Abstract
Purpose
The purpose of this study was to provide the readers with a reliable source of animal models currently being utilized to perform state-of-the-art scoliotic research.
Materials and methods
A comprehensive search was undertaken to review all publications on animal models for the study of scoliosis within the database from 1946 to January 2011.
Results
The animal models have been grouped under specific headings reflecting the underlying pathophysiology behind the development of the spinal deformities produced in the animals: genetics, neuroendocrine, neuromuscular, external constraints, internal constraints with or without tissue injury, vertebral growth modulation and iatrogenic congenital malformations, in an attempt to organize and classify these multiple scoliotic animal models. As it stands, there are no animal models that mimic the human spinal anatomy with all its constraints and weaknesses, which puts it at risk of developing scoliosis. What we do have are a multitude of models, which produce spinal deformities that come close to the idiopathic scoliosis deformity.
Conclusion
All these different animal models compel us to believe that the clinical phenotype of what we call idiopathic scoliosis may well be caused by a variety of different underlying pathologies.
Keywords: Scoliosis, Animal models, Disease models, Pathophysiology, Genetic
Introduction
Adolescent Idiopathic scoliosis affects 1–3 % of the population. Its pathogenesis remains elusive. Animal models have become the corner stone of research in the hope to illicit its etiology, and thus redefine the State of The Art, Current Concepts and Future Perspective of possible treatments for scoliosis [1–3]. To date, many hypotheses have been put forward and studied to establish its pathogenesis. These range from neurologic cause, to genetic mutation, to morphometric alteration leading to disadvantageous biomechanical imbalances through to a probable multifactorial spinal developmental disorder. At the core of many of these hypotheses and research, animal models of all types have been developed with the hope of finally answering the persistent enigma: i.e., What is the pathogenesis of adolescent idiopathic scoliosis? Over the years, while these models have increased medical knowledge, many, however, have in fact raised more questions than answers.
Considering that the spontaneous appearance of scoliosis in animals is rare, great efforts are required to achieve and develop scoliotic animal models. Some quite rare observations of veterinary cases have been reported in domestic quadrupeds (mainly dogs, horses and rabbits), but to date have not resulted in a predictable animal model. Indeed, the only variety in quadrupeds that presents the science regularly with a “spontaneous” scoliosis model is the Spanish King Charles dog, which is known to have Chiari malformations [4]. In response to these constraints, numerous animal models have been developed. Over the last 50 years, almost 200 different scoliotic animal models have been tried, resulting in a very heterogeneous group of animal models. Procedures to induce scoliosis have varied from systemic to local interventions and from species to species. In general, most of the techniques involved animals which had not reached skeletal maturity, reflecting mainly the belief that scoliosis is closely related with growth and an evolving and dynamic process.
In the hope of categorizing these scoliotic animal models, one can start by segregating them into two broad groups. A large subset of models has been developed to elucidate the etiology/pathophysiology of scoliosis, while a second subset has been developed to create animals with abnormal vertebral body geometries mimicking AIS deformity. This second subset of models has provided insight into the possible physiological impact of scoliosis on animals. In addition, as these models tend to be larger, they have also provided greater knowledge of the impact of scoliosis on growth disturbances, bone and disc physiology/degeneration, including the mechanical implications. All of these models have also been used to test novel treatments, including promising new surgical techniques of correction.
The purpose of this study is to provide the readership with a reliable source of animal models currently being utilized to perform state-of-the-art scoliotic research, as well as to report the current concepts and the future applications of the different models. The animal models have been grouped by intent of research to generate a systematic glossary of animal models.
History
Von Lesser [5] in the nineteenth century was the first to report an experimental scoliosis in rabbits after a unilateral dissection of the phrenic nerve. In the 1950s, Nachlas et al. [6] tested the effect of an anterior vertebral stapling in dogs. These researchers obtained inconstantly moderate deformities and corrections from using the same techniques on the opposite side. The conclusions of this work were very optimistic, but probably overestimated. Sawin et al. [7] have described the first model of spinal deformity in rabbits with a genetic inherence, and Carrey [8] was the first to describe and purify a line of scoliotic chickens. Based on these three major principles (genetic, neurologic and musculoskeletal constraints applied to a growing spine), numerous experimental procedures have been performed in various animal species, with varying success rates both to approach human scoliotic patterns and to improve the knowledge of human scoliosis.
Methods
A comprehensive search was undertaken using the OVID search tool for publication on animal models for the study of scoliosis with database queries from 1946 to January 2011. The MEDLINE, BIOSIS and EMBASE databases were mined using the following key words as well as the following headings: scoliosis, animal model, disease models. Our search provided over 610 articles. We excluded articles which were not written in English or French and those which were felt not to be relevant to scoliosis research using animal models. As stated, the animal models will be presented under their original intended purpose with the attempt to facilitate the choice of creating an all-encompassing hypothesis.
Results
Over the last 50 years, many interventions have been performed in many different animal species. Chickens rabbits, rats, mice, frogs, pigs, goats, sheep, dogs and also primates have all been used [9]. In an attempt to organize and classify these multiple scoliotic animal models, we have grouped them under specific headings. These headings represent broad categories reflecting the underlying pathophysiology behind the development of the spinal deformities produced in the animals [10]. As well, these headings to a certain degree represent many hypotheses that have been put forward over the years as the “cause” of adolescent idiopathic scoliosis. As these hypotheses pan out and lead to spinal deformities in certain animals, researchers have tried the same interventions in higher species in an attempt to extrapolate their hypotheses to human subjects. Hence under a particular heading, one may find different experimental animals. One will also find different interventions under the same heading as different researchers address different directions in the cascade of the pathophysiology. The arbitrary broad axis/categories, which we put forward, are as follows: genetics, neuroendocrine, neuromuscular, external constraints, internal constraints with or without tissue injury, vertebral growth modulation and iatrogenic congenital malformations. We have also included an animal model developed to study spinal cord injuries in the face of deformity correction.
Genetics models
Linkage studies have suggested a genetic predisposition for adolescent idiopathic scoliosis. A tremendous volume of genetic research has been conducted mainly using human subjects in an attempt to identify specific genes responsible for AIS. To date, chromosomes 1, 6, 7, 8, 12 and 14 have all been linked to AIS [11]. However, little has been done using animal models to study the genetics of AIS per se. What has been done is an extensive analysis of the mouse and human genome database looking for similar genetic sequence. This process, known as synteny, has been used successfully to confirm genetic mutations in individuals with spinal deformities such as congenital scoliosis in VACTREL for which a mouse model had similar genetic mutation [12]. Giampietro et al. [13] have actively used genetically altered mice with known spine or tail deformities (scoliotic phenotypes) to identify possible loci linked with human congenital or idiopathic scoliosis (Table 1). Out of some 45 loci of interest, 27 had comparative linkage maps both in mice and humans, thus identifying human syntenic regions as possible candidate genes for scoliosis. An example of these genetically altered mice is the (ky) mouse. This knockout mouse is known to have degenerative myopathy and has been found to develop kyphoscoliosis. This specific (ky) mouse model is thought to mimic a neuromuscular kyphoscoliosis and will be discussed under the neurological subheading [14].
Table 1.
Synteny defined candidates for IS and CS
| Mouse mutant or locus | Map position (chromosome cM) | Human syntenic region | Human candidates(s) | Human syndrome(s) |
|---|---|---|---|---|
| Dbf (Pax3, lhh) | 1, 40 | 2q35 | PAX3, lHH | Waardenburg, CFDH |
| Gli2 | 1, 63 | 2q14 | GLl2 | |
| Lmx1a | 1, 88.2 | 1q21-q23 | LMX1.1 | |
| Ltap | 1, 93.4 | 1q21-q23 | VANGL2 | |
| us and Lmx1b | 2, 14 and 21 | 9q34 | LMXlB | NP |
| rh and Hoxd | 2, 38 and 45 | 2q31 | HOXD cluster | |
| Pax1 and dm | 2, 82 and 80 | 20p11 | PAX1 | |
| Jun | 4, 44.6 | 1p31-32 | JUN | |
| sks and sno | 4, 54.6 and 58.3 | 1p33-p32.2 | COL9A2 | MED type 2 |
| ct | 4, 69 | 1p35 | PAX7, CRTM | |
| lx | 5, 22 | 4p16.1 | MSX1 | Wolf-Hirschorn |
| hop | 6, 13 | 7q22-qter | PTN, PAX4 | |
| tc | 6, 35.6 | 2p13-pll | TGFA | |
| Dll3 | 7, 10 | 19q13.2-q13.3 | DLL3 | |
| Tks | 9, 9 | llq22-q24 or 19p13.3-Pl3.2 | MMP cluster or ACP5 | |
| lu | 9, 23 | llq22-q24 | MMP cluster | |
| Aft | 9, 32 | 15q23-q25 | CSK, PML | |
| tk | 9, 48 | 6q12-q13 | COL12A1 | |
| Ky | 9, 56 | 3q21 | MYLK | |
| Wnt3a | 11, 32 | 1q42 | WNT3A | |
| Ts | 11, 73.5 | 17q25 | TIMP2 | |
| Rbt | 11, 74 | 17q25 | TIMP2, CBX2 | |
| Bst | 16, 31.5 | 3q13.2 | COL8A1 | |
| Sim2 | 16, 67.6 | 21q22.2 | SIM2 | |
| mctl | 17, 18.5 | 6p21.3 | COL11A2, RXRB | type 2 stickler, OSMED |
| Fbn-2 | 18, 29 | 5q23.3-q31 | FBN2 | CCA |
| ocd | 19, 6 | llql3 | LTBP3 |
CFDH craniofacial deafness-hand syndrome, NP nail-patella syndrome, MED multiple epiphyseal dysplasia, OSMED otospondylomegaepiphyseal dysplasia syndrome, CCA congenital contractural arachnodactyly
On a different note, as previously stated, there are very few species found in nature with “naturally occurring” spinal deformities “similar” to adolescent idiopathic scoliosis, with one exception. One animal model with a naturally occurring spinal deformity that has been studied and published is the teleost—(Poecilia reticulata) also known as the guppy. The model has been well described. It has been used to investigate morphological spinal changes and mainly the mode of inheritance of this spinal deformity. Researchers have observed parallels between the curveback guppy and AIS suggesting that it may be used to explore primary factors involved in the development of spinal curvature in the absence of “gravity” [15]. Gorman et al. found in teleost a polygenic inheritance mode with a female predominance of greater spinal deformity similar to AIS. They demonstrated that these fish mimic the wide variability in phenotype similar to humans with different age of onset, rate of progression and curve magnitude. In addition, curve progression occurred only during growth. They have even noted some curve regression before skeletal maturity. They also explored the structural and micro-anatomical changes in vertebrae of these teleost, which were in keeping more so with Scheuermann’s kyphosis than AIS [16]. The ease of handling, the straightforward objective, qualitative and quantitative means to measure the deformity, the obviously different phenotype, short generation time and availability of genomic resources make the guppy a practical scoliosis animal model to study genetic inheritance. Selective inbreeding allowed for creation of curveback families enriched with genes for the observed curve magnitudes. Interestingly, the same group of researchers identified in this teleost model a qualitative trait locus (QTL) that controls curve susceptibility [17] (Fig. 1). This locus contains over 100 genes including the melatonin receptor MTNR 1B [18].
Fig. 1.
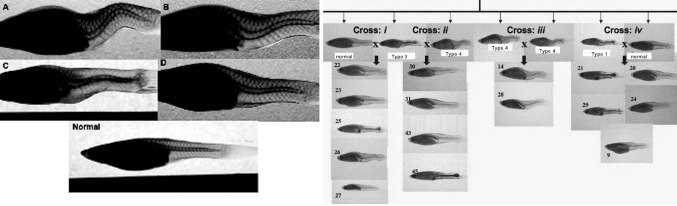
Examples of type of deformities in curveback guppy. a Type 4 progressive curves. b Type 3 moderate curves. c Type 2 slight curve. d Type 1 nearly curved. e Example of exploring inheritance patterns using curveback guppy as animal model for spinal deformities
Neuroendocrine models
Melatonin
The most studied animal model used to explore the pathophysiology of scoliosis has been the pinealectomized chick. This model has led to the hypothesis that the neuroendocrine protein, melatonin, plays an important role in the development of scoliosis. Thillard is credited for introducing the concept over half a century ago [19]. In 1983 Dubousset and Machida [20] popularized the model by drawing a morphological correlation with AIS and newly hatched chickens undergoing pineal or diencephalic lesions. The model was then further validated when melatonin or serotonin was administered to these pinealectomized chicks and they did not develop scoliosis [21–24]. The model was then tested in mammals and showed that pinealectomized rats would only develop a scoliotic deformity if they were rendered bipedal, indirectly confirming that axial loading of the spine plays an integral part in the development of scoliosis. The resulting deformities in these rats were again similar morphologically to AIS with a lordoscoliosis with lateral rotation. However, when this melatonin pathomechanism was tested in higher mammals or in young nonhuman primates, they did not develop scoliosis [25]. In fact, a few papers actually looked at children who have undergone surgical or chemical pineal gland resection for tumor, and none of these children developed scoliotic deformities [26, 27].
In keeping with the same neuroendocrine hypothesis, the C57BL/6J mouse, which is “naturally” melatonin deficient, developed scoliosis when it was rendered bipedal [28, 29]. Akel et al. [30] subsequently demonstrated that by given tamoxifen to these bipedal mice as an anticalmodulin, the progression of scoliosis was significantly decreased compared to controls. Moreover, he found the same results when he gave tamoxifen to pinealectomized chickens.
The role of melatonin in the development of scoliosis in primitive species is compelling. However, in humans, it remains hypothetical [27–31].
Growth disturbances
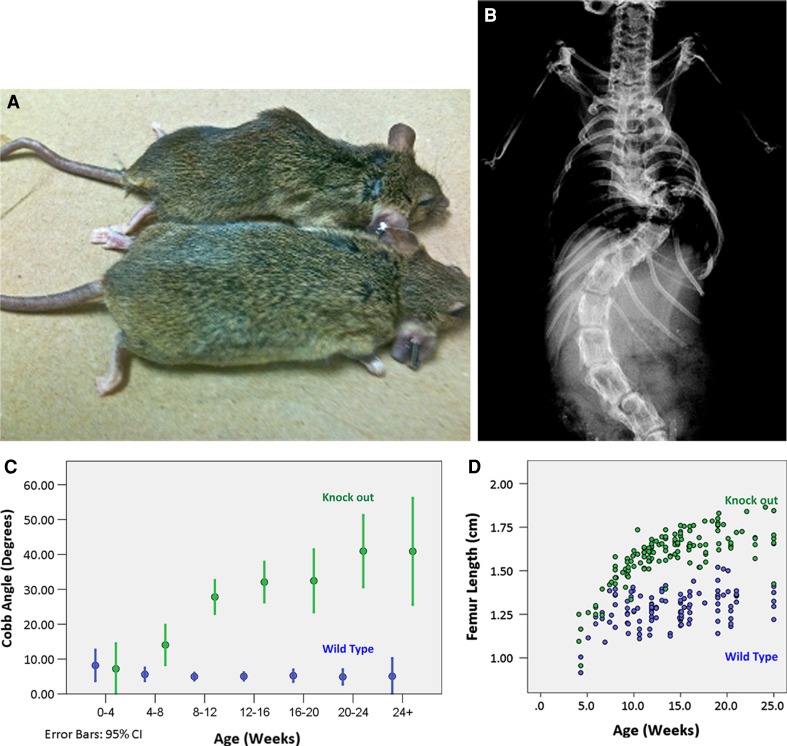
Growth disturbances have also been postulated as an etiopathogenesis of AIS with different growth kinetics between the concave and the convex size [32]. A mismatch between anterior spinal growth in relationship to posterior spinal growth may be the triggering factor to the lordoscoliotic deformity seen in AIS. The FGFR3 knockout mouse is known for skeletal abnormalities (Fig. 2). Its phenotype is associated with kinked tails, greatly expanded growth plates and skeletal overgrowth. Our laboratory has recently characterized the morphogenic spinal deformities found in this scoliotic model, which has features similar to AIS, with unusually accelerated growth for long bones and lumbar spine, 3D spinal deformity and osteoporosis. The spinal deformity occurs simultaneously with peak growth of the femur [article submitted].
Fig. 2.
a Examples of a 14-week-old knockout mouse FGFR3 exhibiting typical spinal deformities. b X-ray of the same mouse. c Diagram demonstrating the development of the primary curve is significant by 8 weeks, plateaus by 25 weeks with mean Cobb angle of 40.9 ± 18.3, while wild-type mice maintained 5.1 ± 4.1° (p < 4 × 10–16). d Diagram illustrating growth disturbance in knockout mice leading to a distinct group with longer femurs before reaching skeletal maturity at approximately 16 weeks (p < 5 × 10–5)
Neurologic models
There has been a series of scoliotic animal models, which have been developed based on the hypothesis that the driving force behind the deformity is a neuromuscular problem [33, 34]. Pincott et al. [35] have shown that monkeys develop scoliosis secondary to an intraspinal injection of a live attenuated oral poliomyelitis vaccine. The spinal deformity was developed incidentally subsequent to a virulence testing protocol. Oddly enough, the spinal cord histology demonstrated greater damage to the spinal cord on the convex side of the scoliotic animal, localized to the posterior horn and Clarke’s column where afferent sensory/proprioceptive nerve endings entered the spinal cord. They concluded that the resulting deformity was thought to be caused by an asymmetrical weakness of the paraspinal muscles due to the loss of proprioceptive innervation, not related to the polio virus itself. The same researcher subsequently undertook a more direct experiment by proceeding with selective dorsal root rhizotomy [36]. The resultant scoliosis developed on the side convex to the damaged side and its severity was dependent on the number of nerve roots cut. Of note, the resulting curves were long C-shape curves similar to neuromuscular curves found in humans afflicted, for example, with cerebral palsy. Another interesting finding was that the monkey that had the L1 nerve root sacrificed developed a greater deformity, which collaborates with the concept that rotational spinal instability across the lower thoracic/lumbar area (weakness induced in this case) may be a key factor in the development of scoliosis (Fig. 3).
Fig. 3.
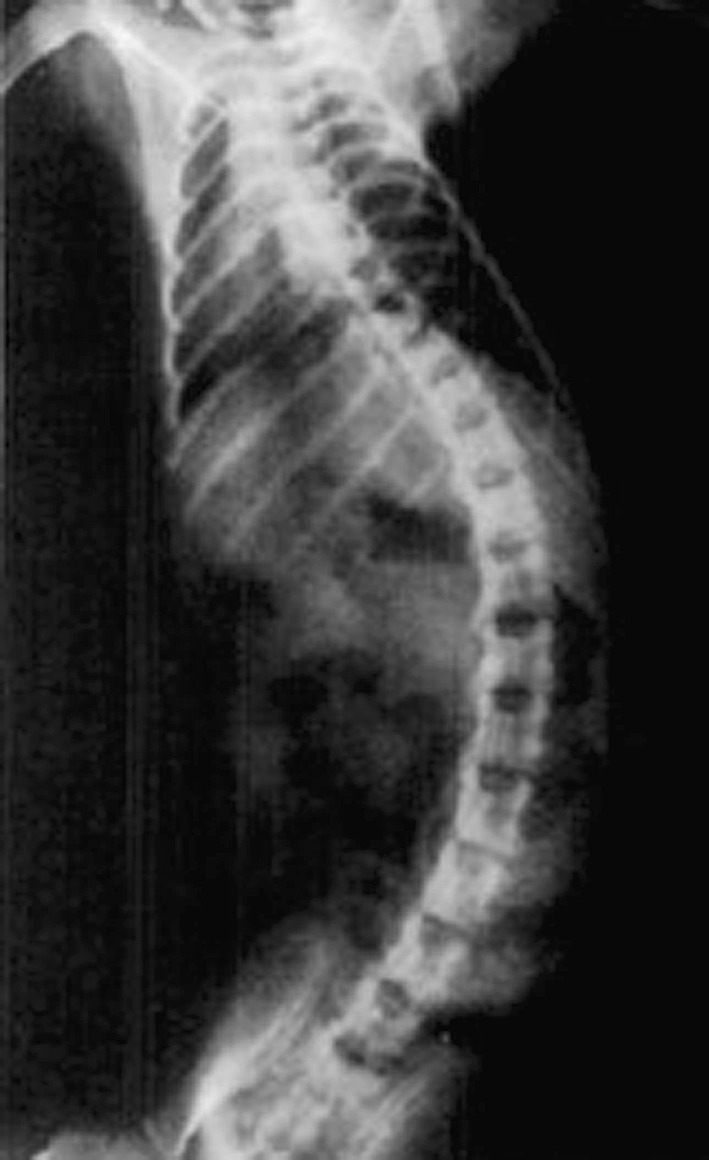
Examples of a neurological animal model. Direct posterior column cord injury post-intraspinal injection of polio virus in a monkey
A different neurological hypothesis possibly linked to the pathoetiology of AIS is the disruption of the central vestibular system (the inner ear). It is hypothesized that there is an imbalance in neural activity in descending locomotor/posture control pathways, which may originate from the inner ear or the central vestibular system. A noteworthy animal model developed by Lambert et al. [37] demonstrated the effect on the axial skeleton if an imbalance is present during growth in a batrachian model. The model consisted of unilaterally removing the labyrinthine end organs of the Xenopus laevis (frog) at its larval stages. They found that after metamorphosis, the young frog spines had developed curvatures in all three planes, including rotation along the long axis of the body with similar characteristics to AIS (Fig. 4). Attempts to reproduce this model in guinea pigs resulted in transient gaze and abnormal limb posturing [38]. However, these transient changes never led to persistent spinal deformity. It has been hypothesized that the rat’s peripheral limb proprioceptive signals substituted for the loss of the inner ear input, leading to the recovery of the abnormal posturing [39]. Lambert et al. [37] concluded that their frog model developed spinal deformities as these animals had persistent asymmetric tone in the axial and limb musculature during growth without compensatory peripheral proprioceptive feedback as they developed in a weightless environment (water). As a consequence, during the metamorphosis, the mostly cartilaginous skeleton of the tadpoles progressively deformed.
Fig. 4.
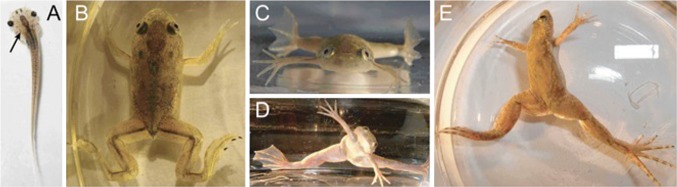
Spinal deformities in (frog) Xenopus laevis after removal of the left labyrinth. aTop view of larva illustrating the induced bending of the body/tail. Top and front views of a young adult control frog (b, c) and after labyrinthine ablation (d, e)
Ky mice, known for a degenerative myopathy, develop over time a thoracolumbar kyphoscoliosis mimicking the neuromuscular scoliosis pattern found in humans [40]. This mouse is deficient in the ky protein, thought to be crucial in the stability of the neuromuscular junction and the ability of muscle to grow and function normally [41]. The ky gene has been localized on chromosome 9 and encodes a novel muscle protein. The human Ky ortholog falls into a conserved synteny region on 3q21. Myosin light polypeptide kinase is localized within this region and could possibly be a candidate gene for congenital and idiopathic scoliosis [13].
Mechanical models
Various local procedures have been developed to induce scoliotic deformities in small and large animals. One can subdivide this research into those using external constraints, internal constraints and direct or indirect spinal tethers with or without tissue injury.
External constraints
The advantages of creating a scoliotic animal model using external constraints are:
(1) they are non-invasive and (2) the creation of scar tissue is minimized around the spine. Various procedures were performed mainly in rabbits and small animals to induce this particular scoliotic model.
Orthopedics constraint/casting–bracings
In accordance with the observation of lordoscoliotic deformity in humans [42, 43], Poussa et al. [44] used in skeleton of immature rabbits an external splint to force lordosis at the thoracolumbar junction. This led to scoliosis in over 50 % of the animals. These results support the idea that lordosis is a prerequisite to developing scoliosis in the rabbit. Hakkarainen [45] produced a scoliosis-like deformity by immobilizing rabbits in a three-point plaster cast for several weeks. The amount of deformity depended on the age and duration of the constraint. A minimal curve of 30° was necessary at the time of termination from immobilization to observe persistent progressive scoliosis. In rats from the same procedure, the influence of gender in the development of a deformity was studied and no difference was noticed [46].
Direct vertebral tether
Following the same principle of external constraints to develop scoliosis, the vertebrae of rat tails were used to study the concept of a mechanically provoked progression of scoliotic deformities according to the Hueter-Volkmann law [47]. In these studies, an external fixator was applied with different constraint protocols resulting in vertebral wedging and production of scoliosis. The authors showed that intervertebral discs underwent remodeling. They also managed to induce vertebral wedging by distraction instead of compression [48]. In addition, vertebral body remodeling has been shown to contribute to the deformity in older rats according to Wolff’s law [49]. Stokes et al. [50] also showed that the imposed reduced mobility caused by the external constraint system is a major source of disc changes. This could possibly explain disc degeneration in scoliosis.
Internal constraints
Indirect spinal tethers
Lateral curvatures in growing rats and rabbits were produced by suturing the inferior angle of the scapula to the contralateral pelvis [51, 52]. The authors reported a number of morphologic and histologic changes characteristic of human scoliosis. Similar results were obtained with the tethering between spinous apophysis and the transverse apophysis with a cauterisation of the laminae to reduce spinal posterior growth [53]. However, the success of this technique was unpredictable. The combination of these techniques with the unilateral resection of a paraspinal muscle speeds up the deformity. However, the histological data showed that a concomitant ischemic spinal cord injury occurred secondary to the cauterization procedure and the authors suggested that the neural damage instead of the muscle or the laminae growth disturbance was responsible for the rapidly progressive deformity. This technique of scoliosis creation by scapula to contralateral-ilium tethering was reactivated recently with bipedal rats and kyphoscoliosis was created [54]. The incidence of vertebral rotation was higher in bipedal rats after tethering release compared to quadruped rats.
Regarding these models, the following conclusions can be drawn: an asymmetric constraint applied in a growing spine in small animals can produce a scoliosis-like deformity. A minimal duration (6–8 weeks in most of the models) of constraint application is mandated to make the deformity structural. However, these techniques that have the advantages of avoiding direct trauma to the spine and simulating human posture to date have never been transposed in large animals.
