Summary
Plasma sphingosine-1-phosphate (S1P) regulates vascular permeability, and plasma and lymph S1P guide lymphocyte egress from lymphoid organs. S1P is made intracellularly, and little is known about how S1P is delivered into circulatory fluids. Here we find that mice without the major facilitator superfamily transporter Spns2 have a profound reduction in lymph S1P, but only a minor decrease in plasma S1P. Spns2-deficient mice have a redistribution of lymphocytes from the spleen to lymph nodes and a loss of circulating lymphocytes, consistent with normal egress from the spleen directed by plasma S1P and blocked egress from lymph nodes directed by lymph S1P. Spns2 is needed in endothelial cells to supply lymph S1P and support lymphocyte circulation. As the first differential requirement for lymph and blood S1P to our knowledge, Spns2 may be an attractive target for immune suppressive drugs.
Introduction
The concentration of sphingosine-1-phosphate (S1P) is high in circulatory fluids. Plasma S1P regulates vascular integrity; loss of plasma S1P causes increased vascular permeability, likely due to loss of signaling through S1P receptor 1 (S1PR1) on endothelial cells(Camerer et al., 2009; Lee et al., 1999). Plasma and lymph S1P guide lymphocyte egress from lymphoid organs into circulation; exit requires an S1P gradient, low in the organ parenchyma compared to the exit site, that is sensed by lymphocytes via S1PR1(Schwab and Cyster, 2007). Although extracellular S1P in tissues is thought to be low in homeostasis, an influx of plasma S1P may be a powerful pro-inflammatory stimulus(Rivera et al., 2008). FTY720, a drug recently approved for treatment of multiple sclerosis, targets four of five S1P receptors(Brinkmann et al., 2010). By inhibiting S1PR1 signaling in lymphocytes, FTY720 traps activated cells in the draining lymph nodes and prevents them from reaching the central nervous system. FTY720 may however also target S1P signaling in endothelial cells and cardiomyocytes, contributing to sideeffects including macular edema and bradycardia (Brinkmann et al., 2010). A major goal of therapies that manipulate S1P signaling is to achieve greater tissue selectivity.
Many questions remain about how S1P distribution is controlled; one outstanding problem is how S1P, made intracellularly by sphingosine kinases, is exported to the extracellular space where it can signal through cell surface receptors. This question is particularly interesting because S1P is thought to be made by all cells as an intermediate in sphingolipid metabolism, hence export capacity may be a determinant of whether this S1P is further metabolized, acts on intracellular targets, or is used for cell-cell communication(Saba and Hla, 2004). Several ABC family transporters have been implicated in S1P secretion in vitro. Knockdown or pharmacological inhibition of ABCC1 inhibits S1P export from vascular endothelial cells, mast cells, MCF-7 breast cancer cells, skin fibroblasts, and rat myometrial cells; knockdown or pharmacological inhibition of ABCG2 further decreases S1P export from MCF-7 cells; deletion or pharmacological inhibition of ABCA1 limits S1P release from vascular endothelial cells and astrocytes(Lee et al., 2007; Mitra et al., 2006; Nieuwenhuis et al., 2009; Sato et al., 2007; Takabe et al., 2010; Tanfin et al., 2011). However, neither Abcc1−/− nor Abca1−/− mice have decreased plasma S1P (Lee et al., 2007). The major facilitator superfamily member Spns2 enables extracellular delivery of S1P, and zebrafish lacking this transporter develop cardia bifida similar to animals lacking S1PR2; Spns2 may transport S1P from the yolk sac into the embryo body to promote myocardial precursor migration to the midline(Kawahara et al., 2009). Spns2 expression by endothelial cells has recently been reported to promote egress of mature T cells from the thymus, likely due to local secretion of S1P at the exit site (Fukuhara et al., 2012; Hisano et al., 2012; Nijnik et al., 2012).
Here we find that Spns2-deficient mice have a profound reduction in lymph S1P, with only a minor change in plasma S1P. Spns2-deficient mice have a redistribution of lymphocytes from the spleen to lymph nodes and loss of lymphocytes in circulation, consistent with normal egress from the spleen directed by plasma S1P and blocked egress from lymph nodes directed by lymph S1P. Spns2 is required in endothelial cells to supply lymph S1P and support lymphocyte circulation.
