Abstract
Hormonal influences on the organization of behavior are apparent to neuroendocrinologists but under-examined in relation to childhood and adolescent mental disorders. A central mystery in the field of developmental psychopathology is the preferential male vulnerability to behavior disorders in childhood and female vulnerability to emotional disorders in adolescence. Relative neglect of a hormonal explanation may be due to lack of simple or unifying conceptual paradigms to guide studies. This paper seeks to stimulate research in this area by drawing upon clinical psychology and neuroscience literatures to offer a heuristic paradigm for clinical research. Two syndromes are selected here for illustration: Attention-Deficit/Hyperactivity Disorder (ADHD) and Major Depressive Disorder (MDD), because they have opposite gender risk profiles. Two guiding theories are evaluated. First, prenatal organizational effects of testosterone may modulate striatally-based dopaminergic circuits in such a way as to place boys at greater risk for early developing inattention and disruptive behavioral disorders. Second, activational effects of estradiol at puberty may modulate amygdalar and other circuitry, with particular effects on serotoninergic pathways, in such a way as to place girls at greater risk for internalizing and mood disorders. Hypotheses from these theories are evaluated based on the current available literature, and limitations of, and future directions for, this literature are discussed.
Keywords: hormones, Attention-Deficit/Hyperactivity Disorder (ADHD), Major Depressive Disorder (MDD), organizational, activational
From a neuroscience perspective, the major role that gonadal hormones play in the organization of the brain and behavioral systems is well-known (e.g., Phoenix, Goy, Gerall & Young, 1959). Hormones have broad-based, and yet specific, ramifications for upstream influences on brain organization and plasticity (Morris, Jordan, & Breedlove, 2004), as well as for downstream behavioral expression at key points in development. In the clinical field of mental disorders, a fundamental puzzle has been characteristic sex differences in the prevalence of psychiatric disorders. Males are more vulnerable to the development of childhood-onset behavior disorders (i.e., Attention-Deficit/Hyperactivity Disorder [ADHD], Oppositional Defiant Disorder [ODD], Conduct Disorder, Autism, as well as learning disabilities). Females are more vulnerable to the development of emotional disorders having later onsets, with modal onset ages in adolescence (i.e., major depressive disorder [MDD], dysthymia, several anxiety disorders, as well as eating disorders; APA, 2000). This phenomenon has led to frequent invocation of sex hormones in relation to these disorders (Arnold, 1996; Geschwind & Galaburda, 1985; Holden, 2005; Zahn-Waxler, Crick, Shirtcliff, & Woods, 2006).
Gonadal hormones may indeed play a role in these sex-biased prevalence rates. Yet surprisingly little research has explored how exactly they might do so. One obstacle has been the lack of a detailed, organizing theory to guide such research in an integrated fashion. Yet some heuristic models of hormone effects on psychopathology exist. For example, an “extreme male brain” theory of autism has been advanced, positing that the behaviors seen in autism are exaggerations of typical sex differences and that exposure to high levels of prenatal testosterone is a risk factor for autism (Knickmeyer & Baron-Cohen, 2005; Knickmeyer et al., 2005). A hormonal model of eating disorders posits that lower levels of prenatal testosterone exposure increases risk for disordered eating (Culbert et al., 2008), while increasing estradiol levels during and after puberty moderate phenotypic and genetic risk for eating disorders (Klump et al., 2006; 2007; 2008). These models illustrate the type of approach we advocate. However, even these models lack an integrative mechanistic explanation of how hormones affect psychopathology or how they might account for the more generalized sex different patterns seen across psychopathology.
In this vein, this essay draws upon themes in basic neuroscience and clinical science of mental disorders to outline likely specific hormonal mechanisms relevant to two major kinds of psychopathology and to provide a basic framework to stimulate research on this problem. To constrain the scope of the discussion, we frame two fundamental theories organized around two common mental disorders: ADHD and MDD. Particular nosological features of these two syndromes are important to bear in mind when considering how hormones may participate in their development. As well, a general perspective on hormonal action is necessary to set the stage for this discussion.
Key Points about Hormonal Effects
There are several categories of gonadal hormones. Two of these categories are particularly relevant to discussion of hormonal effects on psychopathology: androgens and estrogens. Androgens, or C19 steroid hormones, include testosterone, androstenedione, a biochemically reduced form of testosterone 5α- and 5β-dihyrotestosterone (DHT), and dehydroepiandrosterone (DHEA). Estrogens, or C18 steroid hormones, include 17β-estradiol, estrone, and estriol. While other hormones (e.g., progestins) may have effects on psychopathology, androgens and estrogens are the focus of this essay because they have received the most attention in research on hormonal effects on psychopathology. While androgens and estrogens are the broad focus of this review, it should be noted that most work to date has emphasized the specific androgen testosterone and the specific estrogen estradiol.
The effects of these sex hormones fall into two major categories. It has long been recognized that exposure to androgenic hormones early in life, usually around the time of birth up until the fourth month of postnatal life, have permanent, or “organizational,” masculinizing effects on the nervous system and behavior (Phoenix et al., 1959). As a simplifying heuristic, in humans, testosterone is a prominent androgenic hormone that exerts organizational effects. In contrast, exposure to hormones later in development, such as during adolescence, tends to have a more transient effect on neural structure and behavior, which has been characterized as an “activational” effect (although recent work also suggests some organizational effects during this period; Sisk & Zehr, 2005). Although activational effects can be due to many different kinds of hormones, estrogens are commonly implicated. Both organizational and activational effects also may occur in a dynamic fashion over the life span (Arnold & Breedlove, 1985). For the present discussion, hormonal effects are divided into two developmental periods: organizational effects in perinatal development and activational effects at the pubertal transition. Taking this perspective, hypotheses about sex differences in psychopathology naturally arise due to the fact that sex differences in psychopathology prevalence rates primarily arise during, or shortly after, these key developmental periods.
Key Points about Developmental Psychopathology
The current version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; APA, 2000) organizes mental disorders into two major categories: “externalizing” and “internalizing.” So-called “externalizing” disorders include ADHD, ODD, Conduct Disorder, and Antisocial Personality Disorder. “Internalizing” disorders include MDD, dysthymia, and anxiety disorders. For this review, we focus on one exemplar externalizing and one exemplar internalizing disorder: ADHD, which generally has a childhood onset, and MDD which generally has an adolescent onset.
Turning first to ADHD, it is a behavioral syndrome characterized by excessive activity, impulsivity, and/or off-task or “inattentive-disorganized” behavior, is typically identified in early to middle childhood, and is more common in boys (APA, 2000). Youth with this disorder often, but not invariably, exhibit learning and cognitive problems and experience chronic maladjustment. Clinical heterogeneity has long been a challenge to studying the etiology of this disorder. In the DSM-IV-TR (APA, 2000), ADHD was divided into three subtypes: hyperactive-impulsive, inattentive, and combined. However, these subtypes have failed several fundamental tests of validity, perhaps most crucially lack of temporal stability (Lahey et al., 2005). As a result, the subtypes’ validity in their current form has been called into question and numerous alternatives have begun to be evaluated. On the other hand, the behavioral dimensions that comprise ADHD, inattention-disorganization and hyperactivity-impulsivity, have received extensive ongoing validation. They prove robust in large factor analytic studies (Pillow et al., 1998), are highly heritable (Larsson et al., 2006), and are quite stable over time (Lahey et al., 2005; Price et al., 2005).
Building on those findings, theorists have begun to ask whether partially distinct neurobiological determinants may shape the behavioral dimensions of inattention and hyperactiviety-impulsivity (e.g., Barkley, 1997; Nigg, 2005; Sonuga-Barke, 2005). That line of thought has suggested that there may be a distinction between breakdowns in cognitive control supported by dopaminergic circuits (Holroyd & Coles, 2002) and breakdowns in incentive based responding supported by limbic circuitry, converging in the ADHD syndrome (Sonuga-Barke, 2005). Cognitive control may be a mechanism of ADHD that can shed light on these neurobiological determinants of ADHD. Cognitive control is defined as the ability to direct attention and behavior in an effortful manner (i.e., requiring mental resources) and is related to neuropsychological measures of executive control that require the ability to regulate cognition and action in order to attain a goal.
Turning next to MDD, it is characterized by depressed mood (in children, sometimes exhibited as irritability), low energy, negative thoughts, hopelessness, loss of interest or pleasure in previously enjoyed activities, and sleep and appetite changes. MDD has recently begun to be recognized in young children. However, it remains comparatively rare in childhood, with equal risk to boys and girls. At puberty, however, major depression exhibits a dramatic increase in incidence and becomes more common in girls (APA, 2000; Hankin et al., 1998). Several theories have been advanced to explain this phenomenon, including differential physical development and socialization of girls and boys in adolescence (Nolen-Hoeksema, 1994) and abnormalities in serotonin neurotransmission (Cameron, 2004b). However, differential hormonal exposure at puberty remains an important possibility (Angold et al., 1999).
Negative affect (i.e., sadness, anger, anxiety, but not severe or specific enough to be identified as MDD) and negative mood (i.e., a more chronic manifestation of negative affect) are powerful liability markers for depression (Kendler et al., 2006). Negative affect also exhibits sex differences favoring females, shows a rise during puberty, and exhibits changes across the menstrual cycle (Forbes, Williamson, Ryan, & Dahl, 2004).
General Plan for the Review
We first review briefly what is known about organizational hormonal effects on neural development and behavior. Based on this information, we propose a guiding theory of hormonal effects on ADHD. Next, based on a substantial literature review, we critically review what is known about organizational hormonal effects on ADHD and cognitive control mechanisms in order to critically evaluate hypotheses derived from that theory. We follow the same logic with activational hormonal effects, beginning with their effects on neural circuitry and behavior, proceeding to a guiding theory of hormonal effect on MDD, and ending with critical review of what can be concluded about activational hormonal effects on MDD and negative affect in order to evaluate key hypotheses. Finally, we discuss limitations of previous work and provide guidelines for future work on hormones and psychopathology.
Prenatal, Organizational Hormonal Effects
Neural Development
The organizational hypothesis states that prenatal exposure to hormones during a critical period of development permanently affects the developing brain, altering behavior even into adulthood (Phoenix et al., 1959). After genetic (i.e., chromosomal) sex is determined at fertilization, a gene on the Y chromosome, the sex-determining region of the Y chromosome (Sry), causes the gonads to develop into testes (Breedlove & Hampson, 2002; Nelson, 2005b). The testes release several androgenic steroid hormones including testosterone that masculinize the developing body and brain during the prenatal period. Ovaries release little or no hormone prenatally. In girls, the relative absence of androgens is associated with development of a feminine body and brain (Breedlove & Hampson, 2002). Thus, genetic sex is linked to gonadal sex, which is linked to hormonal sex. Hormonal sex is defined as the ratio of circulating estrogen-to-androgen, which is higher in most prenatal and mature females than in males. Sexual differentiation, a term used to discuss how humans (and animals) develop into males and females, is integrally tied with organizational effects.
