 1AN | 62 | 4 | 7 |
| O.C. | 16 | 168 | 38 | 1AN | 70 | 24 | 6 |
| R.L. | 16 | 172 | 64 | 1CN | 52 | 35 | 6 |
| R.W. | 12 | 177 | 64 | 3CN | 68 | 29 | 9 |
| R.A. | 15 | 179 | 56 | 1CN | 55 | 30 | 5 |
| S.R. | 16 | 166 | 81 | 1CN | 58 | 22 | 6 |
| S.L. | 16 | 180 | 56 | 1CN | 48 | 22 | 5 |
| S.D. | 17 | 170 | 46 | 1CN | 55 | 28 | 6 |
| S.N. | 13 | 174 | 52 | 1C− | 66 | 19 | 6 |
| S.C. | 18 | 180 | 63 | 1CN | 58 | 28 | 6 |
| T.C. | 15 | 166 | 57 | 2BN | 67 | 28 | 6 |
| T.L. | 14 | 170 | 51 | 1B+ | 57 | 35 | 6 |
| W.L. | 16 | 172 | 57 | 1B− | 49 | 24 | 6 |
| Y.S. | 19 | 164 | 50 | 1BN | 50 | 20 | 5 |
Table 2.
Raw data of the posterior group
| Patient | Age at surgery in (years) | Height in (cm) | Weight in (kg) | Lenke type | Cobb angle preop (°) | Cobb angle postop (°) | Fusion segments |
|---|---|---|---|---|---|---|---|
| A.E. | 24 | 158 | 50 | 1AN | 47 | 18 | 8 |
| A.B. | 13 | 157 | 65 | 3C+ | 70 | 24 | 11 |
| A.Z. | 12 | 162 | 53 | 1AN | 60 | 22 | 10 |
| B.V. | 13 | 170 | 59 | 1CN | 55 | 30 | 13 |
| D.K. | 12 | 160 | 58 | 1B− | 73 | 35 | 10 |
| E.E. | 14 | 168 | 45 | 2A− | 74 | 27 | 11 |
| F.S. | 40 | 170 | 70 | 3AN | 61 | 36 | 11 |
| G.K. | 19 | 175 | 55 | 2A− | 55 | 24 | 12 |
| G.N. | 13 | 166 | 48 | 2A− | 68 | 30 | 11 |
| H.K. | 16 | 169 | 72 | 2BN | 61 | 35 | 9 |
| H.H. | 18 | 188 | 71 | 2AN | 75 | 38 | 9 |
| H.J. | 14 | 187 | 50 | 2A− | 60 | 18 | 10 |
| K.S. | 18 | 166 | 60 | 2A− | 55 | 22 | 10 |
| M.L. | 13 | 175 | 45 | 3CN | 65 | 20 | 11 |
| M.D. | 14 | 166 | 67 | 2A− | 66 | 28 | 11 |
| O.M. | 28 | 172 | 64 | 1CN | 77 | 44 | 12 |
| R.T. | 15 | 180 | 63 | 4BN | 80 | 22 | 12 |
| R.S. | 39 | 174 | 65 | 1AN | 68 | 28 | 8 |
| S.Ju. | 22 | 162 | 55 | 1A+ | 50 | 21 | 8 |
| S.Si. | 14 | 167 | 65 | 2AN | 46 | 14 | 10 |
| S.D. | 18 | 181 | 63 | 2A− | 66 | 31 | 12 |
| S.Sv. | 21 | 182 | 59 | 2AN | 62 | 20 | 12 |
| S.M. | 19 | 180 | 59 | 2AN | 58 | 20 | 11 |
| S.T. | 19 | 170 | 59 | 1AN | 49 | 17 | 9 |
| S. E. | 16 | 170 | 61 | 1A− | 76 | 29 | 12 |
| S.Ja. | 12 | 172 | 53 | 1CN | 70 | 40 | 11 |
| S.I. | 25 | 176 | 50 | 1AN | 48 | 11 | 9 |
| W.M. | 45 | 170 | 63 | 1A− | 60 | 32 | 8 |
| Y.L. | 17 | 150 | 49 | 3C− | 62 | 17 | 12 |
Treatment
The curve types were classified using the Lenke classification system (Tables 1, 2) [12]. The decision on whether to carry out either an anterior or posterior procedure was taken by the surgeon (U.L. or V.B.) in accordance with the Lenke criteria [12]. Patients were included in group A (anterior) if dual-rod instrumentation via a double thoracotomy was intended or in group P (posterior) if rib hump resection was to be carried out in addition to posterior correction and fusion.
In group A, the anterior approach was performed with single-lung ventilation and with the patient in the left lateral position, with a single skin incision over the seventh rib. The lower thoracotomy is performed between the eight and ninth ribs. This approach allows the lower thoracic 3–4 vertebrae and discs to be addressed. To gain access to the middle and upper thoracic spine, the fifth rib is removed. In two patients an additional phrenotomy was required in order to achieve the upper lumbar spine. After complete disc resection up to the posterior longitudinal ligament, instrumentation and correction are performed with a dual-screw and rod system (Halm-Zielke instrumentation, Depuy Spine, Kirkel-Limbach, Germany) [14].
In group P, posterior instrumentation and correction were performed with the patient the in prone position, with a dual-rod pedicle screw-based system (Expedium, Depuy Spine, Kirkel-Limbach, Germany). After instrumentation and correction were completed, the wake-up test was routinely performed. After the wake-up, test rib hump resection was performed. During rib hump resection, five to six hump ribs were dissected subperiosteally, medial to the angle of the rib. Rib shortening of 1–2 cm was performed, followed by rib osteosynthesis with a fibre. A thoracic drain was placed in all patients.
The patients were evaluated prospectively for both pulmonary function and radiographic changes before and 10 days after surgery, as well as after 3, 6, 12, and 24 months during the follow-up.
Radiographic measurement
Radiographic measurements on the coronal and sagittal plane were carried out using the Cobb method preoperatively, postoperatively (Tables 1, 2), and at the 24 month follow-up examination. The Cobb angle of the main thoracic curve on coronal radiographs and the thoracic kyphosis angle from T5 to T12 were measured on sagittal radiographs of the spine with the patient in the standing position.
Pulmonary function tests (PFTs)
All 69 patients underwent PFTs, using body plethysmography to evaluate pulmonary volume (maximum vital capacity,  ) and flow (forced 1 min expiratory volume, FEV1) before surgery and 10 days, 3, 6, 12, and 24 months, postoperatively. The PFTs were performed with the patient in the standing position. The test was repeated three times, and the highest reading was selected. Absolute values were used for statistical analysis, but percentage predicted values relative to age, sex, height and weight were also evaluated.
) and flow (forced 1 min expiratory volume, FEV1) before surgery and 10 days, 3, 6, 12, and 24 months, postoperatively. The PFTs were performed with the patient in the standing position. The test was repeated three times, and the highest reading was selected. Absolute values were used for statistical analysis, but percentage predicted values relative to age, sex, height and weight were also evaluated.
Intraoperative and postoperative data were documented, particularly with regard to pulmonary complications.
Statistical analysis
Statistical analysis was carried out using the SPSS software package (version 15.0; SPSS, Chicago, Illinois, USA). The Kolmogorov–Smirnov test was used to confirm normal distribution of the data. A paired t test was used for statistical analyses of the two groups. Multivariate tests were used to correlate age, height, weight and radiographic parameters with the pulmonary function results. P ≤ 0.05 was considered statistically significant and P ≤ 0.01 was considered highly statistically significant.
Results (Tables 1, 2)
The mean preoperative thoracic Cobb angle in all 69 patients corrected spontaneously from 62.8° (SD 10°; range 46°–85°) to 38.3° (SD 11.7°; range 15°–72°) on bending films. Postoperatively, the Cobb angle corrected significantly to 26° (SD 7.2°; range 4°–44°), with a mean loss of correction of 1.7° during the 2 year follow-up period to 27.7° (SD 7.3°; range 3°–44°). The mean preoperative sagittal profile for thoracic kyphosis remained unchanged, from 25.1°(SD 13.1; range 1°–49°) to 27.5° at follow-up (SD 11.1°; range 7°–53°).
There were no significant differences in preoperative weight or height between the two patient groups, with a mean preoperative weight of 56.5 kg (SD 8.9 kg; range 38–81 kg) and mean preoperative height of 169 cm (SD 21; range 146–188 cm).
In the anterior group, the vital capacity ( ) was 2.9 L preoperatively (SD 0.7; range 1.78–4.86 L) in comparison with 2.98 L in the posterior group (SD 0.81; range 1.62–4.66 L), with no significant difference between the groups. Ten days postoperatively, a highly significant (P > 0.001) decrease in
) was 2.9 L preoperatively (SD 0.7; range 1.78–4.86 L) in comparison with 2.98 L in the posterior group (SD 0.81; range 1.62–4.66 L), with no significant difference between the groups. Ten days postoperatively, a highly significant (P > 0.001) decrease in  was observed in both groups: group A: 1.5 L (SD 0.5; range 0.67–2.87 L), group P: 1.97 L (SD 0.57; range 1.02–3.12 L). During the follow-up period (3, 6, 12 and 24 months),
was observed in both groups: group A: 1.5 L (SD 0.5; range 0.67–2.87 L), group P: 1.97 L (SD 0.57; range 1.02–3.12 L). During the follow-up period (3, 6, 12 and 24 months),  increased gradually to nearly the preoperative values at the 24-month follow-up examination: group A, 2.8 L (SD 0.7; range 1.8–4.58 L) and group P, 3.08 L (SD 0.73; range 1.83–4.89 L).
increased gradually to nearly the preoperative values at the 24-month follow-up examination: group A, 2.8 L (SD 0.7; range 1.8–4.58 L) and group P, 3.08 L (SD 0.73; range 1.83–4.89 L).
The forced expiratory volume showed a similar course to that of vital capacity in the two groups. In the anterior group, the FEV1 was 2.3 L, preoperatively (SD 0.6; range 1.18–3.46 L), in comparison with 2.27 L (SD 0.57; range 1.4–3.35 L) in the posterior group, with no significant difference between the groups. Ten days postoperatively, a significant (P > 0.001) decrease in the FEV1 was observed in both groups: group A, 1.1 L (SD 0.4; range 0.44–1.78 L), group P, 1.64 L (SD 0.49; range 0.76–2.78 L). During the follow-up period (3, 6, 12 and 24 months), the FEV1 increased gradually to nearly the preoperative values at the 24-month follow-up examination: group A, 2.3 L (SD 0.6; range 1.38–3.61 L) and group P, 2.53 L (SD 0.62; range 1.54–4.1 L) (Tables 3, 4).
Table 3.
Average patient data for maximum vital capacity ( ) and forced expiratory volume after 1 min (FEV1) in litres and percentages in the anterior group
) and forced expiratory volume after 1 min (FEV1) in litres and percentages in the anterior group
| Time point |  |
FEV1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (%) | Standard deviation (%) | Minimum (%) | Maximum (%) | Mean (%) | Standard deviation (%) | Minimum (%) | Maximum (%) | |
| Preoperative | 2.9 (75.3) | 0.7 (16) | 1.78 (48) | 4.86 (105) | 2.3 (71.3) | 0.6 (16.6) | 1.18 (42) | 3.41 (108) |
| Postoperative | 1.5 (38.5) | 0.5 (11.7) | 0.67 (16) | 2.87 (60) | 1.1 (36.1) | 0.4 (12.1) | 0.44 (16) | 1.78 (61) |
| 3-month follow-up | 2.2 (58.5) | 0.6 (11.3) | 1.33 (38) | 3.61 (79) | 1.8 (54.5) | 0.5 (12.6) | 1.04 (30) | 2.89 (83) |
| 6-month follow-up | 2.5 (66.2) | 0.6 (12.4) | 1.57 (44) | 4.3 (91) | 2.0 (63.1) | 0.5 (13) | 1.18 (37) | 3.45 (88) |
| 12-month follow-up | 2.7 (70.6) | 0.7 (14.5) | 1.71 (47) | 4.49 (100) | 2.2 (67.5) | 0.5 (14.3) | 1.28 (41) | 3.72 (93) |
| 24-month follow-up | 2.8 (70.9) | 0.7 (14.2) | 1.8 (45) | 4.58 (102) | 2.3 (67.3) | 0.6 (13.5) | 1.38 (43) | 3.61 (96) |
Percentages are individually measured values referenced by the patient’s age, sex, height and weight in correlation with the index value standard table published by the European Association for Coal and Steel
Table 4.
Average patient data for maximum vital capacity ( ) and forced expiratory volume after 1 min (FEV1) in litres and percentages in the posterior group
) and forced expiratory volume after 1 min (FEV1) in litres and percentages in the posterior group
| Time point | 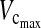 |
FEV1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (%) | Standard deviation (%) | Minimum (%) | Maximum (%) | Mean (%) | Standard deviation (%) | Minimum (%) | Maximum (%) | |
| Preoperative | 2.98 (71.7) | 0.81 (13.5) | 1.62 (48) | 4.66 (99) | 2.27 (65.7) | 0.57 (11.1) | 1.4 (44) | 3.35 (84) |
| Postoperative | 1.97 (47.7) | 0.57 (11.2) | 1.02 (26) | 3.12 (73) | 1.64 (48.4) | 0.49 (10.3) | 0.76 (27) | 2.78 (65) |
| 3-month follow-up | 2.66 (64.1) | 0.58 (9.9) | 1.31 (44) | 3.91 (80) | 2.18 (63.14) | 0.5 (8.77) | 1.22 (46) | 3.18 (80) |
| 6-month follow-up | 2.96 (72.3) | 0.68 (11.3) | 1.62 (51) | 4.55 (94) | 2.4 (69.6) | 0.52 (8.7) | 1.43 (53) | 3.68 (87) |
| 12-month follow-up | 3.04 (74.2) | 0.69 (13.3) | 1.56 (42) | 4.59 (98) | 2.49 (72) | 0.57 (11.6) | 1.36 (42) | 3.79 (96) |
| 24-month follow-up | 3.08 (74.2) | 0.73 (12.7) | 1.83 (44) | 4.89 (92) | 2.53 (71.9) | 0.62 (10.7) | 1.54 (43) | 4.1 (88) |
Percentages are individually measured values referenced by the patient’s age, sex, height and weight in correlation with the index value standard table published by the European Association for Coal and Steel
The multivariate tests showed that the preoperative or postoperative Cobb angle did not have any significant influence on vital capacity or forced expiratory volume. A positive correlation was observed between the amount of thoracic kyphosis and the  and FEV1, both in the preoperative period and during the follow-up period.
and FEV1, both in the preoperative period and during the follow-up period.
In the anterior group, 8 of the 40 patients (20 %) developed pulmonary complications. Four patients developed only pleural effusion after removal of the chest drain and were successfully treated conservatively. One patient developed pneumonia over a follow-up period of 4 months. Three patients developed atelectasis in the left lung. One of these patients was successfully treated with only one bronchoscopy and intensive respiratory exercises. The second patient (K.S.) underwent two bronchoscopy procedures and reintubation, with 12 h of ventilation therapy. The preoperative values—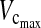 2.95 L (84 %) and FEV1 2.52 L (85 %)—changed to
2.95 L (84 %) and FEV1 2.52 L (85 %)—changed to 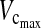 2.56 L (63 %) and FEV1 1.82 L (54 %) during the follow-up. The third patient (S.D.), who developed complete atelectasis of the left lung, required a total of four bronchoscopy procedures, with a new thorax drain on the left side and ventilation therapy for a total of 6 days in the intensive care unit. This patient achieved complete pulmonary recovery during the follow-up. The preoperative values—
2.56 L (63 %) and FEV1 1.82 L (54 %) during the follow-up. The third patient (S.D.), who developed complete atelectasis of the left lung, required a total of four bronchoscopy procedures, with a new thorax drain on the left side and ventilation therapy for a total of 6 days in the intensive care unit. This patient achieved complete pulmonary recovery during the follow-up. The preoperative values— 3.08 L (80 %) and FEV1 2.12 L (84 %)—changed to
3.08 L (80 %) and FEV1 2.12 L (84 %)—changed to  2.99 L (75 %) and FEV1 2.2 L (64 %) during the follow-up.
2.99 L (75 %) and FEV1 2.2 L (64 %) during the follow-up.
In the posterior group, only 3 of the 29 patients (10 %) developed pleural effusion, which was successfully treated with conservative therapy in all cases.
Discussion
The effect of different surgical techniques for correcting spinal deformity in patients with idiopathic adolescent scoliosis has been investigated in several studies [4, 6, 8–10, 13, 20, 23]. The present prospective study was performed in order to compare postoperative pulmonary function in patients with idiopathic thoracic scoliosis who were treated either with an open anterior procedure using a dual-rod technique via a double thoracotomy or with a posterior pedicle screw-based procedure with additional rib hump resection. The hypothesis was that decreasing pulmonary function, postoperatively, might be more severe in patients who were treated with double thoracotomy in comparison with posterior instrumentation with additional rib hump resection.