Results and Discussion
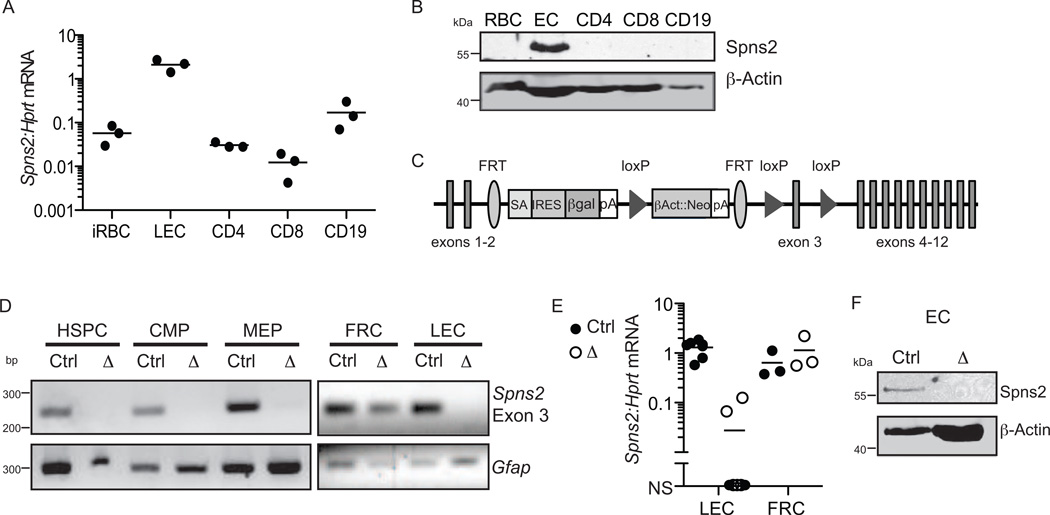
After confirming that murine Spns2 can export S1P (Fig. S1), we asked whether Spns2 is expressed in the cells that supply circulatory S1P. These cells have been identified primarily by lineage-specific deletion of the sphingosine kinases. Hematopoietic cells are the main source of plasma S1P, with red blood cells (RBC) a major contributor (Pappu et al., 2007). RBC have lost most of their mRNA, but Spns2 transcript is scarce in their immediate precursor reticulocytes (Fig. 1A). Spns2 protein was undetectable in RBC (Fig. 1B). Lymphatic endothelial cells are the main source of lymph S1P (Pham et al., 2010). Spns2 mRNA is robustly expressed by lymph node lymphatic endothelial cells (Fig. 1A), and Spns2 protein was readily detected in endothelial cells (Fig. 1B).
Figure 1. Murine Spns2 is expressed by endothelial cells but not RBC.
(A) Expression of Spns2 mRNA by the indicated cell populations, expressed relative to hypoxanthine-guanine phosphoribosyltransferase (Hprt) transcript, assessed by RT-qPCR. Ter119+CD71+ immature RBC (iRBC) were sorted from bone marrow; CD31+gp38+ lymphatic endothelial cells (LEC), CD4+CD62Lhi T cells (CD4), CD8+CD62Lhi T cells (CD8), and CD19+CD62Lhi B cells (CD19) were sorted from lymph nodes. Data compile 3 experiments with mice on a B6 background.
(B) Expression of Spns2 protein by the indicated cell populations, assessed by Western blot. RBC were isolated from blood by differential centrifugation; CD31+ endothelial cells (EC) were isolated from heart and lung by magnetic bead enrichment; and CD4+ T cells, CD8+ T cells, and CD19+ B cells were isolated from lymph nodes by magnetic bead enrichment. Data are representative of 3 experiments with mice on a B6 background.
(C) Spns2-targeted allele. SA: splice acceptor; pA: polyadenylation signal.
(D-F) Efficiency of Spns2 deletion in Spns2f/fTie2-Cre+ mice.
(D) PCR for Spns2 exon 3 in genomic DNA from sorted Spns2f/fTie2Cre+(Δ) or littermate control (Ctrl) cells. Littermate controls maintained one or two intact alleles of Spns2. RBC progenitors were isolated from bone marrow. Hematopoietic stem and progenitor cells (HSPC) were defined as Lin−IL7Rα−c-Kit+Sca1+; common myeloid progenitors (CMP) as Lin−IL7Rα−c Kit+Sca1−FcγRloCD34+; and megakaryocyte erythroid progenitors (MEP) as Lin−IL7Rα−c-Kit+Sca1−FcγRloCD34−. Lymphatic endothelial cells (LEC), defined as CD45−CD31+gp38+, and fibroblastic reticular cells (FRC), defined as CD45−CD31−gp38+ were isolated from lymph nodes. Data are representative of at least 2 experiments. (E) Spns2 mRNA assessed by RT-qPCR of transcripts from sorted LEC and FRC. Data compile 7 pairs of mice analyzed in 7 experiments for LEC and 3 pairs of mice analyzed in 3 experiments for FRC. NS, no signal. (F) Spns2 protein assessed by Western blot of mixed heart and lung endothelial cells (EC). Data are representative of 2 experiments. See also Fig. S1.