Sexual Differentiation of Neural Circuitry and Behavior
As outlined by Hines (2002; Collaer & Hines, 1995), three theories, which are not mutually exclusive, describe sexual differentiation: classical, active feminization, and gradient. The classical theory states that androgens cause masculine development, whereas their absence is linked to feminine development. The active feminization model suggests that ovarian hormones also play a role, actively promoting feminization of neural circuitry and behavior. The gradient model suggests that hormones influence not just behavioral differences between sexes (e.g., in cognition, childhood play, and aggression), but also behavioral variation within each sex. That is, females exposed to higher levels of androgen in utero may exhibit more male-like characteristics (e.g., increased spatial ability) than females exposed to lower levels of androgen. We elaborate briefly on the classical model because it has guided most research on sexual differentiation.
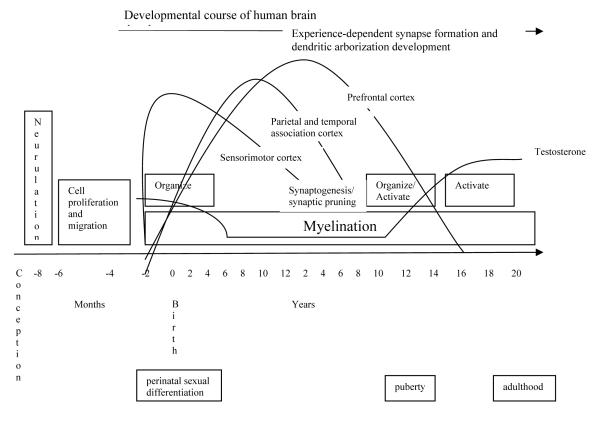
Animal research (mainly with rats) on normal prenatal development suggests that the masculine pattern of exposure to high levels of testosterone in late gestation and early postnatal development may influence neural development via several mechanisms, listed in Table 1 and depicted in Figure 1. As the table lists, high levels of testosterone may have their effects through two routes: direct mechanisms, or “upstream” effects, and indirect mechanisms, or “downstream” effects. In regard to direct or “upstream” effects, high levels of testosterone lead to increased cell death within the right hemisphere of the brain. In addition, high levels of testosterone lead to increased neural lateralization (i.e., specialization of each brain hemisphere) and slower development of the brain (Geschwind & Galaburda, 1985; Goodman, 1991; Lyon & Gadisseux, 1991). Degree of lateral specialization has apparent clinical consequences. For example, women recover language more frequently than men following focal stroke (Inglis, 1982; Yager et al., 2005).
Table 1.
Suggested Organizational Testosterone Effects on Brain Development
| “Upstream” Effects |
| Increased cell proliferation and cell death |
| Slower prenatal development |
| Increased lateralization |
| Modulation of neurotransmission |
| Interaction with genotype |
| “Downstream” Effects |
| Influence on selection of environmental niche |
| Effect on elicitation of environmental response |
Figure 1.
Hormonal and Neural Development
Data from Casey, Tottenham, Liston, & Durston, 2005 and Sisk & Zehr, 2005
Because of the prolonged consolidation of their neural development, males may be more vulnerable to environmental insult and have more variable behavioral outcomes (Lyon & Gadisseux, 1991; Morris, Jordan, & Breedlove, 2004). The effects of early insult such as exposure to toxins and contaminants, anoxia, and poor nutrition may have a stronger effect on males than on females, perhaps for this reason (Goodman, 1991; Levy & Heller, 1992). This idea was presaged in Geschwind’s (1985) theory of cerebral lateralization. He suggested that males are at increased risk for learning disorder and hyperactivity because fetal testosterone slows normal structural development in the left hemisphere of the brain, modifying cerebral lateralization (McManus & Bryden, 1991) and making males vulnerable for a longer period of time to insult and to structural abnormalities in the left hemisphere. Overall, three direct developmental mechanisms related to testosterone appear to increase male vulnerability to subtle insult in the pre- and perinatal period: increased cell proliferation and death, slower prenatal brain development, and increased lateralization of brain function.
Testosterone may have modulatory effects on neural development. For example, testosterone may modulate neurotransmitter activity prenatally. When pregnant rats were exposed to restraint stress, their male pups had reduced testosterone levels, as well as increased dopamine levels in the striatum (Gerardin et al., 2005). This finding suggests that testosterone and dopamine interact in response to developmental events. Testosterone may interact with genotype to influence neural development as well as behavior. A further possibility is that testosterone level influences neural development indirectly via the organism’s selection of experiential niches and the elicitation of environmental response, called “downstream” effects (Morris, Jordan, & Breedlove, 2004). Due to space constraints, we focus herein on relatively direct (“upstream”) effects mediated via alterations in neural circuitry (Table 1).
Sexually Dimorphic Brain Structures, Brain Function, and Behavior
Perhaps the most important upstream effect of hormones, in terms of their eventual influence on behavior, is via their effects on brain structure and brain function. Sexual dimorphism in the brain has been widely observed in animals and linked to the organizational effects of androgens (Breedlove & Hampson, 2002; Tobet & Fox, 1992). Further, in rodents, these dimorphisms are associated with sex differences in behavior, such as aggression, that are analogous to behaviors that appear during early childhood in humans (Morris, Jordan, & Breedlove, 2004; Tobet & Fox, 1992).
One sexually dimorphic neural structure is the neural pathway between the two cortical hemispheres. The corpus callosum (i.e., the primary structure through which synaptic communication between the two hemispheres of the brain is accomplished) and anterior commissure are generally larger in females than in males (Breedlove & Hampson, 2002; Hines, 2002; Nelson, 2005b). The amygdala also may be sexually dimorphic (Tobet & Fox, 1992); the medial nucleus appears to be larger in males than in females. Sex differences in neural structure have been identified in recent longitudinal neuroimaging studies in humans as well, with females showing greater cortical thickness in posterior, temporal and inferior parietal brain regions and greater percentage of gray matter than males (Cosgrove, Mazure, & Staley, 2007; Sowell et al., 2007). Further, imaging studies indicate that human females appear to reach peak gray matter volumes one to two years earlier than males (Rapoport & Gogtay, 2008), whereas males exhibit greater overall brain volume and a higher percentage of white matter (Cosgrove et al., 2007).
Sexual dimorphism in brain function also has been observed in animals and humans. For example, human females appear to display less lateralization (i.e., specialization) of cortical function than do males, illustrated by a lower prevalence of left-handedness (Tobet & Fox, 1992). Becker (1999) found sex differences in the development of dopaminergic function (i.e., a neurotransmitter involved in higher order cognition and behavior) in the striatum and nucleus accumbens in rats. In female rats, extracellular striatal dopamine varies across the estrous cycle such that higher levels of estrogen are related to more amphetamine-induced dopamine release in the striatum. Estrogen and progesterone appear to modulate dopamine in the striatum and nucleus accumbens in females but not in males (Xiao & Becker, 1994). Sex differences also have been noted in the pattern of dopamine receptor density in the striatum, the nucleus accumbens, and the prefrontal cortex during juvenile development in rats, with males exhibiting a much higher rate of receptor increase during early development than females (Andersen & Teicher, 2000).
Cosgrove et al.’s (2007) review noted additional sex differences in neural function in humans. Global cerebral blood flow is higher in women than in men. Sex differences also have been noted in neurotransmission. Whole blood serotonin levels are higher in women, whereas men synthesize serotonin more quickly than women. Dopaminergic function is enhanced in women via increased dopamine transporter availability and higher striatal presynaptic dopamine synthesis. Sex differences in behavior, many of which appear across species, therefore may be influenced by hormones. Boys usually exhibit more rough-and-tumble play and more aggression as children, and less interest in parenting as adults (Collaer & Hines, 1995; Hines, 2002).
Theories of Hormonal Mechanisms of ADHD
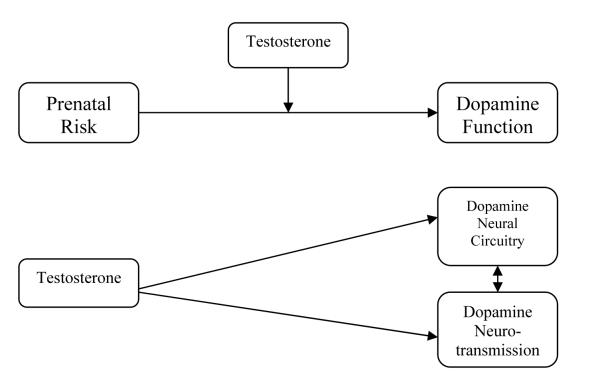
Based on the above review of literature on organizational hormonal effects on neurobiology and behavior, two theories of organizational effects on ADHD were postulated. The first theory states that gonadal hormones may modulate the processes that guide the development of dopaminergic circuitry, influencing corresponding deficits in cognitive control and reward processes in ADHD. High levels of testosterone may affect dopaminergic neural circuitry by slowing down neural development globally and making the dopaminergic components of the brain vulnerable for a longer period of time during prenatal development (e.g., Morris, Jordan, & Breedlove, 2004). In this way, prenatal testosterone levels may moderate the relationship between prenatal risk factors (including genes, contaminants, low birth weight, maternal smoking, etc.) and the developing prenatal neurobiology implicated in ADHD (see Figure 2a). In the second theory of hormonal mechanisms of ADHD, gonadal hormones may act directly on the prenatal development of dopaminergic neural circuitry and dopamine function in the nucleus accumbens, striatum, and prefrontal cortex via its masculinizing effects, which are then evidenced by corresponding deficits in cognitive control and reward processes (see Figure 2a). High levels of testosterone may increase risk for ADHD symptoms via a maturational delay in the development of dopaminergic innervation and metabolism, as well as increased lateralization of underlying dopaminergic neural circuitry and increased reuptake of dopamine neurotransmission (Andersen & Teicher, 2000).
Figure 2a.
Potential Prenatal Testosterone Influence on Dopamine Neurotransmission in ADHD
These theories lead to several specific hypotheses. First, a between-sex difference favoring boys should be evident in the prevalence rate of ADHD, and boys should exhibit more impaired cognitive control than girls. Second, ADHD symptoms and cognitive control should be positively correlated with prenatal testosterone exposure between and within the sexes. Third, prenatal testosterone levels should impact dopaminergic neural circuitry.
We will now subject these hypotheses to empirical test based on the current literature on organizational hormone effects on ADHD and cognitive control. The literature evaluated in the following sections was obtained via searches conducted using PsycInfo and MedLine search engines using combinations of the following terms: organizational hormonal effects, androgen, testosterone, sex/gender differences, neural circuitry, digit ratio (and finger-length ratio), animal models, ADHD, cognition, and executive function.
Organizational Hormonal Effects and ADHD
Hypothesis 1: Sex Differences in the Prevalence Rate of ADHD and in Cognitive Control
ADHD exhibits a prominent sex-biased prevalence rate in childhood with a 3:1 ratio favoring boys (APA, 2000). Thus, the organizational hormonal effects of androgens on childhood ADHD are at least plausible. However, findings of sex differences in cognitive control are less consistent. To provide some background, cognitive control and motivated responding are two of the major candidates for core dysfunctions in ADHD, and they rely on the midbrain dopaminergic system (Holroyd & Coles, 2002). Examination of these cognitive or motivational effects in relation to hormones is therefore relevant to the etiological “signal” of hormones on ADHD behavioral symptoms, as these effects may be closer than other behaviors to the brain alterations attributed to hormone effects. (For reviews of operational behavioral and pharmacological probes of these two major response pathways; see Nigg, 2006; Posner, 2004).
Cognitive control is defined as the ability to direct attention and behavior in an effortful manner (i.e., requiring mental resources). It is related to neuropsychological measures of executive control that require the ability to regulate cognition and action in order to attain a goal. Such measures show moderate weakness in groups of children with ADHD versus typically developing children (Willcutt et al., 2005). These measures involve several component operations (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Friedman & Miyake, 2004; Miyake et al., 2000), which may be able to be exploited in isolating etiological effects. It is expected that cognitive control and motivated responding (including reinforcement learning) are closely related. Interplay of bottom-up signals of expectancy violations and top-down control operations likely helps to maintain adaptive behavior (Nigg & Casey, 2005). Cognitive control involves top-down signaling from the prefrontal cortex modulated by dopamine circuitry, whereas reinforcement response involves ascending dopamine circuits from the limbic system that in turn influences prefrontal response. These top-down and bottom-up effects are partially dissociable and both appear to contribute to ADHD (Nigg, 2006; Sonuga-Barke, 2005).
There are well-established sex differences in several intellectual abilities, but sex differences in cognitive control are less explored. Thus, in typically developing samples, girls on average outperform boys on measures of verbal fluency and memory, information processing, fine motor skills, perceptual speed, and spatial organization, while boys outperform girls on measures of visuospatial reasoning and mathematics during middle childhood (Anderson, 2002; Hines, 2002; Waber et al., 2007). Recent research suggests that these differences are due to sex differences in neural efficiency, or less brain activation (i.e., increased neural efficiency) in brighter individuals (Neubauer, Grabner, Fink, & Neuper, 2005).
However, none of those abilities are hypothesized to be core deficits in ADHD. Data on sex differences in cognitive control, one of the abilities thought to be central to ADHD, are much more limited. Two studies of preschoolers are intriguing, however. Overman (2004) examined sex differences in cognitive tasks, such as object reversal, that are thought to depend on the orbital prefrontal cortex. During toddlerhood, boys performed better than girls, an effect attributed to the more rapid maturation of the orbital prefrontal cortex circuitry in males (Overman, 2004). Kerr and Zelazo (2004) noted that three-year-old girls made more disadvantageous choices than boys on a children’s version of the Gambling Task.
Several studies have shown that girls with ADHD, like boys, show weak cognitive control (Castellanos et al., 2000; Rucklidge & Tannock, 2002; Seidman et al., 2005). In one of the few studies that directly compared boys and girls with ADHD on cognitive control measures, no differences emerged (Seidman et al., 2005). However, Nigg et al. (2002) found that cognitive control was impaired in girls with mild and severe ADHD (Inattentive and Combined subtypes of ADHD), but only in boys with severe ADHD (Combined ADHD subtype), suggesting that girls needed more severe impairment in order to exhibit symptoms. Thus, it is unclear whether boys exhibit more severely impaired cognitive control than girls.
Additional studies directly comparing boys and girls while adjusting for normative sex differences in language or spatial ability would be informative, as would clarification of effects at different ages. It also would be helpful to have data that directly address hormonal effects on cognitive control and reinforcement learning, rather than using sex as a proxy. No such studies are extant, to our knowledge. It is striking that so few data are available to assess sex differences in cognitive control. Few studies have examined relations between prenatal testosterone exposure and indicators of the efficiency of central dopamine circuits at the cognitive or neurobiological level. Study of alternative phenotypes of ADHD, including inattention, hyperactivity-impulsivity, and cognitive control, are important adjuncts to studies testing associations between hormones and symptoms. Tests of these questions would require studies with larger numbers of boys and girls. In addition, direct examination of associations between hormone exposure and cognitive control would be required in order to determine whether cognitive control statistically mediates an association of hormone exposure with ADHD. Finally, prospective studies also are necessary to unpack organizational effects on ADHD. Evaluation of hormone exposure levels during pregnancy is invasive; nonetheless, the direct assessment of prenatal testosterone levels (e.g., from amniocentesis in women who are having amniocentesis for medical reasons) would be informative from a scientific viewpoint, if it could be assumed that this sample was not biased (e.g., being more likely to have children with ADHD). This kind of work also could be conducted within animal models of ADHD, if models with better face validity in regard to sex differences were developed. Evaluation of early sex differences in cognition and behavior during the preschool years in relation to prenatal exposure, followed by tracking into the school age years, will be needed to clarify the developmental sequence by which hormones may predispose to ADHD risk.
Hypothesis 2: ADHD Symptoms and Cognitive Control are Positively Correlated with Prenatal Testosterone Exposure Between and Within the Sexes
Because of the risk and cost of examining prenatal hormone levels before birth in humans, it is difficult to directly examine the relationship between prenatal testosterone exposure and ADHD symptoms. To this end, investigators have identified markers of prenatal hormonal influence that can be observed in children and have the advantages of low risk and cost. Cochlear otoacoustic emissions (i.e., weak sounds produced by the cochlear), dermatoglyphics (i.e., fingerprints), and finger ratios are three such measures (Manning et al., 1998; McFadden et al., 2006; Meier, Sorensen, & Jamison, 1993; reviewed by Cohen-Bendahan, van de Beek, & Berenbaum, 2005). Finger-length ratios have been most studied in relation to ADHD and thus are the focus here.
Smaller ratios of finger length, particularly that of the index finger to ring finger (2D:4D), are thought to be associated with higher prenatal testosterone exposure (Manning et al., 1998), including one study examining amniotic fluid in humans (Lutchmaya et al., 2004). A few studies have directly linked finger-length ratio in childhood to prenatal androgen exposure by examining 2D:4D ratio in girls with congenital adrenal hyperplasia (CAH), a disorder in which girls are exposed to abnormally high levels of androgen prenatally. Girls with CAH exhibit smaller right 2D:4D ratios than girls without CAH; their ratios are comparable with typically developing boys (Brown, Hines, Fane, & Breedlove, 2002; Okten, Kalyoncu, & Yaris, 2002; for null findings, see Buck, Williams, Hughes, & Acerini, 2003). In addition to sex differences, it should be noted that finger-length ratios also show ethnic differences, an important consideration in research examining finger-length ratio associations with psychopathology (Manning, Stewart, Bundred, & Trivers, 2004).
Three studies examined finger-length ratios (and thus, indirectly, prenatal testosterone exposure) in clinically diagnosed samples of children with ADHD. Thus, these studies indirectly addressed the hypothesis that higher levels of prenatal testosterone exposure are related to increased ADHD symptoms. De Bruin, Verheij, Wiegman, and Ferdinand (2006) examined 144 boys with psychiatric disorders and 96 boys without psychiatric disorders. As expected, boys with autism/Asperger syndrome and ADHD/oppositional defiant disorder (comorbid groups) had lower finger-length ratios than boys with anxiety disorders, and boys with autistic spectrum disorders had lower finger-length ratios than boys without psychiatric disorders. McFadden and colleagues (2005) studied finger-length ratios and click-evoked otoacoustic emissions (CEOAEs). Boys and girls (ages 7-15) with the Inattentive subtype of ADHD (n = 29) had smaller CEOAEs and smaller finger-length ratios than those with the Combined subtype of ADHD (n=17) or typically developing control children (n=33), suggesting higher levels of prenatal testosterone in the inattentive group only. Martel and colleagues (2008) examined associations between finger-length ratios (2D:4D) and ADHD, including Combined and Inattentive subtypes, in a larger and clinically well-characterized sample of community-recruited boys and girls aged six to 18 years with ADHD (n=113) and non-ADHD controls (n=137). In boys, but not in girls, ADHD was associated with more masculinized finger-length ratios with most specific effects seen for inattentive symptoms of ADHD.
Several studies have also examined prenatal hormonal effects on childhood behavior relevant to ADHD using finger-length ratios. Williams et al. (2003) examined the right 2D:4D in a community sample of approximately 200 preschool children using the Social Difficulties Questionnaire and the Social Cognition Questionnaire. Low 2D:4D (i.e., high prenatal testosterone exposure) was related to high hyperactivity in girls but not boys. In a sample of 58 Caucasian children from the United Kingdom (25 boys and 33 girls; ages 5 through 7), Fink, Manning, Williams, and Podmore-Nappin (2007) found that caregiver-reported hyperactivity and conduct problems, rated on the Strengths and Difficulties Questionnaire, were correlated with lower 2D:4D in boys, but not in girls. In a sample of 56 Caucasian children (29 boys and 27 girls; ages 6 through 11) from Austria, the authors found lower 2D:4D was related to more externalizing problems in girls and more social problems in boys. Stevenson et al. (2007) examined finger-length ratios in relation to ADHD symptoms, measured by the Wender Utah Rating Scale, in a college sample of 238 women and men between the ages of 18 and 47. More masculine left 2D:4D was related to more inattentive and hyperactive-impulsive ADHD symptoms, but only in females. Overall, all studies found a relationship between more masculine ratios (or increased prenatal testosterone exposure) and increased ADHD symptoms, although this relationship was sometimes significant only within one sex or the other.
One personality study is relevant. Low 2D:4D and high concurrent levels of testosterone were related to increased sensation-seeking (Fink, Neave, Laughton, & Manning, 2006), a trait which shows sex differences that favor boys and that has been related to externalizing disorders (Aluja & Torrubia, 2004; Rosenblitt, Soler, Johnson, & Quadagno, 2001; Zuckerman, 2005). However, no work has assessed relations between cognitive control and prenatal testosterone either directly or indirectly (via 2D:4D). However, clues about hormone effects on cognitive control may be obtained from findings in Turner Syndrome. Females with Turner Syndrome, or a single X chromosome, have ovaries that produce less prenatal and postnatal levels of estrogen (Ross et al., 2002). These females also have a characteristic cognitive profile (i.e., poor visual-motor integration, pattern recognition, face recognition, motor speed and coordination) that might be explainable by poor spatial processing. Yet they also show difficulties in alertness and planning (i.e., Test of Variables of Attention, Familiar Figures Test, and the Tower of Hanoi; Ross et al., 2002), as well as in right-lateralized, spatially–demanding executive tasks (Liben et al., 2002; Ross et al., 2002), similar to findings in children with ADHD (Willcutt et al., 2005).
Taken together, research on finger-length ratios may suggest that prenatal testosterone exposure is positively related to ADHD symptoms, at least in boys, and possibly to correlated traits such as externalizing problems and sensation seeking. Thus, there is some support for the hypothesis that prenatal levels of testosterone are positively correlated with ADHD symptoms. However, there is little information about the impact of prenatal testosterone on cognitive control. Thus, future work should address the possibility that there are associations between prenatal testosterone (even measured indirectly via finger-length ratios) and cognitive control.
Hypothesis 3: Prenatal Testosterone Levels Impact Dopaminergic Neural Circuitry
To date, no animal models have directly manipulated prenatal hormone exposure to examine its effect on ADHD behavioral symptoms. However, suggestive animal evidence is available. The spontaneously hypertensive rat (SHR) is the most commonly used animal model of ADHD (Sagvolden, Johansen, Aase, & Russell, 2005). Yet the SHR model has uncertain applicability for examining sex differences in ADHD (see Davids, Zhang, Tarazi, & Baldessarini, 2003), in large part because the animals do not show the expected sex differences in behavioral symptoms that are seen in humans. One study found that female SHR rats were more impulsive (defined as premature bar pressing) than males, especially during diestrus (i.e., a short period of hormonal quiescence between two estrus periods; Sagvolden & Berger, 1996).
However, other research with the SHR rat addresses the hypothesis that androgens (and testosterone specifically) affect dopaminergic neural circuitry. Research with the SHR suggests that early androgen treatment affects neurobiology, specifically catecholamine innervation (King, Barkley, Delville, & Ferris, 2000). When male SHR rats were exposed to elevated androgen levels early in postnatal development, they demonstrated decreased catecholamine (including dopamine) innervation in the frontal cortex and deficits in spatial memory (reminiscent of deficits in working memory seen in children with ADHD), as measured by the water maze. This could suggest that early exposure to high androgen levels may alter dopaminergic neurotransmission, increasing risk for ADHD in a similar manner as in humans. Other work is also consistent with hormonal effects on learning in the SHR rat. Gonadectomy enhanced Pavlovian conditioning of a visual stimulus paired with food in male and female SHR, but not in the comparison strain, suggesting that hormones may impair conditional learning in this strain more so than other strains (Bucci et al., 2008b).
These findings provide interesting evidence for hormonal modulation of dopaminergic neural circuitry and cognition, if not ADHD symptoms, in the SHR. Even so, existing animal models of ADHD have several limitations in regard to elucidating sex differences or hormonal effects. Future research might directly examine sex differences, manipulate prenatal hormone exposure within SHR rats, and incorporate analogue tests of inattention, hyperactivity, and impulsivity (e.g., Bucci et al., 2008a). An animal model that showed a human-like sexual dimorphism of behavior would be of keen interest.
Summary
During the prenatal period, gonadal hormones influence the sexual differentiation of the body, brain, and behavior. A theory of organizational effects on ADHD posits that prenatal androgens (specifically testosterone) exert organizational effects on ADHD by influencing dopaminergic neural circuitry (i.e., striatum and nucleus accumbens and ascending projections to prefrontal cortex). Preliminary data suggest some support for three hypotheses generated by this theory. First, ADHD exhibits a sex difference favoring boys. Second, increased prenatal testosterone exposure (measured indirectly via finger-length ratios) also appears to be related to increased ADHD symptoms, at least in boys (Martel et al., 2008; McFadden et al., 2005), although across sex effects are less clear. Evidence from Turner’s Syndrome suggests that higher testosterone:estrogen ratios relate to cognitive control problems similar to those seen in ADHD, at least in girls. However, more work on associations between prenatal testosterone exposure and cognitive control is desperately needed. Third, organizational androgen effects target striatum, including caudate, and related dopamine circuits (Gerardin et al., 2005; King, Barkley, Delville, & Ferris, 2000). Since the caudate is among the regions that appears to develop abnormally in ADHD beginning early in life (Castellanos et al., 2002), these links are quite heuristic (although circumstantial) in suggesting that prenatal organizational effects may pertain to alterations in dopamine signaling in very early childhood, and to ADHD itself in middle childhood.
Unfortunately, this interesting conclusion must remain quite tentative. Much remains unknown about links between hormonal mechanisms, cognitive control, and ADHD symptoms. For example, it is unclear whether there are sex differences in cognitive control, a key prediction of the theory. In part this is due to lack of data rather than to disconfirming data. Thus, future work should examine sex differences in cognitive control and possible hormonal effects on cognitive control to evaluate the claims made here. In addition, prospective studies should directly address the effects of prenatal testosterone exposure on ADHD. Finally, the development of animal models with better face validity would be helpful.
Pubertal, Activational Hormonal Effects
Neural Alteration and Activation
While the organizational hypothesis of hormonal effects states that early exposure to hormones affects the developing brain permanently, the activational hypothesis states that differential exposure to hormones at puberty alters and activates previously-organized neural circuits. Puberty is a prototypical activating event. At puberty, luteinizing hormone (LH) and follicle stimulating hormone (FSH) stimulate the production of androgen, estrogen, and progesterone (Sisk & Foster, 2004). Although sex hormones are present at low levels during childhood, their levels dramatically increase at puberty; males experience an 18-fold increase in testosterone, and females experience an eight-fold increase in estradiol levels (Susman, Nottelmann, Inoff-Germain, Dorn, & Chrousos, 1987). For women, puberty is only the first life event during which hormone levels fluctuate. The menstrual cycle, pregnancy, and menopause also are linked with large changes in hormone levels, leading to potential activational effects on neural circuitry and behavior.
Some nuance is important, however, in defining organizational versus activational effects at puberty. Changes in hormones during puberty also may result in organizational effects on the brain (Arnold & Breedlove, 1985; Bancroft, 1991; Sisk, Schulz, & Zehr, 2003; Sisk & Zehr, 2005). For example, several types of reproductive and social behaviors (e.g., sexual behavior, scent marking, social interactions) are impaired in male rodents if they are castrated before puberty (Schulz & Sisk, 2006). Hormone replacement in adulthood does not reverse these deficits, indicating that puberty is a critical period for these effects. Thus, activational effects of hormones on phenotypes such as Major Depressive Disorder (MDD) may be related to transient changes, permanent changes, or both, in neural circuitry that continue beyond the pubertal period.
Sex Differences in Behavior and Neural Circuitry during Puberty
Sex differences in negative affect that coincide with the advent of puberty are apparent. Whereas girls and boys at the pre-to-early stage of pubertal development do not differ in negative affect, girls at the mid-to-late stage of pubertal development exhibit significantly higher levels of negative affect than do boys at the corresponding stage of pubertal development (Forbes, Williamson, Ryan, & Dahl, 2004). The differential activation of androgen, estrogen, and progesterone at puberty may influence developing neural circuitry and behavior relevant to depression differently in the sexes, increasing females’ vulnerability to the development of depression. For example, estradiol and testosterone may regulate gene expression and neurotransmission (e.g., serononin, GABA) across development (Amin et al., 2006; Dubrovsky, 2006; Eser et al., 2006; Rubinow et al., 1998). Research in animals and humans shows that estradiol at puberty targets the amygdala, orbital and medial prefrontal cortical areas, and hippocampus (McEwen, 2001; Walf & Frye, 2006), as well as interacting with the hypothalamic-pituitary-adrenal (HPA) axis (Shansky et al., 2004).
Sex differences in neural structure and function support these ideas. Girls have smaller amgydala volumes than boys, even after controlling for overall brain volume (Durston et al., 2001). During adolescence and adulthood, men and women exhibit sex differences in affective processing (Hofer et al., 2007; Killgore, Oki, & Yurgelun-Todd, 2001). Between the ages of nine and 17, females show an increase in prefrontal relative to amygdala activation in the left hemisphere, whereas males do not exhibit this pattern (Killgore, Oki, & Yurgelun-Todd, 2001).
These same brain regions have been associated with depression (Drevets, 1999; Gabbay et al., 2005; Rosenberg, MacMaster, Mirza, & Easter, 2006). Increased glucose metabolism in the amygdala is associated with MDD and with increased depression severity in individuals with MDD (Drevets, 1999; Neumeister et al., 2006). In addition, animal research suggests that pubertal hormones, particularly estradiol, amplify the stress response in the prefrontal cortex by affecting the relative levels of serotonin, noradrenaline, and dopamine differentially in males and females (Koshibu & Levitt, 2006; Shansky et al., 2004). Thus, there is considerable circumstantial evidence that pubertal hormone levels influence brain circuitry differentially in males and females in a manner that is consistent with the effects seen in depression.
Theories of Hormonal Mechanisms of MDD
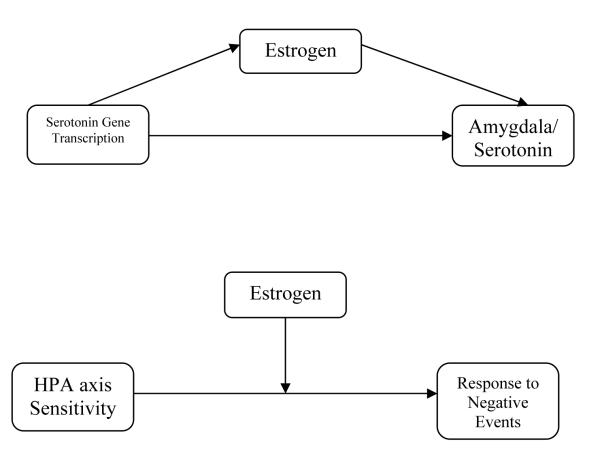
Based on a review of literature on activational hormonal effects on neurobiology and behavior, we formulated two theories regarding activational effects of gonadal hormones on MDD. In the first theory, rapidly fluctuating levels of hormones during puberty or declining levels of estrogen during the menstrual cycle may respectively exert or remove an activational effect on the development of MDD by influencing serotonin activity through the serotonin receptor (see Figure 2b). Estrogen is known to be a potent regulator of several serotonergic systems (e.g., the 5HT2a receptor; Andrade, Nakamuta, Avanzi, & Graeff, 2005; Birzniece et al., 2006). Rapidly-changing levels of estrogen at puberty may therefore confer risk by directly influencing serotonin gene transcription which then leads to amygdala abnormalities and low serotonin levels. In this hypothesis, estrogen may directly modulate serotonin activity. Abnormal development of the amygdala and low levels of serotonin then may lead to increased sensitivity of the HPA axis and increased negative affect. This HPA axis sensitivity and increased negative affect in turn may confer vulnerability for the development of a major depressive episode, especially in the presence of stressful social events. In the second theory of activational hormonal effects on MDD, estrogen may interact with the HPA axis, increasing its sensitivity to stress and modulating its activity during puberty (see Figure 2b). Altered HPA activity may make individuals more sensitive to stress and consequent major depressive episodes (Shansky et al., 2004).
Figure 2b.
Potential Pubertal Estrogen Influence on Serotonergic Neurotransmission in Depression
These theories lead to at least four specific hypotheses. First, a between-sex difference favoring girls should be evident in the prevalence rate of MDD and negative affect, beginning at puberty. Second, MDD symptoms and negative affect should be positively correlated with estrogen levels or estrogen level changes between and within the sexes. Third, estrogen should impact serotonin gene transcription and activity. Fourth, estrogen should interact with HPA activity. We will now subject these hypotheses to empirical test based on the current literature on activational hormone effects on MDD and negative affect. The literature evaluated in the following sections was obtained via searches conducted using PsycInfo and MedLine search engines using combinations of the following terms: activational hormonal effects, puberty, estrogen, estradiol, sex/gender differences, neural circuitry, animal models, MDD, and negative affect/mood.
Activational Hormonal Effects and MDD
Hypothesis 1: Sex Differences in the Prevalence Rate of MDD and Negative Affect Beginning at Puberty
Sex differences in the prevalence rate of depression begin to emerge at the age of 13 (Hankin et al., 1998). Negative affect (i.e., sadness, anger, anxiety, but not severe or specific enough to be identified as major depression) and negative mood (i.e., a more chronic manifestation of negative affect) are powerful liability markers for depression (Kendler et al., 2006). They appear to be one promising component of MDD that may be influenced by hormonal mechanisms. Negative affect also exhibits sex differences favoring females, shows a rise during puberty, and exhibits changes across the menstrual cycle (Forbes, Williamson, Ryan, & Dahl, 2004). Thus, there appear to be sex differences in MDD prevalence and negative affect beginning at puberty so hormonal effects on MDD and negative affect appear to be at least plausible.
Hypothesis 2: MDD Symptoms and Negative Affect are Positively Correlated with Estrogen Levels or Estrogen Level Changes Between and Within the Sexes
Hormonal effects on depression and negative affect are highly complex. Two key and not mutually exclusive hypotheses regarding hormone-psychopathology relations should be considered. First, optimal level of hormones might be protective in regard to depression. Second, fluctuating levels of hormones may increase risk for psychopathology.
Three longitudinal studies examined activational hormonal effects on MDD and negative affect at puberty. Initial studies were non-confirmatory (Nottelmann et al., 1987; Susman et al., 1987; Susman, Dorn, & Chrousos, 1991) but either had modest-size samples (fewer than 100 girls) or failed to take into account the presence or absence of menstruation and normal variations in hormone levels across the menstrual cycle. In landmark studies from the Great Smoky Mountains Study, Angold and colleagues (1998; 1999) did include such controls in a larger study of about 400 girls and 400 boys, with two important findings. First, pubertal status (i.e., stage of pubertal morphological development), rather than pubertal timing (i.e., timing of pubertal morphological development compared to same-age peers), was associated with an increased risk for depression. Girls were more likely to be depressed than boys only after the transition to mid-puberty (i.e., Tanner Stage III and above), regardless of age. Second, levels of estradiol and testosterone statistically accounted for the effect of pubertal status on depressive symptomatology in the girls. Susman et al. (1991) found that girls with higher levels of negative affect were characterized by higher levels of testosterone, higher cortisol, and lower adrenal hormones, but not altered estradiol levels. The relative importance of estradiol versus testosterone in these effects still needs examination, however.
Further, it is unclear whether hormonal effects outweigh social effects, as suggested by Angold et al. (1999). Brooks-Gunn and Warren (1989) studied 100 girls from age 10 to 14 years. Negative affect increased during periods characterized by the most rapid increases in hormone levels (also see Paikoff et al., 1991). However, hormones (i.e., estradiol, LH, FSH, and testosterone) accounted for only 4% of the variance in negative affect, whereas social factors accounted for approximately 10%. Using the same sample at the same ages, Graber, Brooks-Gunn, and Warren (2006) confirmed an association between higher circulating estradiol and increased depressive symptoms, but relative size of effects was not revisited.
Although it is possible to directly manipulate hormones in humans, it is much more difficult to implement and interpret the results of such a study (e.g., it is questionable whether exogenous hormones can mimic natural feedback systems; Becker et al., 2005). Therefore, periods of dramatic hormonal activity (besides puberty)-- including the menstrual cycle, pregnancy, and menopause-- are examined as times during which activational hormonal effects may influence depression or, more broadly, negative affect or mood.
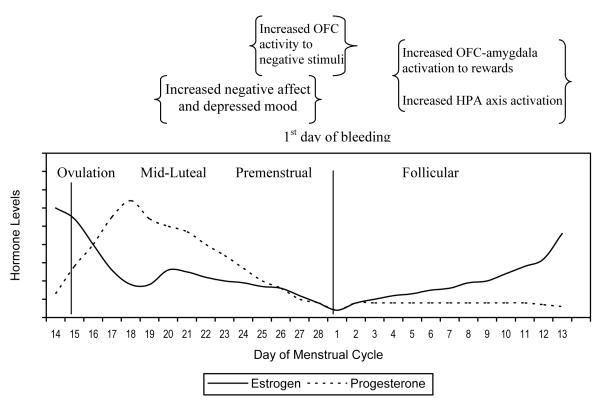
In normally menstruating girls/women, ovarian hormones exhibit highly predictable changes in magnitude across the menstrual cycle, shown in Figure 3. Estradiol and progesterone levels are relatively low during menstrual bleeding. Estradiol levels rise during the follicular phase until the LH surge, at which time ovulation occurs. After ovulation, estradiol levels decline (Rosenfield, 1991) while progesterone levels rise steadily. There is a second peak in estradiol during the mid-luteal phase, but then both progesterone and estradiol decline throughout the premenstrual phase. At this point, menstrual bleeding begins, completing the cycle. Menstrual changes allow for a powerful test of hormone-behavior relationships because they occur in a predictable fashion and are experienced in all regularly menstruating, post-pubertal women.
Figure 3.
Hormones, Mood, Affect, and Brain Activation Across the Menstrual Cycle
Note. OFC=orbitofrontal cortex. HPA=hypothalamic-pituitary-adrenal axis.
Depressed mood symptoms vary across these menstrual cycle fluctuations in a systematic fashion, depicted schematically in Figure 3. Women are most likely to develop mood symptoms (i.e., depressed mood, apathy) during the mid to late-luteal phase of their menstrual cycle, a period during which progesterone levels are peaking while estradiol levels are declining (Nelson, 2005a). Negative affect also appears to vary across the menstrual cycle, with the most negative affect occurring before or during the menstrual phase (vs. ovulatory and premenstrual phases; Herrera et al., 1990). However, depression and negative affect have yet to be studied together to determine whether they have a phased or sequential likelihood.
These cyclical changes in mood or negative affect appear to be related to fluctuating hormone levels. In a study of female university students, use of oral contraceptives altered day-to-day affect variability in mood (Oinonen & Mazmanian, 2001). Triphasic (i.e., an oral contraceptive with three phases of hormone variation) users experienced more variability in affect compared to non-users, presumably due to the greater variability in their hormone levels across the menstrual cycle. Consistent with this idea, symptoms of depressed mood typically develop during the premenstrual period when estradiol and progesterone are declining (Poromaa, Smith, & Gulinello, 2003). Mood symptoms of premenstrual syndrome improved in response to ovarian function suppression (via leuprolide) and reoccurred following estradiol or progesterone replacement in a subset of women, suggesting that premenstrual mood symptoms may be due to an abnormal response to hormonal changes (Schmidt et al., 1998). In fact, women with premenstrual dysphoria displayed an abnormal gonadotrophin reponse to estradiol challenge compared to other women (Erikkson et al., 2006).
Some additional clues may be obtained by considering two additional changes in hormone levels, even though they do not occur during the activating period of adolescence. First, approximately 15% of women develop symptoms of MDD in the first six months after childbirth when sex hormones (including estradiol) are rapidly and dramatically declining (Clayton, 2004; Norman & Litwack, 1997). Research has linked the sharp decline of circulating estradiol and progesterone during the postpartum period with the development of MDD in a subset of women with a history of postpartum depression (Bloch et al., 2000). Second, many women experience increased irritability and depression at the beginning of menopause when estrogen levels also are declining (reviewed by Buchanan, Eccles, & Becker, 1992; Cohen et al., 2006) versus before menopause begins or after it is completed (Freeman et al., 2004; Freeman, Sammel, Lin, & Nelson, 2006).
Treatment with estradiol during the postpartum period, as well as during perimenopause and menopause, has been related to decreased depression, but not consistently (for positive findings, see Ahokas et al., 2001; Cohen et al., 2003; Miller et al., 2002; Soares et al., 2001; for negative findings, see Almeida et al., 2006; Goldstein et al., 2005; Morrison et al., 2004; Pefanco et al., 2007; Schmidt et al., 2002). A meta-analysis on the effect of hormone replacement therapy on depressed mood during menopause suggested that estrogen significantly reduced depressed mood (Zweifel & O’Brien, 1997). Estradiol treatment also may accelerate the effects of antidepressants among non-responders during menopause (Rasgon et al., 2007). The combination of these findings suggests that low levels of estrogen, or else dramatic baseline changes in estrogen, confer risk for depression.
Animal research provides a particularly powerful way to test questions of activational hormonal influences because hormonal activation can be induced and controlled by ovariectomizing animals and then directly infusing hormones. Some animal studies suggest that the withdrawal of estradiol, following high levels (similar to the pattern seen during the menstrual cycle), may be related to MDD. Withdrawal of estradiol in rats that have been exposed to high levels of estradiol and progesterone (to mimic levels during pregnancy) led to more time spent immobile during the forced swim test, behaviors thought to simulate depressive symptoms (Galea, Wide, & Barr, 2001; Stoffel & Craft, 2004). Further, infusion of estradiol in rats leads to increased mobility, suggesting an anti-depressive effect of estradiol administration (Estrada-Camarena, Fernandez-Guasti, & Lopez-Rubalcava, 2003). Confirming this impression, immobility in the forced swim task in rats following ovariectomy is reversed by estradiol treatment (Bekku & Yoshimura, 2005). However, these findings also could be interpreted as suggesting a curvilinear relationship such that an optimal level of estradiol may be protective for females, while both low and high levels of estradiol increase depressive symptoms.
Further, estrogen may have effects on brain-behavior relationships which depend on the specific neural circuitry and structure that are exposed to estrogen effects. Direct infusion of estrogen into the hippocampus and amygdala of adult, ovariectomized rats increases exploration of the open field, time spent on the light side of a chamber, and decreases time spent immobile during the forced swim test, behaviors believed to be characteristic of low anxiety and depression (Walf & Frye, 2006). However, administration of estrogen to the hippocampus, but not the amygdala, of intact female rats increased anxious and depressed behavior.
Overall, there appears to be some support for a relationship between estradiol and mood symptoms and negative affect. However, such relations appear complex. Estradiol may show a curvilinear relationship with mood symptoms and negative affect, such that insufficient or excess estrogen activation at puberty increases depression risk. Low levels or large fluctuations may be most risky between major developmental transitions (e.g., increase during puberty and decrease during menopause). The effects of circulating estrogen on depression could also differ at different transitional periods: at puberty, between puberty and menopause, at menopause, and after menopause. Further, a subset of women may be differentially sensitive to hormones in such a way as to predispose them to premenstrual dysphoric disorder and postpartum mood disorders (Bloch et al., 2005).
However, methodological limitations of prior work on hormonal associations with depressed mood and negative affect are notable. First, the complexity of effectively accounting for normal menstrual cycle variations in ovarian hormones, negative affect, and MDD symptoms in menstruating girls and women is an issue. Many previous analyses of hormone levels in adolescent girls may be looking at general indices of symptomatology and a very specific, potentially cyclical, measure of gonadal hormone. If a girl has begun menses and her overall negative affect for the last month is compared to an estradiol level taken on day 14 of her cycle, a relationship between these two indices may be missed when, in fact, it exists. Daily measures across the menstrual cycle are needed to examine these relations over a 30 or 60 day period.
Statistical analyses of hormones will require larger sample sizes so that both girls and boys can be examined with attention to potential differential effects within sex during puberty. This will be particularly important if hormonal effects are small or unknown. Girls who have and have not started menstruation will most likely need to be considered separately because hormone levels in the menstruating group will vary depending on their current phase within the menstrual cycle. Pubertal status, chronological age, and timing of puberty also will need to be examined in analyses of girls and boys.
Short-term longitudinal studies will be necessary to fully characterize dynamic hormonal organizational and activational influences that are defined by short-lived critical periods. For example, activational effects may occur only during very specific periods, such as during puberty or during other transitional periods, and effects may be dependent on hormone fluctuations rather than absolute levels during some developmental periods. Thus, research that does not examine hormone levels over time or ignores developmental stage (e.g., pubertal or menopausal transition) may not find these more dynamic kinds of effects in females. Salivary measures of hormones may aid in this endeavor as a noninvasive method of collecting hormones that will increase participant compliance and cut down on burden (Edler, Lipson, & Keel, 2007).
Hypothesis 3: Estrogen Impacts Serotonin Gene Transcription and Activity
Some research has addressed estrogenic effects on serotonergic neural circuitry and interactions between estrogen activity and HPA axis function. Variations in mood across the menstrual cycle may be related to hormonally influenced changes in neurobiological activity, as measured by functional magnetic resonance imaging. Menstrual cycle variation was related to variation in activation of orbitofrontal cortex (OFC) in response to emotional stimuli (Protopopescu et al., 2005). OFC activity was increased for negative (vs. neutral) stimuli premenstrually (when estrogen levels are decreasing), but not postmenstrually (i.e., during the follicular phase, when estrogen levels are increasing). This finding suggests that declining levels of estrogen are related to increased sensitivity of the OFC to negative stimuli, although the direction of effect is unclear. In addition, women appear to exhibit increased reactivity of the reward system (i.e., the orbitofrontal cortex and amygdala), measured using a monetary reward task, during the midfollicular phase of the menstrual cycle, a phase characterized by rising estradiol levels (Dreher et al., 2007). Estradiol levels were positively associated with activation in the amygdalar-hippocampal complex in women, and women exhibited stronger activation of the anterior medial prefrontal cortex at the time of reward delivery than did men. Other fMRI research suggests that estrogen may enhance affective regulation by increasing cortical control over the amygdala (Goldstein et al., 2005), providing protection against negative mood. Emotional arousal was positively correlated with brain activity in the amygdala during the late follicular/midcycle phase (when estrogen is relatively high) but not during the early follicular phase (when estrogen and progesterone are relatively low).
Estrogen modulates the central neurotransmitter systems implicated in depression, particularly that of serotonin (Bertrand et al., 2005; for reviews see Buchanan, Eccles, & Becker, 1992; Cameron, 2004a; 2004b; Hayward & Sanborn, 2002; Steiner, Dunn, & Born, 2003). Such modulation may be one route to altered depression sensitivity in girls. Primate research suggests that changes in levels of ovarian hormones interact with stressful experiences to alter neurotransmission. Specifically, cessation of ovarian hormones through surgical menopause in combination with the stressful experience of social subordination appears to down-regulate serotonin neurotransmission in the amygdala, suggesting that it might influence depressed behavior (Beyer & Feder, 1987; Shively & Bethea, 2004). Finally, behavioral genetics research on depression indicates that the heritability estimate of depression increases during puberty, providing some indirect evidence that estrogen may be having an effect on risk genes during the pubertal period (Silberg et al., 1999).
In summary, estrogen appears to affect serotonergic neural circuitry, although its subsequent effects on depression remain unclear. Future work should examine estrogen effects on serotonergic neural circuitry during key activational periods, such as during puberty, over the menstrual cycle, and during pregnancy and menopause. If these effects could also be related to MDD symptoms and negative affect that would be helpful. Further, interactions between estrogen, serotonin, HPA activity (discussed below), and stressful experiences should be explored.
Hypothesis 4: Estrogen Interacts with HPA Activity
These effects of estrogen appear to be moderated by stress or by level of HPA axis activation. The HPA axis regulates responses to stressful or challenging experiences. Recent research in animals suggests that estrogen, specifically estradiol, amplifies the stress response (i.e., increases catecholamine release) in the prefrontal cortex. These effects may make adult females more sensitive to the negative effects of stressful experiences on depression and cognition (Shansky et al., 2004). For example, in one experiment, adult male and female rats had to perform a working memory task after the administration of a medication (benzodiazepine inverse agonist; anxiogenic drug) that activated stress systems in the brain (Shansky et al., 2004). In the high estrogen phase of their cycle (i.e., proestrus), female rats given lower doses of the drug exhibited more impaired performance than male rats given the same medication dose. This effect was not seen in females during the estrus (i.e., low estrogen) phase of their cycle. Ovariectomized female rats also showed increased stress responses only after estrogen replacement (Shansky et al., 2004). Further, when rats were restrained under lights for 45 minutes, a stressful experience, injections of estradiol in ovariectomized rats did not lead to anti-depressive effects, suggesting that estradiol may not have anti-depressive effects under conditions of stress (Frye & Wawrzycki, 2003). The anti-depressive effects of estradiol also may depend on an optimal dose of corticosterone, suggesting an interaction between estradiol effects and HPA axis tone (Walf & Frye, 2005). Arnsten and Shansky (2004) suggested that estrogen may lower the threshold for the prefrontal cortical dysfunction that results from stressful experiences. Thus, levels of estrogen, specifically estradiol, may increase risk for depression by altering prefrontal activation thresholds in response to stress.
Research examining a primate model of depressive behavior (defined as slumped or collapsed body posture accompanied by a lack of responsiveness to external stimuli) found that depressed adult macaque monkeys have over-reactive HPA function and poor ovarian function (i.e., lower mean peak luteal-phase progesterone concentrations) compared to typical monkeys (Shively & Bethea, 2004). In a series of studies validating this primate model of depression, Shively et al. (1997; 2002; 2005) found that socially subordinate female monkeys who exhibited behaviors consistent with behavioral depression had lower progesterone levels than dominant monkeys. The peak of luteal phase progesterone concentrations was significantly lower in the depressed, socially subordinate monkeys versus the non-depressed monkeys (Shively, Laber-Laird, & Anton, 1997; Shively, Williams, Laber-Laird, & Anton, 2002). Although promising, animal models of depression are limited in face validity since they rely on behavioral markers that also can be due to fatigue, social status, or other factors and do not isolate the subjective mood prominent in human depression. However, one study in humans indicated that activation of the HPA axis was increased during the early follicular phase when estradiol was rising and cortical control over the amygdala was reduced (Goldstein et al., 2005). Thus, estradiol may moderate the functioning of the limbic-OPFC circuit and the HPA axis, in turn moderating risk for depression.
In summary, estradiol appears to have moderated effects on depression via interaction with stressful life events and the HPA axis, potentially explaining mixed findings in regard to estradiol relations with depression.
Summary
Hormones appear to exert direct and indirect activating, transient, and long-term effects on neural circuitry, neurotransmission, and behavior at puberty, as well as during the monthly menstrual cycle, pregnancy, and menopause (Berenbaum, 1998; Sisk & Zehr, 2005). A theory of hormonal effects on MDD and negative affect posits that hormones at puberty exert an activational effect on symptoms of negative affect, by influencing serotoninergic neurotransmission and related neural structures including the amygdala and the HPA circuitry. Initial support for four main hypotheses generated from this theory is promising, but suggest complex effects. Strong support was garnered for the first hypothesis; MDD symptoms and negative affect show sex differences favoring girls that increase during puberty. However, support for the second hypotheses establishing links between estrogen, MDD symptoms, and negative affect were less clear. Findings were mixed, possibly suggesting links between MDD symptoms, negative affect, and low (or declining), non-optimal, or fluctuating levels of hormones during puberty, across the menstrual cycle, and during pregnancy and menopause. This body of research exhibits several notable limitations including small sample size, lack of attention to normal menstrual cycle variation, and reliance on cross-sectional design to study dynamic hormonal effects. Preliminary evidence also supports the third and fourth hypotheses that estradiol impacts serotonergic neural circuitry and interacts with the HPA axis. However, more research on these last two hypotheses is needed.
Overall Summary
ADHD and MDD have sex differences in prevalence rates that begin in childhood and adolescence respectively, suggesting the possibility of hormonal influences. Although developmental neuroscience of hormonal mechanisms provides one valuable lens through which to view normal and abnormal developmental processes, research on childhood and adolescent psychopathology seldom includes this level of analysis. Initial research on hormonal mechanisms of ADHD and MDD reveal preliminary tantalizing effects. In regard to ADHD, the links between prenatal, organizational hormonal effects and ADHD are promising, though the observed effect may depend on child age, with somewhat clearer preliminary findings in preschool than in school age youth. In addition, hormonal effects on specific ADHD symptoms domains would be helpful. The little research that has examined hormonal mechanisms of ADHD, measured indirectly via finger-length ratios, is generally supportive of links between hormones and ADHD (e.g., Martel et al., 2008; McFadden et al., 2005), although longitudinal studies examining the relationship between prenatal hormone exposure, measured directly, and childhood psychopathology would be illustrative.
In regard to MDD, links between activational gonadal hormone effects, MDD, and negative affect are better-established, in part because they are more easily studied. Research suggests links between low (or declining), non-optimal, or fluctuating levels of estrogen and MDD, as well as the negative affect that characterizes individuals with MDD. Further, hormones appear to influence and interact with environmental factors such as high stress, influencing the sensitivity of the HPA axis and neurotransmission within the brain (Buchanan, Eccles, & Becker, 1992; Protopopescu et al., 2005; Shansky et al., 2004; Shively & Bethea, 2004). However, an articulation of potential mechanisms of estrogen (e.g., through effect on limbic-prefrontal circuitry or through influence on serotoninergic neurotransmission) remains poorly articulated.
Future Directions: Theoretical Considerations
Research on hormonal effects on psychopathology would benefit from several theoretical considerations. First, non-linear relations between hormones and behavior need continued consideration. Hormones may have curvilinear relations with behavior. For example, low or non-optimal levels of estrogen may be related to more MDD symptoms. Alternatively, fluctuating levels of estrogen may be related to MDD symptoms. In the case of ADHD, prenatal testosterone: estradiol ratios may be better predictors of ADHD symptoms than testosterone alone. Second, animal models of psychopathology and other experimental work with animals, as well as clinical research with humans, now provide a foundation for the generation of specific theories of hormonal mechanisms of psychopathology. Theories of psychopathology should examine directly hormonal mechanisms, incorporating existing theory of organizational and activational hormonal effects. One preliminary step might be to develop a new animal model that mimics sex differences in prevalence; this may require moving the ADHD animal models into species other than rodents (e.g., canines or primates). In addition, the face validity of animal models of psychopathology should be carefully evaluated. Finally, and perhaps most importantly, specific theory, hypotheses, and predictions should guide studies more than they have to date.
Conclusion
Links between hormones and developmental psychopathology are intriguing and conceptually compelling when one considers patterns of risk that differ by sex. First, organizational effects may influence dopamine and other key neurotransmitters and so may be candidates for disorders such as ADHD that involve breakdowns in striatal dopamine circuity and related cognitive operations. Second, activational effects may influence serotonin and HPA axis responses to stress and so may be candidates for disorders such as MDD that involve breakdowns in these circuits. Nonetheless, demonstration of these ideas remains largely circumstantial, so the present essay aimed to highlight key findings and to stimulate more research on these theories. Further study of hormonal mechanisms of developmental psychopathology has the potential to illuminate developmental, environmental, and genetic processes that confer risk and protection for clinical disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen defiency om severe postpartum depression: Successful treatment with sublingual physiologic 17β-estradiol: A preliminary study. J Clin Psychol. 2001;62(5):332–336. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: Effect on mood, cognition, and quality of life. Neurobiol Aging. 2006;27(1):141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Aluja A, Torrubia R. Hostility-aggressiveness, sensation-seeking, and sex hormones in men: Re-exploring their relationship. Neuropsychobiology. 2004;50(1):102–107. doi: 10.1159/000077947. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Fourth ed. American Psychiatric Association; Washington, D.C.: 2000. text rev. [Google Scholar]
- Amin Z, Mason GF, Cavus I, Krystal JH, Rothman DL, Epperson CN. The interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacol, Biochem, and Behav. 2006;84:635–643. doi: 10.1016/j.pbb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8(2):71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Nakamuta JS, Avanzi V, Graeff FG. Anxiolytic effect of estradiol in the median raphe nucleus mediated by 5-HT-sub(1A) receptors. Behav Brain Res. 2005;163(1):18–25. doi: 10.1016/j.bbr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29:1043–1052. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello, Worthman CM. Puberty and depression: The role of age, pubertal status, and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Arnold LE. Sex differences in ADHD: Conference Summary. J Abnorm Psychol. 1996;24(5):555–569. doi: 10.1007/BF01670100. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav. 1985;19(4):469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Shansky RM. Adolescence: Vulnerable period for stress-induced prefrontal cortical function? Ann N Y Acad Sci. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Bancroft J. Reproductive hormones. In: Rutter M, Casaer P, editors. Biological Risk Factors for Psychosocial Disorders. Cambridge Univ Press; Cambridge: 1991. pp. 260–310. [Google Scholar]
- Barkely RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Becker J. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KP, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: Prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology. 2005;183(3):300–307. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA. How hormones affect behavioral and neural development: Introduction to the special issue on “Gonadal hormones and sex differences in behavior.”. Dev Neuropsychol. 1998;14(2/3):175–196. [Google Scholar]
- Bertrand PP, Paranavitane UT, Chavez C, Gogos A, Jones M, van den Buuse M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: A behavioral and electrochemical study. Brain Res. 2005;1064:10–20. doi: 10.1016/j.brainres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Beyer C, Feder HH. Sex steroids and afferent input: Their roles in brain sexual differentiation. Annu Rev. 1987;49:349–364. doi: 10.1146/annurev.ph.49.030187.002025. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Backstrom T, Johansson I, Lindblad C, Lundgren P, Lofgren M, et al. Brain Res Rev. 2006;51:212–239. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rotenberg N, Koren D, Klein E. Risk factors associated with the development of postpartum mood disorders. J Affect Disord. 2005;88:9–18. doi: 10.1016/j.jad.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Hampson E. Sexual differentiation of the brain and behavior. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. Second Ed MIT Press; Cambridge, MA.: 2002. pp. 75–114. [Google Scholar]
- Brooks-Gunn J, Warren MP. Biological and social contributions to negative affect in young adolescent girls. Child Dev. 1989;60(1):40–55. doi: 10.1111/j.1467-8624.1989.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane BA, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Horm Behav. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Hopkins ME, Keane CS, Sharma M, Orr LE. Sex differences in learning and inhibition in spontaneously hypertensive rats. Behav Brain Res. 2008a;187:27–32. doi: 10.1016/j.bbr.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Hopkins ME, Nunez AA, Breedlove SM, Sisk CL, Nigg JT. Physiol Behav. 2008b;93(3):651–657. doi: 10.1016/j.physbeh.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JA, Becker JB. Are adolescents the victims of raging hormones: Evidence for activational effects of hormones on moods and behaviors at adolescence. Psychol Bull. 1992;111(1):62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio—comparisons between healthy controls and females with classic congenital adrenal hyperplasia. Hum Reprod. 2003;18(5):976–979. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: Understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. In: Dahl RE, Spear LP, editors. Adolescent brain development: Vulnerabilities and opportunities. Vol. 1021. New York Academy of Sciences; New York: 2004a. pp. 110–123. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: Complex relationships exist between reproductive hormones, stress-related hormones, and the activity of neural systems that regulate behavioral affect. In: Dahl RE, Spear LP, editors. Adolescent brain development: Vulnerabilities and opportunities. Vol. 1021. New York Academy of Sciences; New York: 2004b. pp. 134–142. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with Attention-Deficit/Hyperactivity Disorder. JAMA. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Marvasti FF, Ducharme JL, Walter JM, Israel ME, Krain A, et al. Executive function oculomotor tasks in girls with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39(5):644–650. doi: 10.1097/00004583-200005000-00019. [DOI] [PubMed] [Google Scholar]
- Clayton AH. Postpartum mood disorders. Prim Psychiatry. 2004;11(9):20–21. [Google Scholar]
- Cohen LS, Soares CN, Poitras JR, Prouty J, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: A preliminary report. Am J Psychiatry. 2003;160(8):1519. doi: 10.1176/appi.ajp.160.8.1519. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychol Bull. 1995;118(1):55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry. 2008;65(3):329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarinin RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Rev. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- De Bruin E, Verhiej F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder-not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965. doi: 10.1017/S0012162206002118. [DOI] [PubMed] [Google Scholar]
- Dreher J, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. In: McGinty JF, editor. Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug use: In honor of Lennart Heimer. New York Academy of Sciences; New York: 1999. pp. 614–637. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol Biochem Behav. 2006;84:644–655. doi: 10.1016/j.pbb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff HE, Casey BJ, Giedd JN, Buitelaar JK, vanEngelan H. Anatomical MRI of the developing human brain: What have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37(1):131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Eriksson O, Backstrom T, Stridsberg M, Hammarlund-Udenaes M, Naessen T. Differential response to estrogen challenge test in women with and without premenstrual dysphoria. Psychoneuroendocrinology. 2006;31:415–427. doi: 10.1016/j.psyneuen.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Eser D, Schule C, Baghai TC, Romeo E, Uzunov DP, Rupprecht R. Neuroactive steroids and affective disorders. Pharmacol Biochem Behav. 2006;84:656–666. doi: 10.1016/j.pbb.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- Fink B, Manning JT, Williams JHG, Podmore-Nappin C. The 2nd to 4th digit ratio and developmental psychopathology in school-aged children. Pers Individ Diff. 2007;42:369–379. [Google Scholar]
- Fink B, Neave N, Laughton K, Manning JT. Second to fourth digit ratio and sensation seeking. Pers Individ Diff. 2006;41:1253–1262. [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and negative affect in depression: Influence of sex and puberty. In: Dahl RE, Spear LP, editors. Adolescent brain development: Vulnerabilities and opportunities. Vol. 1021. New York Academy of Sciences; New York: 2004. pp. 341–347. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent-variable analysis. J Exp Psychol: Gen. 2004;133(1):101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Silva RR, Castellanos FX, Rabinovitz B, Gonen O. Structural and functional neuroimaging of pediatric depression. Prim Psychiatry. 2005;12(9):51–57. [Google Scholar]
- Galea LAM, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Gerardin DCC, Pereira OCM, Kempinas WG, Florio JC, Moreira EG, Bernardi MM. Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav. 2005;84:97–104. doi: 10.1016/j.physbeh.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein KM, Harpole LH, Stechuchak KM, Coffman CJ, Bosworth HB, Steffens DC, et al. Hormone therapy does not affect depression severity in older women. Am J Geriatr Psychiatry. 2005;13(7):616–623. doi: 10.1176/appi.ajgp.13.7.616. [DOI] [PubMed] [Google Scholar]
- Goodman R. Developmental disorders and structural brain development. In: Rutter M, Casaer P, editors. Biological Risk Factors for Psychosocial Disorders. Cambridge University Press; Cambridge: 1991. pp. 260–310. [Google Scholar]
- Graber JA, Brooks-Gunn J, Warren MP. Pubertal effects on adjustment in girls: Moving from demonstrating effects to identifying pathways. J Youth Adolesc. 2006;35(3):413–423. [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Examining gender differences in a 10-year longitudinal sample. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J Adolesc Health. 2002;30S:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Herrera E, Gomez-Amor J, Martinezz-Selva J, Ato M. Relationship between personality, psychological and somatic symptoms, and the menstrual cycle. Pers Individ Diff. 1990;11(5):457–461. [Google Scholar]
- Hines M. Sexual differentiation of human brain and behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Academic Press; Boston: 2002. pp. 425–462. [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Tettenbacher MA, Verius M, Felber S, Fleischhacker WW. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychol Med. 2007;37(1):109–119. doi: 10.1017/S0033291706008919. [DOI] [PubMed] [Google Scholar]
- Holden C. Sex and the suffering brain. Science. 2005;308:1574–1577. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Inglis J. Sex differences in the cognitive effects of unilateral brain damage. Cortex. 1982;18(2):257–275. doi: 10.1016/s0010-9452(82)80007-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: A Swedish longitudinal, population-based twin study. Arch Gen Psychiatry. 2006;63:1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Kerr A, Zelazo PD. Development of “hot” executive function: The children’s gambling task. Brain Cogn. 2004;55:148–157. doi: 10.1016/S0278-2626(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Oki M, Yrugelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport: Rapid Commun Neurosci Res. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- King JA, Barkley RA, Delville Y, Ferris CF. Early androgen treatment decreases cognitive function and catecholamine innervation in an animal model of ADHD. Behav Brain Res. 2000;107(1-2):35–43. doi: 10.1016/s0166-4328(99)00113-8. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating:” Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychol Med. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol. 2005;21(10):825–845. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Raggat P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46(2):198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Koshibu K, Levitt P. Transforming growth factor-α induces sex-specific neurochemical imbalance in the stress- and memory-associated brain structures. Neuropharmacology. 2006;50:807–813. doi: 10.1016/j.neuropharm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62(8):896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson J. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2006:973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Levy J, Heller W. Gender differences in human neuropsychological function. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of Behavioral Neurobiology: Sexual Differentiation. Vol. 11. Plenum Press; New York: 1992. pp. 245–274. [Google Scholar]
- Liben LS, Susman EJ, Finkelstein JW, Chinchilli VM, Kunselman S, Schwab J, Dubas JS, Demers LM, Lookingbill G, D’Arcangelo MR, Krogh HR, Kulin HE. The effects of sex steroids on spatial performance: A review and an experimental clinical investigation. Dev Psychol. 2002;38(2):236–253. [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggett P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Human Development. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lyon G, Gadisseux J. Structural abnormalities of the brain in developmental disorders. In: Rutter M, Casaer P, editors. Biological Risk Factors for Psychosocial Disorders. Cambridge University Press; New York: 1991. [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13(11):3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Manning J, Stewart A, Bundred P, Trivers R. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Hum Dev. 2004;80(2):161–168. doi: 10.1016/j.earlhumdev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Martel MM, Gobrogge KL, Breedlove SM, Nigg JT. Evidence for organization effects of steroid hormones on childhood ADHD in boys but not girls. Behav Neurosci. 2008;122(2):273–281. doi: 10.1037/0735-7044.122.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Estrogens effects on the brain: Multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Raper J, Lange HS, Wallen K. Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta) Horm Behav. 2006;50(2):274–284. doi: 10.1016/j.yhbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- McFadden D, Westhafer JG, Pasanen EG, Carlson C, Tucker DM. Physiological evidence of hypermasculinization in boys with the inattentive type of Attention-Deficit/Hyperactivity Disorder (ADHD) Clin Neurosci Res. 2005;5:233–245. [Google Scholar]
- McManus IC, Bryden MP. Geschwind’s theory of cerebral lateralization: Developing a formal, causal model. Psychol Bull. 1991;110(2):237–253. doi: 10.1037/0033-2909.110.2.237. [DOI] [PubMed] [Google Scholar]
- Meier RJ, Sorenson JC, Jamison PL. Prenatal testosterone effect on dermatoglyphic development in rhesus macaques. Int J Primatol. 1993;60(3):164–168. doi: 10.1159/000156685. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Conney JC, Rasgon NL, Fairbanks LA, Small GW. Mood symptoms and cognitive performance in women estrogen users and nonusers and men. J Am Geriatr Soc. 2002;50(11):1826–1830. doi: 10.1046/j.1532-5415.2002.50511.x. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Kallan MJ, Have TT, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: A randomized, controlled trial. Biol Psychiatry. 2004;55(4):406–412. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An Introduction to Behavioral Endocrinology. Third Edition Sinauer; Sunderland, MA.: 2005a. Hormones and affective disorders; pp. 773–816. [Google Scholar]
- Nelson RJ. An Introduction to Behavioral Endocrinology. Third Edition Sinauer; Sunderland, MA.: 2005b. Sex differences in behavior: Sex determination and differentiation; pp. 109–168. [Google Scholar]
- Neubauer AC, Grabner RH, Fink A, Neuper C. Intelligence and neural efficiency: Further evidence of the influence of task content and sex on the brain-IQ relationship. Cogn Brain Res. 2005;25:217–225. doi: 10.1016/j.cogbrainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Neumeister A, HU X, Luckenbaugh DA, Schwartz M, Nugent AC, Bonne O, Herscovitch P, et al. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and control. Arch Gen Psychiatry. 2006;63(9):978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- Nigg JT. What causes ADHD? Understanding what goes wrong and why. The Guilford Press; New York: 2006. [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41(1):59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. An interactive model for the emergence of gender differences in depression in adolescence. J Res Adolesc. 1994;4(4):519–534. [Google Scholar]
- Norman AW, Litwack G. Hormones. 2nd Ed Academic Press; San Diego: 1997. [Google Scholar]
- Nottelmann ED, Susman EJ, Inoff-Germain G, Cutler GB, Loriaux DL, Chrousos GP. Developmental processes in early adolescence: Relationships between adolescent adjustment problems and chronologic age, pubertal stage, and puberty-related serum hormone levels. J Pediatr. 1987;110(3):473–480. doi: 10.1016/s0022-3476(87)80521-8. [DOI] [PubMed] [Google Scholar]
- Oinonen KA, Mazmanian D. Effects of oral contraceptives on daily self-ratings of positive and negative affect. J Psychosom Res. 2001;51:647–658. doi: 10.1016/s0022-3999(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Okten A, Kalyoncu M, Yaris N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Hum Dev. 2002;70(1-2):47–54. doi: 10.1016/s0378-3782(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Paikoff RL, Brooks-Gunn J, Warren MP. Effects of girls’ hormonal status on depressive and aggressive symptoms over the course of one year. J Youth Adolesc. 1991;20(2):191–215. doi: 10.1007/BF01537608. 191. [DOI] [PubMed] [Google Scholar]
- Pefanco MA, Kenny AM, Kaplan RF, Kuchel G, Walsh S, Kleppinger A, et al. The effect of 3-year treatment with 0.25 mg/day of micronized 17β-estradiol on cognitive function in older postmenopausal women. J Am Geriatr Soc. 2007;55(3):426–431. doi: 10.1111/j.1532-5415.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pillow DR, Pelham WE, Hoza B, Molina BSG, Stultz CH. Confirmatory factor analyses examining Attention Deficit Hyperactivity Disorder symptoms and other childhood disruptive behaviors. J Abnorm Child Psychol. 1998;26(4):293–309. doi: 10.1023/a:1022658618368. [DOI] [PubMed] [Google Scholar]
- Poromaa IS, Smith S, Gulinello M. GABA receptors, progesterone, and premenstrual dysphoric disorder. Arch Womens Mental Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- Posner MI. Cognitive Neuroscience of Attention. Guilford Press; New York: 2004. [Google Scholar]
- Price TS, Simonoff E, Asherson P, Curran S, Kuntsi J, Waldman I, Plomin R. Continuity and change in preschool ADHD symptoms: Longitudinal genetic analysis with contrast effects. Behav Genet. 2005;35(2):121–132. doi: 10.1007/s10519-004-1013-x. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. PNAS. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N. Brain neuroplasticity in healthy, hyperactive, and psychotic children: Insights from neuroimaging. Neuropsychopharmacology. 2008;33:181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Dunkin J, Fairbanks L, Altshuler LL, Troung C, Elman S, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: A pilot study. J Psychiatric Res. 2007;41(3-4):338–343. doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Mirza Y, Easter PC. Imaging and neurocircuitry of pediatric major depression. Clin Neuropsychiatry: J Treatment Eval. 2006;3(3):219–229. [Google Scholar]
- Rosenblitt JC, Soler H, Johnson SE, Quadagno DM. Sensation-seeking and hormones in men and women: Exploring the link. Horm Behav. 2001;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Puberty and its disorders in girls. Endocrinol Metab Clin North Am. 1991;20(1):15–42. [PubMed] [Google Scholar]
- Ross JL, Stefanatos GA, Kushner H, Zinn A, Bondy C, Roeltgen D. Persistent cognitive deficits in adult women with Turner syndrome. Neurology. 2002;58:218–225. doi: 10.1212/wnl.58.2.218. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: Implications for affective regulation. Biol Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. J Child Psychol Psychiatry. 2002;43(8):988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Berger DF. An animal model of Attention Deficit Disorder: The female shows more behavioral problems and is more impulsive than the male. Eur Psychologist. 1996;1(2):113–122. [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of Attention-Deficit/Hyperactivity Disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St. Clair LS. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. 2002;27(8):907–920. doi: 10.1016/s0306-4530(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006:254–255. 120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Valera E, Doyle AE, Faraone SV. Impact of gender and age on executive functioning: Do girls and boys with and without attention deficit hyperactivity disorder differ neuropsychologically in preteen and teenage years? Dev Neuropsychology. 2005;27(1):79–105. doi: 10.1207/s15326942dn2701_4. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. ILAR J. 2004;45(2):189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior ad physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (macaca fascicularis) Biol Psychol. 2005;69(1):67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, et al. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: A finishing school for male social behavior. Ann N Y Acad Sci. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Causal models of Attention-Deficit/Hyperactivity Disorder: From common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: From menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Stevenson JC, Everson PM, Williams DC, Hipskind G, Grimes M, Mahaoney ER. Attention Deficit/Hyperactivity Disorder (ADHD) symptoms and digit ratios in a college sample. Am J Hum Biol. 2007;19:41–50. doi: 10.1002/ajhb.20571. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Chrousos GP. Negative affect and hormone levels in young adolescents: Concurrent and predictive perspectives. J Youth Adolesc. 1991;20(2):167–190. doi: 10.1007/BF01537607. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Inoff-Germain, Nottelmann ED, Loriaux DL, Cutler GB, Chrousos GP. Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Dev. 1987;58:1114–1134. [PubMed] [Google Scholar]
- Tobet SA, Fox TO. Sex differences in neuronal morphology influenced hormonally throughout life. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of Behavioral Neurobiology: Sexual Differentiation. Vol. 11. Plenum Press; New York: 1992. pp. 41–83. [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, et al. The NIH MRI study of normal brain development: Performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Greenhalgh KD, Manning JT. Second to fourth finger ratio and possible precursors of developmental psychopathology in preschool children. Early Hum Dev. 2003;72(1):57–65. doi: 10.1016/s0378-3782(03)00012-4. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: Effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180(2):155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. A new model for determining the influence of age and sex on functional recovery following hypoxic-ischemic brain damage. Dev Neurosci. 2005;27(2-4):112–120. doi: 10.1159/000085982. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Cliff NR, Shirtcliff EA, Woods KE. The origins and development of psychopathology in females and males. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Second Edition. Vol. 1. John Wiley & Sons; Hoboken, NJ: 2006. pp. 76–138. [Google Scholar]
- Zuckerman M. Neurobiology of Personality. 2nd Ed Cambridge University Press; Cambridge, New York: 2005. [Google Scholar]
- Zweifel JE, O’Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22(3):189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]