During the postoperative period, the 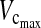 and FEV1 decreased significantly in both the anterior and posterior groups. However, the difference between preoperative and postoperative values was 1.4 L for
and FEV1 decreased significantly in both the anterior and posterior groups. However, the difference between preoperative and postoperative values was 1.4 L for 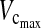 and 1.2 L for FEV1 in the anterior group, in comparison with only 1.01 L for
and 1.2 L for FEV1 in the anterior group, in comparison with only 1.01 L for 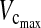 and 0.63 L for FEV1 in the posterior group. Preoperative lung function values were reached in the posterior group by the 6-month follow-up examination, whereas in the anterior group, preoperative values were not reached until the 24-month follow-up examination.
and 0.63 L for FEV1 in the posterior group. Preoperative lung function values were reached in the posterior group by the 6-month follow-up examination, whereas in the anterior group, preoperative values were not reached until the 24-month follow-up examination.
There were no significant differences between the two groups preoperatively with regard to age, height, weight, Cobb angle, sagittal thoracic profile or pulmonary function. The decrease in vital capacity and forced expiratory volume during the immediate postoperative period was more severe in the anterior group than in the posterior group. After 2 years’ follow-up, the preoperative values were nearly achieved in both groups again.
A significant correlation between degree of preoperative thoracic scoliosis, hypokyphosis and length of the thoracic curve was reported in a study of 631 patients with idiopathic scoliosis [19]. Newton et al. [19] also found that patients with hypokyphosis were more likely to have pulmonary impairment, whereas thoracic hyperkyphosis ≥41° was associated with improved pulmonary function. In the present study, no correlation was observed between pulmonary function and the Cobb angle, but a positive correlation between the amount of thoracic kyphosis and pulmonary function was also observed.
The present study shows that pulmonary function is impaired during the immediate postoperative period. Postoperative recovery of pulmonary function was observed earlier in the posterior group than in the anterior group. Other authors have also reported a decline in pulmonary function following an open thoracotomy approach [4, 8, 10, 20, 23], and some authors therefore recommend that a thoracoscopic procedure should be used in patients with moderate curves in order to protect the vital capacity. Several studies have reported that thoracoscopic curve correction does not significantly worsen pulmonary function, but also does not improve it [6, 11, 13, 15, 20, 24].
Thoracotomy has a deleterious effect on pulmonary function, but in all previous papers, the authors did not make any distinction with only one thoracotomy or double thoracotomy. The present study underlines the fact that particularly when double thoracotomy is carried out, reduced pulmonary function during the first 2 years of the postoperative period has to be accepted. This may be associated with postoperative respiratory complications. The influence of different types of surgical intervention on pulmonary function has been prospectively evaluated in several studies [3–6, 8–10, 13, 20, 23]. Preoperative pulmonary function was measured in an evaluation of 133 patients with adolescent idiopathic scoliosis who underwent posterior instrumentation [22]. Pulmonary function was classified as normal when forced vital capacity and FEV1 were in the range 80–120 %, mild in the range 65–80 %, moderate at 50–65 %, and severe when less than 50 %, with a mean Cobb angle of 48° (range 20°–92°). In the study by Vedantam and Crawford, 73 % of preoperative pulmonary function tests in patients with moderate scoliosis were normal. In this group, the additional thoracoplasty (n = 8) was the only reason for a higher incidence of postoperative pulmonary complications [22].
In the present study, minor pulmonary complications were observed in both the anterior and posterior groups. Major complications, with atelectasis followed by repeated bronchoscopy and reintubation with ventilation therapy, were only observed in the anterior group. The anterior approach with double thoracotomy thus increases the risk of pulmonary complications.
Kim et al. [9] reported on 139 patients with an average Cobb angle of 60° (range 40°–91°) who were treated with posterior instrumentation alone, without rib hump resection; this led to improved pulmonary function at the 2-year follow-up evaluation (FVC 79 % preoperatively and 81 % postoperatively; FEV1 75 % preoperatively and 77 % postoperatively). In this study, there was also no correlation between the Cobb angle correction and impairment of pulmonary function [9].
There has been debate on whether opening the chest itself may be responsible for pulmonary function impairment, with conflicting views on whether open thoracotomy with single-lung ventilation and the patient in the lateral decubitus position is associated with poorer results than rib hump resection after posterior instrumentation with double-lung ventilation and the patient in a prone position. Suk et al. retrospectively compared pulmonary function after posterior pedicle instrumentation with and without additional rib hump resection. No differences in pulmonary function were found in their evaluation over an average 6-year follow-up period [21]. There was a negative correlation in the study with the preoperative thoracic Cobb angle and a significant positive correlation with the preoperative thoracic kyphosis [21]. In a prospective clinical investigation, Kim et al. compared open thoracotomy with an anterior thoracolumbar open approach in thoracolumbar curves in patients with thoracic scoliosis. At the 2-year follow-up examination, there was a persistent significant impairment in FVC and FEV1 in the thoracic group and no difference in the thoracolumbar group [10]. Newton et al. [20] also reported a modest decline in pulmonary function tests 2 years after adolescent idiopathic scoliosis surgery in relation to anterior thoracotomy or thoracoplasty.
Conclusions
Opening the chest wall in thoracic scoliosis surgery leads to impairment of pulmonary function for up to 2 years after the operation. Severe pulmonary complications were only observed in the anterior group in the present study. Postoperative recovery of pulmonary function was observed earlier in the posterior group (6 months) than in the anterior group (24 months). The Cobb angle correction was not found to have a significant influence on vital capacity in the postoperative period in this study. Thoracic kyphosis also has a significant positive influence on vital capacity both in the preoperative period and also in the postoperative period. It may be better to treat patients who are at risk for pulmonary complications with posterior surgery without opening the chest wall rather than anterior approaches, as the long period of reduced vital capacity in the postoperative period may lead to more pulmonary complications.
Conflict of interest
None.
References
- 1.Bullmann V, Halm HF, Niemeyer T, Hackenberg L, Liljenqvist U. Dual-rod correction and instrumentation of idiopathic scoliosis with the Halm-Zielke instrumentation. Spine. 2003;28:1306–1313. doi: 10.1097/01.BRS.0000065571.58058.68. [DOI] [PubMed] [Google Scholar]
- 2.Chen SH, Huang TJ, Lee YY, Hsu RW (2002) Pulmonary function after thoracoplasty in adolescent idiopathic scoliosis. Clin Orthop Relat Res 399:152–161 [DOI] [PubMed]
- 3.Gitelman Y, Lenke LG, Bridwell KH, Auerbach JD, Sides BA. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure: a 10-year follow-up analysis. Spine. 2011;36:1665–1672. doi: 10.1097/BRS.0b013e31821bcf4c. [DOI] [PubMed] [Google Scholar]
- 4.Graham EJ, Lenke LG, Lowe TG, Betz RR, Bridwell KH, Kong Y, Blanke K. Prospective pulmonary function evaluation following open thoracotomy for anterior spinal fusion in adolescent idiopathic scoliosis. Spine. 2000;25:2319–2325. doi: 10.1097/00007632-200009150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Greggi T, Bakalouidis G, Fusaro I, Di Silvestre M, Lolli F, Martikos K, Vommaro F, Barbanti-Brodano G, Cioli A, Giacomini S. Pulmonary function after thoracoplasty in surgical treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2010;23:e63–e69. doi: 10.1097/BSD.0b013e3181d268b9. [DOI] [PubMed] [Google Scholar]
- 6.Izatt MT, Harvey JR, Adam CJ, Fender D, Labrom RD, Askin GN. Recovery of pulmonary function following endoscopic anterior scoliosis correction: evaluation at 3, 6, 12, and 24 months after surgery. Spine. 2006;31:2469–2477. doi: 10.1097/01.brs.0000238659.12918.b5. [DOI] [PubMed] [Google Scholar]
- 7.Kaneda K, Shono Y, Satoh S, Abumi K. Anterior correction of thoracic scoliosis with Kaneda anterior spinal system. A preliminary report. Spine. 1997;22:1358–1368. doi: 10.1097/00007632-199706150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Lenke LG, Bridwell KH, Kim KL, Steger-May K. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J Bone Joint Surg Am. 2005;87:1534–1541. doi: 10.2106/JBJS.C.00978. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Lenke LG, Bridwell KH, Cheh G, Whorton J, Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis: is there a relationship between major thoracic curve correction and pulmonary function test improvement? Spine. 2007;32:2685–2693. doi: 10.1097/BRS.0b013e31815a7b17. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Lenke LG, Bridwell KH, Cheh G, Sides B, Whorton J. Prospective pulmonary function comparison of anterior spinal fusion in adolescent idiopathic scoliosis: thoracotomy versus thoracoabdominal approach. Spine. 2008;33:1055–1060. doi: 10.1097/BRS.0b013e31816fc3a5. [DOI] [PubMed] [Google Scholar]
- 11.Kishan S, Bastrom T, Betz RR, Lenke LG, Lowe TG, Clements D, D’Andrea L, Sucato DJ, Newton PO. Thoracoscopic scoliosis surgery affects pulmonary function less than thoracotomy at 2 years postsurgery. Spine. 2007;32:453–458. doi: 10.1097/01.brs.0000255025.78745.e6. [DOI] [PubMed] [Google Scholar]
- 12.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169–1181. [PubMed] [Google Scholar]
- 13.Lenke LG, Newton PO, Marks MC, Blanke KM, Sides B, Kim YJ, Bridwell KH. Prospective pulmonary function comparison of open versus endoscopic anterior fusion combined with posterior fusion in adolescent idiopathic scoliosis. Spine. 2004;29:2055–2060. doi: 10.1097/01.brs.0000138274.09504.38. [DOI] [PubMed] [Google Scholar]
- 14.Liljenqvist UR, Bullmann V, Schulte TL, Hackenberg L, Halm HF. Anterior dual rod instrumentation in idiopathic thoracic scoliosis. Eur Spine J. 2006;15:1118–1127. doi: 10.1007/s00586-005-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonner BS, Auerbach JD, Estreicher MB, Bert RR, Crawford AH, Lenke LG, Newton PO. Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J Spinal Disord. 2009;22:551–558. doi: 10.1097/BSD.0b013e318192d8ad. [DOI] [PubMed] [Google Scholar]
- 16.Lowe TG, Enguidanos ST, Smith DA, Hashim S, Eule JM, O’Brien MF, Diekmann MJ, Wilson L, Trommeter JM. Single-rod versus dual-rod anterior instrumentation for idiopathic scoliosis: a biomechanical study. Spine. 2005;30:311–317. doi: 10.1097/01.brs.0000152376.09501.ae. [DOI] [PubMed] [Google Scholar]
- 17.Min K, Waelchli B, Hahn F. Primary thoracoplasty and pedicle screw instrumentation in thoracic idiopathic scoliosis. Eur Spine J. 2005;14:777–782. doi: 10.1007/s00586-005-0977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muschik MT, Kimmich H, Demmel T. Comparison of anterior and posterior double-rod instrumentation for thoracic idiopathic scoliosis: results of 141 patients. Eur Spine J. 2006;15:1128–1138. doi: 10.1007/s00586-005-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. A study of six hundred and thirty-one patients. J Bone Joint Surg Am. 2005;87:1937–1946. doi: 10.2106/JBJS.D.02209. [DOI] [PubMed] [Google Scholar]
- 20.Newton PO, Perry A, Bastrom TP, Lenke LG, Betz RR, Clements D, D’Andrea L. Predictors of change in postoperative pulmonary function in adolescent idiopathic scoliosis: a prospective study of 254 patients. Spine. 2007;32:1875–1882. doi: 10.1097/BRS.0b013e31811eab09. [DOI] [PubMed] [Google Scholar]
- 21.Suk SI, Kim JH, Kim SS, Lee JJ, Han YT. Thoracoplasty in thoracic adolescent idiopathic scoliosis. Spine. 2008;33:1061–1067. doi: 10.1097/BRS.0b013e31816f2888. [DOI] [PubMed] [Google Scholar]
- 22.Vedantam R, Crawford AH. The role of preoperative pulmonary function tests in patients with adolescent idiopathic scoliosis undergoing posterior spinal fusion. Spine. 1997;22:2731–2734. doi: 10.1097/00007632-199712010-00006. [DOI] [PubMed] [Google Scholar]
- 23.Vedantam R, Lenke LG, Bridwell KH, Haas J, Linville DA. A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine. 2000;25:82–90. doi: 10.1097/00007632-200001010-00015. [DOI] [PubMed] [Google Scholar]
- 24.Verma K, Lonner BS, Kean KE, Dean LE, Valdevit A. Maximal pulmonary recovery after spinal fusion for adolescent idiopathic scoliosis: how do anterior approaches compare? Spine. 2011;36:1086–1095. doi: 10.1097/BRS.0b013e3182129d62. [DOI] [PubMed] [Google Scholar]