Based on these results, we hypothesized that if Spns2 were important in secretion of circulatory S1P, it might have a selective role in supplying S1P to lymph. To test this, we obtained Spns2- mutant mice from the NIH Knockout Mouse Project. A splice acceptor preceding exon 3 prematurely terminates the transcript, and we refer to this allele as Spns2tr. After flippase-mediated recombination, the original sequence is restored except that exon 3 remains flanked by loxP sites, and we refer to this allele as Spns2f. After Cre-mediated recombination, the loss of exon 3 results in a truncated non-functional protein (Fig. 1C). We crossed Spns2f/f animals with mice carrying Cre recombinase under the Tie2 promoter, which is expressed in hematopoietic and endothelial cells(Ficara et al., 2008; Kisanuki et al., 2001; Srinivasan et al., 2007). In Spns2f/fTie2Cre+ mice, exon 3 of Spns2 was efficiently deleted in genomic DNA of hematopoietic stem cells, common myeloid progenitors, and megakaryocyte-erythrocyte progenitors (red blood cells and reticulocytes are anucleate), as well as lymph node lymphatic endothelial cells, but not lymph node fibroblastic reticular cells (Fig. 1D). Furthermore, Spns2 transcript was lost in Spns2f/fTie2-Cre+ lymph node lymphatic endothelial cells but not lymph node fibroblastic reticular cells (Fig. 1E), and Spns2 protein was lost in Spns2f/fTie2-Cre+ endothelial cells (Fig. 1F). The loss of Spns2 mRNA and protein was substantial but not complete in Spns2tr/tr mice (data not shown), hence for most experiments we used Spns2f/fTie2-Cre+ animals.
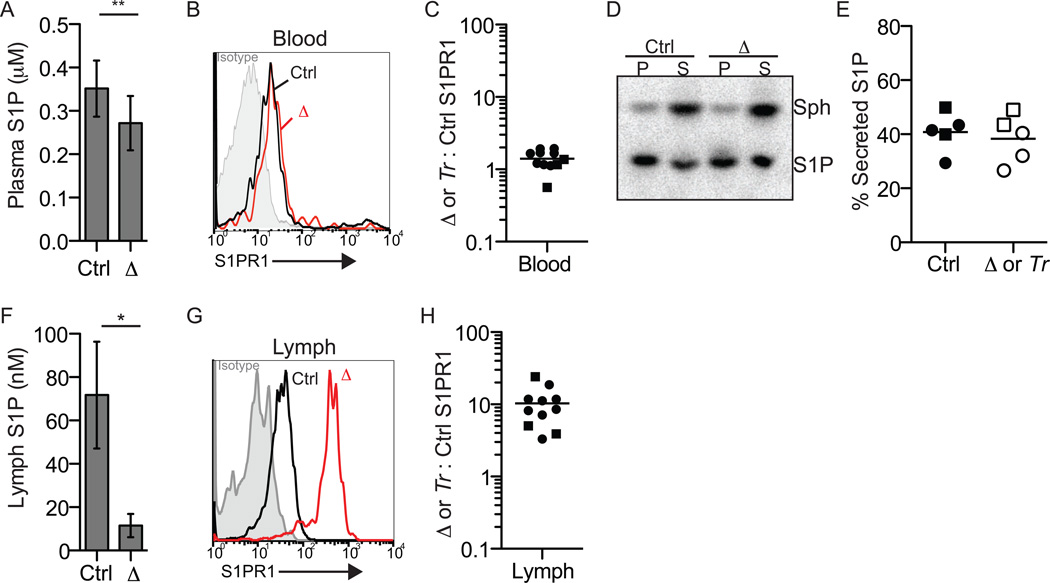
We first assessed whether blood plasma S1P was altered in Spns2-deficient mice. Tandem mass spectrometry measurements revealed little reduction (~23%) of plasma S1P in Spns2f/fTie2-Cre+ mice compared to littermate controls (Fig. 2A), similar to previous reports (Fukuhara et al., 2012; Hisano et al., 2012; Nijnik et al., 2012). As a complementary measure of S1P, we examined surface S1PR1 expression on naïve T cells circulating in blood; S1PR1 is exquisitely sensitive to ligand-mediated internalization, and its surface expression has been used extensively as a measure of cell exposure to S1P (Liu et al., 1999; Pappu et al., 2007; Pham et al., 2010; Schwab et al., 2005). Naïve T cells in the blood exhibited similarly low receptor levels in Spns2tr/tr mice, Spns2f/fTie2-Cre+ mice, and littermate controls, suggesting that lymphocytes sense equivalent S1P (Fig. 2B,C). