Abstract
The EGFR-directed antibody cetuximab has proven, albeit modest, clinical benefit as monotherapy in head and neck and colorectal cancer. In a recent study, Yonesaka et al. uncovered a new mechanism of cetuximab resistance mediated by increased ERBB2 signaling via amplification of ERBB2 or increased levels of the ERBB3/ERBB4 ligand heregulin.
The advent of targeted biologic agents has revolutionized treatment for many types of cancer by focusing therapy on specific aberrant signaling pathway components. One such target the epidermal growth factor receptor (EGFR, or ERBB1) is overexpressed in many cancers, including colorectal (CRC), head and neck, bladder, and non-small cell lung cancer (NSCLC), and overexpression correlates with poor outcomes. Cetuximab, a monoclonal antibody directed against the ligand-binding domain of the EGFR, can inhibit cell growth and survival in a number of malignancies, and is FDA-approved for the treatment of head and neck squamous cell carcinoma and metastatic CRC.
Treatment response to cetuximab, however, has not been uniform as a subset of patients show poor response upfront while others develop resistance after initial benefit. Well-established causes of de novo cetuximab resistance include activating mutations in KRAS codons 12 and 13 and BRAF, while the impact of PIK3CA and NRAS mutations is less clear. A recent study by Yonesaka et al. has identified a new mechanism of de novo and acquired resistance to cetuximab therapy via increased signaling through ERBB2, a member of the ErbB family of receptor tyrosine kinases (Yonesaka et al., 2011). Importantly, they demonstrate that either amplification of ERBB2 or increased levels of the ERBB3/ERBB4 ligand heregulin are present in patients with CRC that exhibited de novo or acquired cetuximab resistance.
Resistance to EGFR-targeted therapy is typically mediated though alternate means of extracellular signal-regulated kinase 1/2 (ERK1/2) activation that bypass EGFR either via alternative receptors at the plasma membrane or constitutively-active downstream components. By generating cetuximab-resistant cell lines, Yonesaka et al. first identified multiple clones that exhibited less effective suppression of ERK1/2 phosphorylation in the presence of cetuximab. Further analysis of these clones revealed amplification of ERBB2 with corresponding increases in total and phospho-ERBB2 levels. Subsequent depletion of ERBB2 in the resistant clones restored sensitivity to cetuximab, confirming the importance of ERBB2 in the resistant phenotype.
In addition to ERBB2 amplification, Yonesaka et al. uncovered a subset of cetuximab-resistant clones where acquired resistance was mediated instead by increased levels of heregulin, a ligand that binds ERBB3 and ERBB4. These elevated levels of heregulin resulted in increased association between ERBB2 and ERBB3 and subsequent activation of downstream targets. Interestingly, this is analogous to hormone-refractory breast cancer where heregulin induces estrogen independence and metastasis in part through an increase in ERK1/2 and suspected ERBB activity (Atlas et al., 2003). The expression levels of other ERBB ligands were not addressed by Yonesaka et al. and so their potential contribution to ERBB2-mediated resistance in this experimental system is unclear. The role, if any, of these other ligands would be interesting as upregulation of some ligands may also lead to ERBB2 activation (and hence, cetuximab resistance), while upregulation of other ligands (i.e. EREG and AREG) in the pre-treatment setting has been shown to correlate instead with sensitivity to cetuximab (Bertotti et al., 2011; Khambata-Ford et al., 2007)
As a complement to these in vitro results, Yonesaka and colleagues were able to demonstrate that alterations in ERBB2 signaling correlate with acquired resistance to cetuximab in a clinical setting. Though the number of analyzed patient samples was limited, amplification of ERBB2 and increased heregulin levels were observed after patients became non-responsive to cetuximab therapy. Yonesaka et al. also presented more comprehensive clinical data indicating that ERBB2 amplification and elevated heregulin play a role in de novo resistance as well. In CRC patients treated with cetuximab, levels of serum heregulin protein and tumor heregulin mRNA, though widely variable, were significantly higher in patients with stable or progressive disease. These higher levels of heregulin appeared to correlate with reduced progression-free and overall survival. The presence of ERBB2 amplification in a larger patient cohort also correlated with worse overall survival. Of the 233 CRC patients examined, approximately 5.5% showed amplification of ERBB2, which is analogous to the 5.2% of metastatic CRC and 3.6% of head and neck squamous cell carcinomas with ERBB2 amplification found in other cohorts (Personeni et al., 2008; Stransky et al., 2011). Importantly, elevated heregulin or ERBB2 amplification resulted in worse survival among patients with wild-type KRAS when examined independently. Overall, the specificity of these correlations is somewhat uncertain since we do not yet know if ERBB2 amplification or elevated heregulin levels are poor prognostic factors independent of cetuximab therapy. Furthermore, data on progression-free and overall survival should be interpreted with caution as these are retrospective analyses on a heterogeneous group of patients.
In a complementary report by Bertotti et al. using 85 patient-derived CRC xenografts, ERBB2 was amplified in 36% of KRAS/NRAS/BRAF/PIK3CA wild-type tumors that were resistant to cetuximab (Bertotti et al., 2011). This amplification was greatly enriched in this population as ERBB2 was amplified in only 2.7% of unselected CRCs.
Similar to the mechanism of resistance reported by Yonesaka et al., resistance through bypass signaling is seen with other EGFR-targeted agents. In NSCLC, amplification of MET was associated with resistance to the reversible EGFR tyrosine kinase inhibitor gefitinib via ERBB3 activation (Engelman et al., 2007). In HER2 (ERBB2) amplification-positive breast cancers, resistance to trastuzumab, a humanized antibody directed against the extracellular domain of HER2, can be mediated by overexpression of EGFR and EGFR ligands EGF, TGF-α, and HB-EGF (Ritter et al., 2007). These studies and that presented by Yonesaka et al. illustrate that the effectiveness of monotherapy targeting one member of the ErbB family may be compromised by compensatory upregulation or activation of another ERBB receptor or related receptor tyrosine kinases (Figure 1).
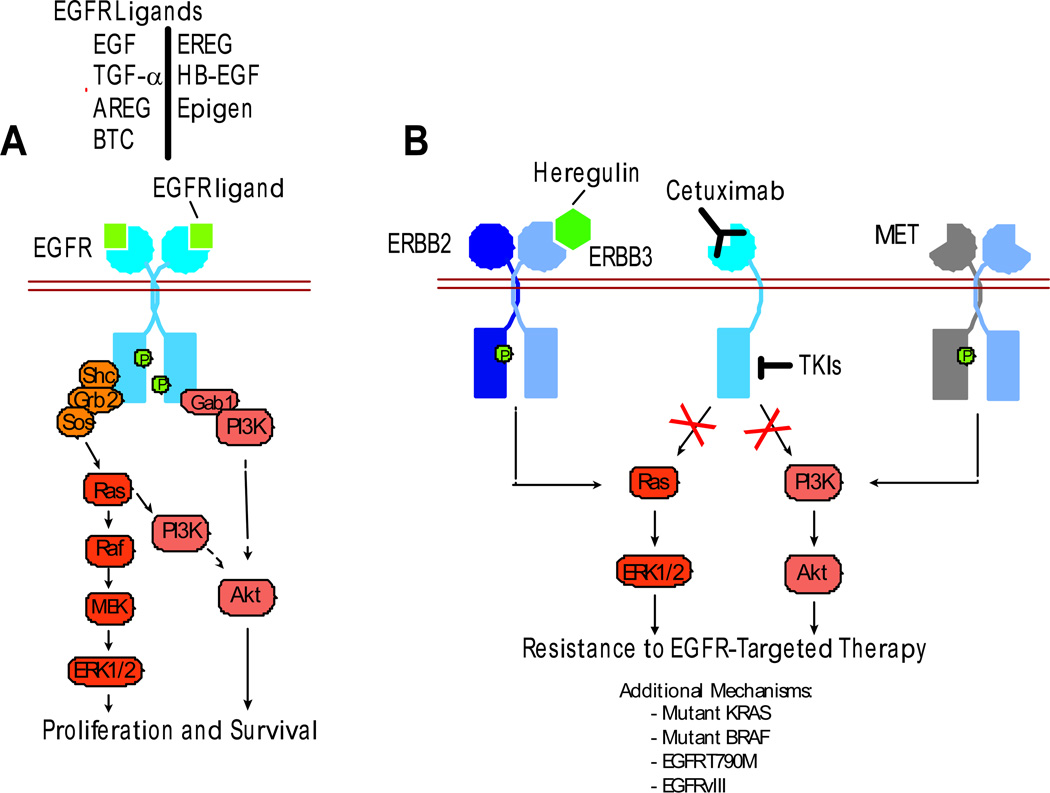
Figure 1. Mechanisms of Resistance to EGFR-Targeted Therapy.
(A) Seven different mammalian EGFR ligands bind and activate EGFR, initiating complex signaling cascades that include activation of ERK1/2 (proliferation) and Akt (survival). (B) In solid tumors, monoclonal antibodies that block EGFR ligand binding (e.g. cetuximab) or EGFR-selective tyrosine kinase inhibitors (TKIs) prevent activation of downstream signaling components. De novo or acquired resistance to cetuximab may develop through ERBB2 amplification and increased levels of the ERBB3/ERBB4 ligand heregulin (Yonesaka et al., 2011), or to TKIs through MET amplification (Engelman et al., 2007) - in both instances, ERBB3 is activated. Additional mechanisms of resistance to EGFR targeted therapies may exist de novo (mutant KRAS, BRAF, EGFRvIII) or be selected for (EGFR T790M).
The identification of ERBB2 as a mediator of de novo and acquired cetuximab resistance suggests that a strategy of combined targeted therapy in EGFR-driven malignancies might improve treatment outcomes in a select group of patients. In preclinical in vitro studies, combined treatment with cetuximab and an ERBB2-targeted agent inhibited growth of cetuximab-resistant cells (Quesnelle and Grandis, 2011; Yonesaka et al., 2011). Additionally, treatment of cetuximab-resistant, KRAS/NRAS/BRAF/PIK3CA wild-type, ERBB2-amplified patient-derived xenografts with combined EGFR and ERBB2-inhibition resulted in significant tumor regression (Bertotti et al., 2011). Early clinical studies thus far with combined ERBB receptor inhibition, however, have been more equivocal. In ERBB2-amplified breast cancers, combined trastuzumab and gefitinib treatment was well tolerated, but the addition of gefitinib seemed to provide no obvious benefit (Arteaga et al., 2008). Whether a strategy of combined EGFR and ERBB2 inhibition will prove successful in malignancies amenable to EGFR blockade remains to be seen and is the subject of on-going clinical trials. Consideration of ERBB2 amplification status with regard to optimal timing of treatment (i.e. upfront combination or delayed addition of ERBB2-targeted therapy) may provide an additional means to improve outcomes in this patient population. Ultimately, the question remains as to whether dual EGFR/ERBB2 inhibition will itself be limited by de novo or induced upregulation of the remaining ERBB receptors. This answer will become more evident as we begin to examine the clinical outcome of combined EGFR and ERBB2-targeted therapy.
References
- Arteaga CL, O'Neill A, Moulder SL, Pins M, Sparano JA, Sledge GW, Davidson NE. A phase I-II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2)-overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14:6277–6283. doi: 10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas E, Cardillo M, Mehmi I, Zahedkargaran H, Tang C, Lupu R. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–175. [PubMed] [Google Scholar]
- Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C, et al. A molecularly annotated platform of patient-derived xenografts ('xenopatients') identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. In Cancer Discovery. 2011 doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- Personeni N, Fieuws S, Piessevaux H, De Hertogh G, De Schutter J, Biesmans B, De Roock W, Capoen A, Debiec-Rychter M, Van Laethem JL, et al. Clinical usefulness of EGFR gene copy number as a predictive marker in colorectal cancer patients treated with cetuximab: a fluorescent in situ hybridization study. Clin Cancer Res. 2008;14:5869–5876. doi: 10.1158/1078-0432.CCR-08-0449. [DOI] [PubMed] [Google Scholar]
- Quesnelle KM, Grandis JR. Dual Kinase Inhibition of EGFR and HER2 Overcomes Resistance to Cetuximab in a Novel In Vivo Model of Acquired Cetuximab Resistance. Clin Cancer Res. 2011;17:5935–5944. doi: 10.1158/1078-0432.CCR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, et al. Activation of ERBB2 Signaling Causes Resistance to the EGFR-Directed Therapeutic Antibody Cetuximab. Sci Transl Med. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]