Indirect spinal tether with tissue injury
A symmetric growth of the chest is necessary to obtain the harmonious growth of the spine. The stimulation or inhibition of the costal growth could induce a spinal deformity. Sevastikoglou et al. [55] were the first to report spinal deformities (lordoscoliosis from 30° to 60°) in rabbits. An osteotomy with a fixed override of four consecutive ribs and contralateral rib fractures resulted in progressive scoliosis. The rib head removal was efficient in some quadrupeds. In New Zealand rabbits, an original model was reportedly based on an isolated section of the intercostal nerves. For the authors, the sections led to a vasodilatation caused by sympathectomy and an overgrowth of the vertebral endplate nearby. Scoliosis was induced by asymmetric growth, though not the paresis of trunk muscles [56]. Sevastik et al. [57] observed the correction of these experimentally induced scoliotic curves either by rib elongation with a metallic expander or further resection of intercostal nerves on the opposite side. The section of the ligaments around the proximal fourth and fifth without damaging the ligamenta tuberculi costae or the section of all ligaments around the costovertebral joint led to a scoliosis of 50°–115° in approximately 50 % of animals, notably pigs and rabbits [58]. Hemilaminectomy performed on five consecutive thoracic vertebrae also produced scoliosis in rabbits. The impacts of inadvertent concomitant adjacent tissue injury (costotransverse ligament, nerve root or subtle spinal cord injury) were raised as possible confounding variable in these studies [59, 60].
In all these techniques, an overlap between mechanic and neurologic means is usually observed. For reasons that are unclear, the same procedures transposed in primates were ineffective; unilateral resection of the rib heads, excision of the intercostal nerves, division of the costotransverse ligaments, various muscle resection, denervation have been attempted with little or no success [61]. Deformities were observed only after a large bilateral excision of the costal elements [62]. The clinical correlates of such animal model can be seen in children who have had thoracic burns or partial chest wall resection for tumors, due to which they developed spinal deformities.
With the concept of thoracic insufficiency syndrome described by Campbell, models affecting both spinal and thoracic structures were developed recently. Metha et al. [63] had reported a rabbit model with bilateral thoracic procedures. The ventilatory compliance was affected by the thoracic scars altering the ventilatory mechanics. In a more recent study, the researchers studied the effect of expansion thoracoplasty, not only on lung volume but also on alveolar structures. They observed an improvement of the alveolar perfusion and of the airspace fraction in treated rabbits. The effects of a thoracic fusion on thorax and lung development were studied in rabbits [64].
Direct spinal tethers
These models directly affect vertebral growth and the surrounding tissues. They have attracted a large amount of interest in recent years. The safety and the efficacy of fusionless scoliosis treatment, growth modulation, disc behavior, permeability and remodeling of the vertebral endplate were studied and interesting information were extracted from these scoliotic spinal models. Surgical techniques have evolved from rigid to flexible constructs and with minimizing the violation of the surrounding tissue to preserve the spine in a pristine state and to be closer to human mechanic conditions. Spinal deformities were sustained more easily in young animals. However, several limitations have to be considered: Mechanical forces acting on the spine are different in quadrupeds than in bipeds, degenerative changes observed are for a short period and growth rates are different (e.g., T1–S1 length increase of 20 cm within 2 months in a landrace piglet). Most of these studies reported geometric and histological changes. Little data on cellular and molecular biology of the structures responsible for spine growth and mobility are available as yet.
Neurocentral cartilage
The theory proposed initially by Berguistain [65] that an asymmetrical growth in the neurocentral cartilage of the vertebrae could give a vertebral rotation and thus lead to scoliosis was tried in pigs, whose neurocartilages were active beyond the age of 1 year in the thoracic area. A selective epiphysiodesis of the neurocentral cartilage performed on four to five consecutive vertebrae produced a structural scoliosis in pigs with characteristic vertebral wedging and rotation. This technique requires rather important residual growth to obtain a deformity (piglets of 1 or 2 months old). The use of two pedicle screws across the neurocentral cartilage was more effective [66]. This technique could be used to reverse the deformity in an immature pig model [67]. In a study comparing neurocentral screwing by anterior or posterior approach, they reported that screw insertion by anterior thoracotomy was unable to create scoliosis despite production of a shorter pedicle and a narrower canal. In case of posterior insertion, the screws violated mostly the intervertebral foramina with evidence of nerve root damage. They suggested that a neuropathic mechanism may be necessary to initiate the development of scoliosis that can be maintained by inhibition of the neurocentral synchondrosis growth [68].
Asymmetric tether
With the goal of developing surgical techniques to preserve vertebral motion and growth, vertebral asymmetrical constraint was studied in larger animals. Goats, calves and pigs were mostly used. Successful methods to create scoliosis deformity have required more or less extensive vertebral and costal asymmetric posterior tethering. The fixation of the implants onto the spine was a concern at the beginning of these studies and was resolved by using screws (pedicle or anterolateral body). The surgical techniques were minimized to respect the anatomical structures responsible for growth and mobility of the spine.
Anterior asymmetric tether
Anterolateral flexible tether or shape memory alloy staples over consecutive vertebrae were studied in pristine state and in deformed animals to identify a method of controlling progressive scoliosis. Newton et al. [69, 70] have reported on disc and vertebral changes after tethering in different static and dynamic constructs in an immature bovine model. Important disc changes and wedging were observed in static constructs. The differences between static and dynamic fixation were studied. In dynamic constructs, morphology, motion and hydration of the discs were preserved despite global disc height decrease. Of note, nonmechanical means also induce asymmetric growth. Placing unilateral electrodes delivering 50 mA across the growth plate led to scoliosis in rabbits.
Posterior asymmetric spinal ± costal tether
These methods were used mainly in larger animals. In large quadruped animals, a thoracic procedure was usually necessary in association with the posterior spinal tether to obtain a spinal deformity. Nevertheless, an isolated costal ligature had never led to a spinal deformity in large animals. Braun et al. [71] were the first to report a consistent model in young sheep with a rigid vertebral tether and a thoracic procedure (concave rib cage ligament tethering and convex rib resection). A mean increase of 20° of the initial curves was obtained in a mean 12 weeks of growth. In a second study, greater deformities were observed (55°–74°) with the use of a flexible tether [72]. Schwab et al. [73] also generated significant curves with a flexible tethering in a porcine model, and more recently. Zhang et al. [74] in goats with a less invasive procedure on the rib cage. The thoracic procedure is associated with an increase of the postoperative morbidity (up to 30 %). Odent et al. [75] reported a porcine model of early-onset scoliosis without thoracic procedure with an offset flexible tether (Fig. 5) The spinal deformities obtained with these models approach accurately the typical AIS deformity with an inverse relation between spinal rotation and vertebral tilt. The induction of the “vicious cycle” theory proposed by Stokes [48] was confirmed with a persistence of the curves after tether release. The curve evolution was inconstant and proportional to the amount of the vertebral wedging for a similar Cobb angle [76].
Fig. 5.
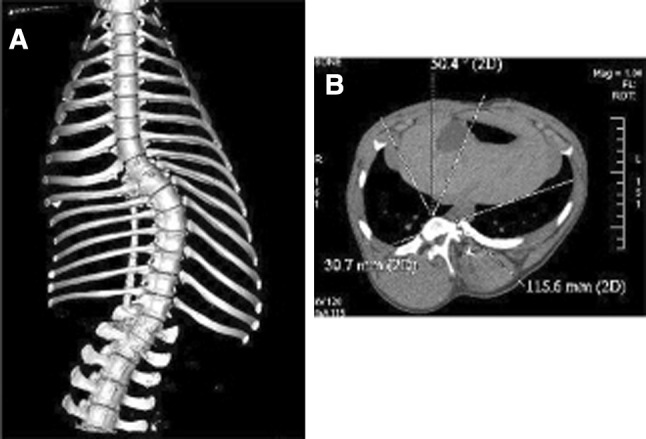
Landrace pig model induced by posterior asymmetrical spinal tether. a Anteroposterior X-ray view and b coronal CT scan view of the spine and thorax
Teratogenic/congenital scoliotic (CS) models
Pregnant mice exposed to teratogens have led to a reliable congenital scoliotic animal model. Rivard et al. [77] induced hypobaric hypoxia during the 10th day of gestation in white mice, which led to 90 % lumbar spinal deformities consistent with congenital scoliosis. They found failure of segmentation in 34 % of the deformities (unilateral bars and bloc vertebrae) and 65 % of failure of formation (hemivertebrae, butterfly vertebrae, agenesis). Similar thoracic malformations were obtained on maternal exposure to carbon monoxide at 9 days of gestation in mice [78].
Two genetically altered mice, the TBX6 knockout, and the Sim2, have phenotypes with congenital scoliosis. Both of these scoliotic animal models have contributed to identifying possible candidate genes involved in the cascade leading to congenital scoliosis in humans. Fei Q et al. [79] studied 127 patients with congenital scoliosis to 127 match-paired control group and found that genetic variants of TBX6 gene were significantly associated with the presence of congenital scoliosis in the Chinese Han population. The Sim2 mutant mice have a phenotype similar to a child with thoracic insufficiency syndrome. In addition to congenital scoliosis, they have chest wall abnormality [80].
Spinal cord injury models
Well-established spinal cord injury (SCI) animal models have been utilized intensively to study the pathophysiology of spinal cord injuries. The vast majority of these small animal models mimic well the mechanism of injury seen in direct blunt cord trauma typically seen in spinal fracture. The classic model consists of a fixed weight dropped from a set height onto an exposed spinal cord of a rodent who had undergone a laminectomy. Other models consisted of transection or unidirectional distraction injuries. However, to study spinal cord injury in the setting of spinal deformity correction, one must consider the mechanism of injury that is quite different from traumatic SCI. The differentiation between the two models is important, as the subsequent response to the initial insult may vary. The current belief related to intraoperative spinal cord injury during scoliosis surgery is more in keeping with either a direct canal encroachment with spinal instrumentation, or more commonly the consequence of spinal cord distraction leading to an ischemic event. Seifert et al. [81] recently described a bidirectional distraction spinal cord injury animal model. The procedure essentially uses rats in the animal model and an external device. This device is calibrated and directly attached to the rat’s spine and allows for direct manipulation of the spine as SSEP and MEP are done.
Discussion
As the etiopathogenesis of AIS is still unknown, it is not surprising that currently there does not exist an animal model that replicates this deformity in its multiple characteristics. Notwithstanding this, multiple models in a multitude of animals have been developed successfully mimicking scoliotic deformities. These models tend to provide but a fraction of the true pathology of this complex condition in terms of etiopathogenesis, anatomy or biomechanics. Many of these animal models have contributed to concepts which continue to be investigated. The accumulation of these models allows us to draw general conclusions for which the etiopathogenesis of AIS will be most likely found.
Genetics/etiopathogenesis
Despite significant advancement in gene mapping and a series of specific chromosomal loci identified in humans as being “associated” with the development of scoliotic curves in adolescence, there are still no clear modes of inheritance or indication of which gene actually confers this condition. Specific loci have been found in large families confirming the autosomal dominant inheritance patterns. Yet, different groups of researchers have found these on different genes [82]. With such divergent results, one can only conclude that AIS phenotype results from a variety of different genotypes; hence, the reason why we have not identified the single gene but rather a series of loci associated with AIS. Such findings are much more in line with the current evidence of AIS being multifactorial. As novel technology continues to decrease the time spent on genetic processing, the use of genetically altered mice and synteny will undoubtedly further identify genes linked to the pathophysiology of spinal deformities such as AIS.
There are obvious drawbacks to teleost as a scoliotic animal model. While the deformity is more so in the sagittal plane than in the coronal plane, this however becomes somewhat irrelevant as the fish’s spine is not under the classic bipedal gravitational pull. Being able to investigate specific subsets of factors contributing to spinal deformity using such primitive animal models may well allow us to break down the individual factors and start understanding better the pathophysiology of the disease. Interestingly, from a developmental point of view, humans and teleosts share most pathways, physiological mechanisms and organ systems. It is likely that there are common primitive pathways which may be involved in the development of spinal deformities.
The neuroendocrine models (that rely mostly on pinealectomized chickens) undoubtedly produce spinal deformities in chickens. The literature confirms that in these species, melatonin is an integral part of the pathophysiology process of developing scoliosis; however, the underlying neural injury in the process is not to be negated. Over time, this model has been less in favor as it was never transposed to large animals and primates. An explanation for its refractory transposition to humans is that chickens have many melatonin receptors in a wide tissue distribution situation including the brainstem and the dorsal gray matter of the spinal cord; this is not the case in primates. In addition, these receptors differ in their interaction in humans [83].
The neurological hypothesis with an aberrant proprioceptive input correlates well with human spinal deformities in different conditions with a high incidence of scoliosis. One needs only to look at scoliotic deformities in patients with cerebral palsy. Even in AIS, researchers have found that they have impaired postural and locomotor performance [84, 85] and poor extraoculor motor control including positional nystagmus [86, 87]. Such findings are indeed interesting, as the central vestibular frog model or the primate dorsal rhizotomy model confirm that disruption in one of the pathways indeed leads to spinal deformity.
Anatomy–growth–spinal motion
In general, it is easier to create a deformity in immature animals. The younger the animal, the easier it is to create a deformity. The methods used to create the spinal deformities are often described as purely mechanical in nature. However, many studies have confirmed that in reality, the deformities are the result of several pathways often without the author’s own knowledge. More often than not, the procedures trigger a neurological process, which also contributes to the development of the spinal deformities. This is true not only for the spinal tethers, but also for the neuroendocrine model. As one critically looks at the anatomical variations of the vertebrae across the animal models, one realizes that subtle anatomical changes have a major impact on the models. Despite many similarities in the basic spinal anatomy in all vertebrae, there exist important differences in terms of anatomy compared to humans, e.g., for chicken, the spine contains only eight vertebrae, all lumbar vertebrae are fused and the disc anatomy is different: only two intervertebral discs are mobile (between the sixth and seventh and between seventh and eighth vertebrae) and the other discs are of the amphiarthrosis type. The rodents (rats and mice) and rabbits are higher vertebrates and are closer to humans than chickens. However, the properties of their intervertebral disc and vertebrae vary significantly in different locations within the spine of the same animal.
In large animals, the anatomy is much closer to humans. However, many differences persist. The organization of the vertebral ossification centers is not similar between species. In most mammals, the central core body forms the ossification center. Secondary centers arise in these at the cranial and caudal areas of the vertebral body, forming a complete osseous plate above and below the physeal plate, like the epiphysis of a long bone. In humans, the secondary center is restricted to the circumferential parts of the vertebrae, forming an epiphyseal ring at the outer edge of the vertebral column (Fig. 6). For disc cell and matrix composition, variation exists, e.g., the notochord cells of the nucleus pulposus decreases very rapidly after birth in humans, contrary to other species including cats, mice, rats, dogs and pigs, all of which retain them throughout much of their adult life [88]. The speed and amount of growth also differ: the amount of growth of the length of a spine in a landrace pig can be up to 10 cm a month. Within 3 months, some species exhibit the same amount of spinal growth that a human does in 20 years (25 cm).
Fig. 6.
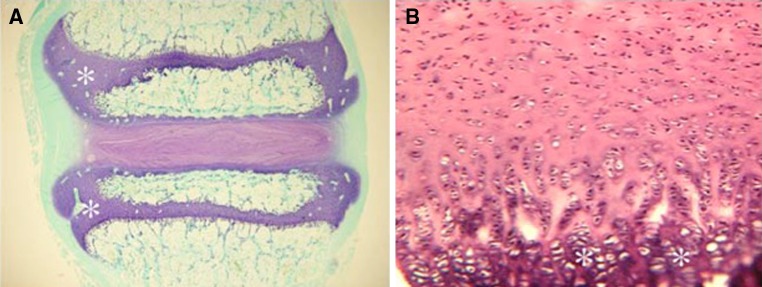
Histology slide demonstrating fundamental differences between animal and human vertebral growth plates. a Sheep’s growth plates (asterisks) are distinct and separate from the intervertebral disc, unlike in the b human where the growth plate is an extension of the cartilaginous endplate between the disc and vertebral body
The thorax anatomy of pigs is the closest to human morphology compared to sheep and goats. The pig thorax is more cubical than in goats and the ribs have a rounded shape as in humans. In ruminants, for unknown reasons, the ribs are large in the cranio-caudal plane and narrow in the coronal plane. Comparing the segmental range of motion of animal species and humans, certain similarities as well as differences can be detected. For example, the absolute range of motion is smaller, particularly in flexion/extension in the lumbar spine, for calves, sheep and pigs compared to humans [89]. In the available animals, the ones closest to humans in anatomy and genetics are the primates. Their use is limited due to high costs and the limited place available to study them. Strikingly, many procedures which were successful to produce deformities in quadrupeds failed in primates.
Despite these species differences, 3D spinal deformities that mimic scoliosis could be induced at the expense of destroying structures involved in regular growth of spine and thorax, neuromuscular balance and anatomical structures. While all these perturbations limit the etiopathogenesis studies, these models can be used to investigate novel treatments for AIS. Granted that the more recent models are achieved with much less tissue injury, there still remains work to be done to standardize curve progression, particularly in curve evolution after tethering release. A lot of information can be extracted for improving our knowledge of the structures responsible for growth and spinal mobility. In cases of installed deformities, many studies are still necessary to explain the vicious cycle of scoliosis progression. Factors such as time of initial constraint, muscular actions and gravity loads, amount of disc and vertebral wedging, and histological and biochemical changes will need to be studied to determine the “functional activity zone” of the structures responsible for spinal motion, balance and mobility. Also, the threshold values of this “vicious cycle” have to be determined. To date, for curve correction and motion preservation studies, the reported results are parsimonious. The behavior of curves after tether release has to be studied more and documented before drawing conclusions on fusionless treatment action. The studies involve mainly geometrical results but not tissue formation such as proteins, signaling pathways, etc. In fact, a large gap exists between genetic research (made mostly by scientists) and the models focused on the geometric aspect of the deformity (made mostly by surgeons). Limited data exist on tissue formation, structural proteins studies and signaling pathway analysis. These changes that are well documented for degenerative spinal disease have to be surely performed in the scoliotic research models.
The main objective of this research field is to study the possibility of restoring growth in the concavity to further correct the deformity. The first results reported by Nachlas were probably overestimated and further studies showed the difficulty of obtaining growth in the concavity [90]. Results on curve correction were poor with staples in a goat model with an important back-outing rate [91]. Better results were obtained with vertebral bone anchors connected with a flexible ligament [92]. However, only coronal corrections were obtained with loosening over time. Three-dimension corrections through modification of vertebral morphology were obtained in a porcine model with an anterolateral tethering correction surgical device [93].
Biomechanics
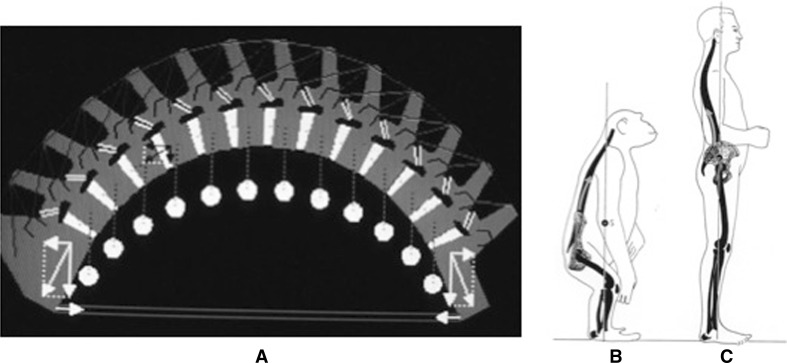
The absence of spontaneous scoliosis in nature and the fact that bipedal rats and mice consistently showed higher incidences of scoliosis in different experimental models compared with their quadruped counterparts clearly showed that the upright position had a role in the development of scoliosis. The loads applied on the spine of quadrupeds are different (“acting like suspension bridge principle”) and probably protect the spine from developing a rotational deformity (Fig. 7). As a consequence, an important external constraint or a significant perturbation of the musculoskeletal equilibrium or disruption in the homeostasis of efferent–afferent neural impulse is required in any type of quadruped animal to induce a scoliotic deformity.
Fig. 7.
Differences of acting loads on spine between a quadrupeds (acting in flexion, like a suspension bridge), b primates (with body center mass in front of the pelvis) and c humans (with body center mass above the pelvis and shear load posteriorly directed)
The distribution of the loads on spinal segment is different between species; most of the weight of the upper body acts on the lumbar spine in humans which is not the case in quadrupeds. Indeed, the load necessary to stabilize a horizontally aligned spine requires higher muscle forces and passive tension than stabilizing an almost balanced vertically aligned spine. Although there are no directly measured forces acting on the spine reported in the literature, we could think that the fourfold higher bone mineral density found in sheep, calf and pig vertebral bodies compared to humans relates to these differences in the amount of loading necessary to stabilize the spine [94].
For erect vertebrae (natural or created), an important difference lies in the fact that humans are the only vertebrate that ambulate in a fully erect position, with the upper body’s center of mass positioned directly above the pelvis. The biped vertebrates ambulate with flexed hips and knees and, therefore, have a more horizontally positioned spine, thus putting the upper body’s center of mass in front of the pelvis [95]. Due to these anatomic features, the human spine is subject to posteriorly directed shear loads. These particular loads have been shown to render it less stable in rotation [96]. Such findings may explain why the higher pinealectomized mammals (including monkeys) do not develop scoliosis as their spine resists better rotational instability.
Choice of the preferred scoliotic animal model
All of the mentioned scoliotic animal models have certain characteristics for which they are ideal to study scoliosis. However, no single model is a true adolescent idiopathic scoliotic model. Hence, researchers must ask themselves what they wish to study and then choose the model best suited for their objectives. For example, if a researcher is looking for an animal model to test a new spinal implant to treat early-onset scoliosis, then the model best suited for this is either the mini pig model described by Odent et al. [75] or the immature goat model described by Braun et al. [71]. This model provides a relatively economical robust animal model having a lordoscoliotic spine with comparable dimension of a 6- to 8-year-old infant. The tether is away from the spinal elements, thus minimizing confounding factors, which may alter the therapeutic response to the corrective surgery. In contrast if the researchers are looking for a scoliotic model to study the pathophysiology/etiology of AIS, then the variety of knockout mice models will most probably offer the best model. Because of the ease of manipulation, their low cost and rapid generation turnover, these mice models allow easy study signaling pathways and histology among other things. For example, if one wants to concentrate on the altered bone growth/remodeling, then one could explore the FGFR3 dysplastic mouse models. Obviously, the conclusions of such animal models need to keep in perspective that until AIS human syntenic regions are identified, there may remain possible pathways that can lead to AIS. For example, one cannot conclude that an aberrant FGFR3 pathway is the cause of AIS simply because the FGFR3 knockout mouse develops scoliosis.
Conclusion and perspectives
There are no animal models which mimic the human spinal anatomy with all its constraints and weaknesses which puts it at risk of developing scoliosis. What we do have are a multitude of models, which produce spinal deformities that come close to the idiopathic scoliosis deformity. The combination of all these models confirms that there are a multitude of causes that can produce scoliosis and that the clinical phenotype of what we call idiopathic scoliosis is most likely caused by a variety of underlying, different pathologies. The culmination of all these animal models leads us to believe that AIS develops subsequently to predisposition to spinal instability in the face of a disruption in the homeostasis of efferent–afferent neural impulse. Future treatment modalities of AIS will most likely originate from these and future animal models. With the help of genetic research, eventually the pathoetiology of AIS will be discovered, thus allowing us to treat the cause of the scoliosis rather than the end result.
Acknowledgment
The institution of the author receives fellowship research funding from AO Spine North America unrelated to this work. The author has had commercial associations in the form of contractual consultancies work with Synthes.
Conflict of interest
None.
Contributor Information
Jean Ouellet, Email: jean.ouellet@Muhc.mcgill.com, Email: jean.ouellet@muhc.mcgill.ca.
Thierry Odent, Email: Thierry.odent@nck.aphp.fr.
References
- 1.Arkin AM. The mechanism of structural changes in scoliosis. J Bone Joint Surg Br. 1949;66:519–528. [PubMed] [Google Scholar]
- 2.Dickson RA, Lawton JO, Archer IA, et al. The pathogenesis of idiopathic scoliosis. Biplanar spinal asymmetry. J Bone Joint Surg Br. 1984;66:8–15. doi: 10.1302/0301-620X.66B1.6693483. [DOI] [PubMed] [Google Scholar]
- 3.Murray DW, Bulstrode CJ. The development of adolescent idiopathic scoliosis. Eur Spine J. 1996;5:251–257. doi: 10.1007/BF00301328. [DOI] [PubMed] [Google Scholar]
- 4.Couturier J, Rault D, Cauzinille L. Chiari malformation and syringomyelia in normal cavalier King Charles Spaniels. A multiple diagnostic imaging approach. J Small Animal Prac. 2008;49:438–443. doi: 10.1111/j.1748-5827.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 5.Von Lesser L. Experimentelles und Klinischeses über Skoliose. Virchows Arch. 1888;113:10–46. doi: 10.1007/BF03015465. [DOI] [Google Scholar]
- 6.Nachlas IW, Borden JN. Experimental scoliosis; the role of the epiphysis. Surg Gynecol Obstet. 1950;90:672–680. [PubMed] [Google Scholar]
- 7.Sawin PB, Crary DD. Genetics of skeletal deformities in the domestic rabbit (Oryctolagus cuniculus) Clin Orthop Relat Res. 1964;33:71–90. doi: 10.1097/00003086-196400330-00007. [DOI] [PubMed] [Google Scholar]
- 8.Carrey M. Genetics of scoliosis in chicken. J Hered. 1981;72:6–12. doi: 10.1093/oxfordjournals.jhered.a109428. [DOI] [PubMed] [Google Scholar]
- 9.Janssen MA, de Wilde RF, Kouwenhoven JM, Castelein RM. Experimental models in scoliosis research: a review of the literature. Spine. 2011;11:347–358. doi: 10.1016/j.spinee.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Mc Ewen GD. Experimental scoliosis. Clin Orthop Relat Res. 1973;93:69–74. doi: 10.1097/00003086-197306000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Raggio CL, Giampietro PF, et al. A novel locus for adolescent idiopathic scoliosis on chromosome 12p. J Orthop Res. 2009;27(10):1366–1372. doi: 10.1002/jor.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giampeietro PF, Raggio CL et al (2006) DLL3 as a candidate gene for vertebral malformations. Am J Med Genet Part A. 140(22):2447–2453 [DOI] [PubMed]
- 13.Giampietro PF, Blank RD, et al. Congenital and idiopathic scoliosis. Clinical and genetic aspects. Clin Med Res. 2003;1(2):125–136. doi: 10.3121/cmr.1.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco G, Coulton GR, et al. The kyphoscoliotic (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle specific protein. Hum Mol Genet. 2001;10(1):9–16. doi: 10.1093/hmg/10.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Gorman KF, Tredwell SJ, Breden F. The mutant guppy syndrome curveback as a model for human heritable spinal curvature. Spine. 2007;32(7):735–741. doi: 10.1097/01.brs.0000259081.40354.e2. [DOI] [PubMed] [Google Scholar]
- 16.Gorman KF, Handrigan GR, et al. Structural and micro-anatomical changes in vertebrae associated with idiopathic-type spinal curvature in the curveback guppy model. Scoliosis. 2010;7:5–10. doi: 10.1186/1748-7161-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorman KF, Christians JK, et al. A major QTL controls susceptibility to spinal curvature in the curveback guppy. BMC genet. 2011;12(1):16. doi: 10.1186/1471-2156-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu XS, Tang NL, et al. Melatonin receptor 1B(MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine. 2007;32(16):1748–1753. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]
- 19.Thillard MJ. Vertebral column deformities following epiphysectomy in the chick. CR Hebd seances Acad Sci. 1959;248:1238–1240. [PubMed] [Google Scholar]
- 20.Dubousset J, Queneau P, Thillard M. Experimental scoliosis induced by pineal and diencephalic lesions in young chickens: its relation with clinical findings. Orthop Trans. 1983;7:7–12. [Google Scholar]
- 21.Machida M, Dubousset J, Imamura Y, et al. Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patient with idiopathic scoliosis. J Pediatr Ortop. 1994;14:329–335. doi: 10.1097/01241398-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Machida M, Dubousset J, Imamura Y, et al. Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Joint Surg Br. 1995;77:134–138. [PubMed] [Google Scholar]
- 23.Machida M, Murai I, et al. Pathogenesis of idiopathic scoliosis. Experimental study in rats. Spine. 1999;24:1985–1989. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 24.O’Kelly C, Wang X, et al. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine. 1999;24:35–43. doi: 10.1097/00007632-199901010-00009. [DOI] [PubMed] [Google Scholar]
- 25.Cheung KM, Wang T, et al. The effect of pinaelectomy on scoliosis development in young nonhuman primates. Spine. 2005;30:2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 26.Day GA, Mc Phee IB, et al. Idiopathic scoliosis and pineal lesions in Australian children. J Orthop Surg (Hong-Kong) 2007;15(3):327–333. doi: 10.1177/230949900701500318. [DOI] [PubMed] [Google Scholar]
- 27.Grivas TB, Savvidou OD. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis. 2007;2:6. doi: 10.1186/1748-7161-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyama J, Murai I, et al. Bipedal ambulation induces experimental scoliosis in C57BL/6J mice with reduced plasma and pineal melatonin levels. J Pineal Res. 2006;40:219–224. doi: 10.1111/j.1600-079X.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 29.Machida M, Dubousset J, Yamada T, Kimura J, Saito M, Shiraishi T, Yamagishi M (2006) Experimental scoliosis in melatonin-deficient C57BL/6J mice without pinealectomy. J Pineal Res 41(1):1–7 [DOI] [PubMed]
- 30.Akel I, Demirkiran G, et al. The effect of calmodulin antagonist on scoliosis: bipedal C57BL/6J mice model. Eur Spine J. 2009;18:499–505. doi: 10.1007/s00586-009-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardo M, Bettini N, et al. The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis (AIS) Eur Spine J. 2011;20(1):S68–S74. doi: 10.1007/s00586-011-1750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Qiu Y, et al. Histomorphological study of the spinal growth plates from the convex side and the concave side in adolescent idiopathic scoliosis. J Orthop Surg. 2007;2:19. doi: 10.1186/1749-799X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacEwen GD. Experimental scoliosis. Isr J Med Sci. 1973;6:714–718. [PubMed] [Google Scholar]
- 34.Suk SI, Song HS, et al. Scoliosis induced by anterior and posterior rhizotomy. Spine. 1989;14:692–697. doi: 10.1097/00007632-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Pincott JR, Taffs LF. Experimental scoliosis in primates: a neurological cause. J Bone Joint Surg Br. 1982;64:503–507. doi: 10.1302/0301-620X.64B4.6284765. [DOI] [PubMed] [Google Scholar]
- 36.Pincott JR, Davies JS, et al. Scoliosis caused by section of dorsal spinal nerve roots. J Bone Joint Surg Br. 1984;66:27–29. doi: 10.1302/0301-620X.66B1.6693473. [DOI] [PubMed] [Google Scholar]
- 37.Lambert FM, Malinvaud D, et al. Vestibular asymmetry as the cause of idiopathic scoliosis: a possible answer from Xenopus. J Neurosci. 2009;29:12477–12483. doi: 10.1523/JNEUROSCI.2583-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Waele C, Graf W, et al. A radiological analysis of the postural syndromes following hemilabyrinthectomy and selective canal and otolith lesions in the guinea pig. Exp Brain Res. 1989;77(1):166–182. doi: 10.1007/BF00250579. [DOI] [PubMed] [Google Scholar]
- 39.Dieringer N. “Vestibular compensation”: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol. 1995;46(2–3):97–129. [PubMed] [Google Scholar]
- 40.Mason RM, Palfrey AJ. Intervertebral disc degeneration in adult mice with hereditary kyphoscoliosis. J Orthop Res. 1984;2(4):333–338. doi: 10.1002/jor.1100020405. [DOI] [PubMed] [Google Scholar]
- 41.Blanco G, Coulton GR, et al. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet. 2001;10(1):9–16. doi: 10.1093/hmg/10.1.9. [DOI] [PubMed] [Google Scholar]
- 42.Roaf R. The basic anatomy of scoliosis. J Bone Joint Surg Br. 1966;48:786–792. [PubMed] [Google Scholar]
- 43.Dickson RA. The aetiology of spinal deformities. Lancet. 1988;331:1151–1155. doi: 10.1016/S0140-6736(88)91963-0. [DOI] [PubMed] [Google Scholar]
- 44.Poussa M, Schlenzka D, Ritsilä V. Scoliosis in growing rabbits induced with an extension splint. Acta Ortop Scand. 1991;62:136–138. doi: 10.3109/17453679108999241. [DOI] [PubMed] [Google Scholar]
- 45.Hakkarainen S. Experimental scoliosis: production of structural scoliosis by immobilization of young rabbits in a scoliotic position. Acta Orthop Scand Suppl. 1981;192:1–57. doi: 10.3109/ort.1981.52.suppl-192.01. [DOI] [PubMed] [Google Scholar]
- 46.Wynarsky G, Schultz A. Effects of age and sex on the external induction of scoliosis in rats. Spine. 1987;12(10):974–977. doi: 10.1097/00007632-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Mente PL, Stokes IA, et al. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine. 1997;22:1292–1296. doi: 10.1097/00007632-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 48.Stokes IA, Spence H, et al. Mechanical modulation of vertebral body growth. Implications for scoliosis progression. Spine. 1996;21:1162–1167. doi: 10.1097/00007632-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 49.Aronsson DD, Stokes IA, et al. The role of remodeling and asymmetric growth in vertebral wedging. Stud Health Technol Inform. 2010;158:11–15. [PubMed] [Google Scholar]
- 50.Stokes IA, Mc Bride CA, et al. Intervertebral disc changes in an animal model representing altered mechanics in scoliosis. Stud Health Tech Inform. 2008;140:273–277. [PubMed] [Google Scholar]
- 51.Kalleimeier PM, Buttermann GR, et al. Validation, reliability, and complications of a tethering scoliosis model in the rabbit. Eur Spine J. 2006;15:449–456. doi: 10.1007/s00586-005-1032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarwark JF, Dabney KW, et al. Experimental scoliosis in the rat. I. Methodology, anatomic features and neurologic characterization. Spine. 1988;13:466–471. doi: 10.1097/00007632-198805000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Somerville EW (1952) Rotational lordosis; the developmentof single curve. J Bone Joint Surg Br 34-B:421–427 [DOI] [PubMed]
- 54.Liu L, Zhu Y, et al. The creation of scoliosis by scapula-to-contralateral ilium tethering procedure in bipedal rats: a kyphoscoliosis model. Spine. 2011;36(17):1340–1349. doi: 10.1097/BRS.0b013e3181f3d164. [DOI] [PubMed] [Google Scholar]
- 55.Sevastikoglou JA, Aaro S, et al. Experimental scoliosis in growing rabbits by operations on the rib cage. Clin Orthop Related Res. 1978;136:282–286. [PubMed] [Google Scholar]
- 56.Sevastik B, Agadir M, et al. Vascular changes in the chest wall after unilateral resection of the intercostal nerves in the growing rabbit. J Orthop Res. 1990;8:283–290. doi: 10.1002/jor.1100080218. [DOI] [PubMed] [Google Scholar]
- 57.Sevastik J, Agadir M, Sevastik B (1990) Effects of rib elongation on the spine. II. Correction of scoliosis in the rabbit. Spine 15(8):826–829 [PubMed]
- 58.Langenskiold A, Michelsson JEA. Experimental progressive scoliosis. Acta Orthop Scand Suppl. 1962;59:1–26. [PubMed] [Google Scholar]
- 59.Alexander MABunch WH, et al. Can experimental dorsal rhizotomy produce scoliosis? J Bone Joint Surg Am. 1972;54:1509–1513. [PubMed] [Google Scholar]
- 60.Barrios C, Tuñón MT, De Salis JA, Beguiristain JL, Cañadell J (1987) Scoliosis induced by medullary damage: an experimental study in rabbits. Spine (Phila Pa 1976) 12(5):433–439 [DOI] [PubMed]
- 61.Robin GC, Stein H (1975) Experimental scoliosis in primates. Failure of a technique. J Bone Joint Surg Br 57(2):142–145 [PubMed]
- 62.Thomas S, Dave PK. Experimental scoliosis in monkeys. Acta Orthop Scand. 1985;56:43–46. doi: 10.3109/17453678508992978. [DOI] [PubMed] [Google Scholar]
- 63.Metha HP, Snyder BD, et al. Expansion thoracoplasty improves respiratory function in a rabbit model of postnatal pulmonary hypoplasia: a pilot study. Spine. 2010;35(2):153–161. doi: 10.1097/BRS.0b013e3181c4b8c7. [DOI] [PubMed] [Google Scholar]
- 64.Olson JC, Kurek KC, et al. Expansion thoracoplasty affects lung growth and morphology in a rabbit model: a pilot study. Clin Orthop Relat Res. 2011;469(5):1375–1382. doi: 10.1007/s11999-011-1807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beguiristain JL, De Salis J, et al. Experimental scoliosis by epiphysiodesis in pigs. Int Orthop. 1980;3:317–321. doi: 10.1007/BF00266028. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Sucato DJ, et al. Neurocentral synchondrosis screw epiphysiodesis of the neurocentral synchondrosis. Production of idiopathic-like scoliosis in an immature animal model. J Bone Joint Surg Am. 2008;90:2460–2469. doi: 10.2106/JBJS.G.01493. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Sucato DJ. Neurocentral synchondrosis screws to create and correct experimental deformity: a pilot study. Clin Orthop Relat Res. 2010;469(5):1383–1390. doi: 10.1007/s11999-010-1587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Sucato DJ (2011) Anterior vs posterior approach of neurocentral cartilage hemiepiphysiodesis to create experimental scoliosis. 19th IMAST congress Copenhagen
- 69.Newton PO, Farnsworth CL, et al. Spinal growth modulation with an anterolateral flexible tether in an immature bovine model: disc health and motion preservation. Spine. 2008;33:724–733. doi: 10.1097/BRS.0b013e31816950a0. [DOI] [PubMed] [Google Scholar]
- 70.Braun JT, Ogilvie JW, Akyuz E, Brodke DS, Bachus KN, Stefko RM (2003) Experimental scoliosis in an immature goat model: a method that creates idiopathic‐type deformity with minimal violation of the spinal elements along the curve. Spine (Phila Pa 1976) 28(19):2198–2203 [DOI] [PubMed]
- 71.Braun JT, Ogilvie JW. Experimental scoliosis in an immature goat model: a method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine. 2003;28:2198–2203. doi: 10.1097/01.BRS.0000085095.37311.46. [DOI] [PubMed] [Google Scholar]
- 72.Braun JT, Ogilvie et al (2006) Creation of an experimental idiopathic-type scoliosis in an immature goat model using a flexible posterior asymmetric tether. Spine 31(13):1410–1414 [DOI] [PubMed]
- 73.Schwab F, Patel A, et al. A porcine model for progressive thoracic scoliosis. Spine. 2009;34:E397–E404. doi: 10.1097/BRS.0b013e3181a27156. [DOI] [PubMed] [Google Scholar]
- 74.Zhang YG, Zheng GQ, et al. Scoliosis model created by pedicle screw tethering in an immature goats: the feasibility, reliability, and complications. Spine. 2009;34:2305–2310. doi: 10.1097/BRS.0b013e3181b1fdd0. [DOI] [PubMed] [Google Scholar]
- 75.Odent T, Cachon T. Porcine model of early onset scoliosis based on animal growth created with mini-invasive posterior offset tethering: a preliminary report. Eur Spine J. 2011;20(11):1869–1876. doi: 10.1007/s00586-011-1830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel A, Schwab F, et al. Does removing the spinal tether in a porcin scoliosis model result in persistent deformity? A pilot study. Clin Orthop Relat Res. 2011;469(5):1368–1374. doi: 10.1007/s11999-010-1750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rivard C (1982) Chir Pedia
- 78.Farley FA, Hall J, et al. Characteristics of congenital scoliosis in a mouse model. J Pediatr Orthop. 2006;26:341–346. doi: 10.1097/01.bpo.0000203011.58529.d8. [DOI] [PubMed] [Google Scholar]
- 79.Fei Q, Wu Z, et al. The association analysis of TBX6 polymorphism with susceptibility to congenital scoliosis in a Chinese Han population. Spine. 2010;35(9):98308. doi: 10.1097/BRS.0b013e3181bc963c. [DOI] [PubMed] [Google Scholar]
- 80.Goshu E, Jin H, et al. Sim@ mutants have developmental defects not overlapping with those of Sim1 mutants. Mol Cell Biol. 2002;22(12):4147–4157. doi: 10.1128/MCB.22.12.4147-4157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seifert J, Bell, et al. Characterization of a novel bidirectional distraction spinal cord injury animal model. J Neurosci Meth. 2011;197(1):97–103. doi: 10.1016/j.jneumeth.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Salehi LB, Mangino M, et al. Assignment of a locus for autosomal dominant idiopathic scoliosis to human chromosome 17p11. Human Genet. 2002;111:401–404. doi: 10.1007/s00439-002-0785-4. [DOI] [PubMed] [Google Scholar]
- 83.Mahood JK, Jiang H, et al. Melatonin levels in idiopathic scoliosis. Spine. 1997;21:1974–1978. doi: 10.1097/00007632-199609010-00006. [DOI] [PubMed] [Google Scholar]
- 84.Sahlstrand T, Petruson B. A study of labyrinthine function in patietns with adolescent idiopathic scoliosis. An electro-nystagmographic study. Acta Orthop Scan. 1979;50:759–769. doi: 10.3109/17453677908991307. [DOI] [PubMed] [Google Scholar]
- 85.Mallau S, Bollini G, et al. Locomotor skills and balance strategies in adolescents idiopathic scoliosis. Spine. 2007;32:E14–E22. doi: 10.1097/01.brs.0000251069.58498.eb. [DOI] [PubMed] [Google Scholar]
- 86.Wiener-Vacher SR, Mazda K. Asymmetric otolith vestibulo-occular responses in children with idiopathic scoliosis. J Pediatr. 1998;132(6):1028–1032. doi: 10.1016/S0022-3476(98)70403-2. [DOI] [PubMed] [Google Scholar]
- 87.Sahlstrand T, Petruson B. A study of labyrinthine function in patients with idiopathic scoliosis. I. An electro-nystagmographic study. Acta Ortop Scand. 1979;50(6):759–769. doi: 10.3109/17453677908991307. [DOI] [PubMed] [Google Scholar]
- 88.Alini M, Eisenstin SM, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine. 2008;J17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White AA, Panjabi MM (1990) Clinical biomechanics of the spine. 2nd edn. JB Lippincott Company
- 90.Nachlas IW, Borden JN. Experimental scoliosis; the role of the epiphysis. Surg Gynecol Obstet. 1950;90(6):672–680. [PubMed] [Google Scholar]
- 91.Braun JT, Ogilvie JW, et al. Fusionless scoliosis correction using a shape memory alloy staple in the anterior thoracic spine of the immature goat. Spine. 2004;29:1980–1989. doi: 10.1097/01.brs.0000138278.41431.72. [DOI] [PubMed] [Google Scholar]
- 92.Braun JT, Akyuz E, et al. Three-dimensional analysis of 2 fusionless scoliosis treatment: a flexible ligament tether versus a rigid-shape memory alloy staple. Spine. 2006;31:262–268. doi: 10.1097/01.brs.0000197569.13266.fe. [DOI] [PubMed] [Google Scholar]
- 93.Lafage V, Schwab V et al (2011) Three dimensions corrections of scoliosis in a porcine model with an anterolateral tethering correction surgical device. 19th IMAST congress Copenhagen
- 94.Wilke HJ, Kettler et al (1999) Is the lumbar sheep spine an adequate model for the human spine? A comparison of biomechanical properties, macroscopic and microscopic anatomy and bone mineral density. In proceedings of the 26th annual meeting, Hawaii, p 24
- 95.D’Aout K, Aerts P, et al. Segment and joint angles of hind limb during bipedal and quadrupedal walking of the bonobo. Am J Phys Anthrop. 2002;119(37):51. doi: 10.1002/ajpa.10112. [DOI] [PubMed] [Google Scholar]
- 96.Castelein RM, van Dieen JH, et al. The role of dorsal shear forces in the pathogenesis of adolescent idiopathic scoliosis: a hypothesis. Med Hypothesis. 2005;65:501–508. doi: 10.1016/j.mehy.2005.03.025. [DOI] [PubMed] [Google Scholar]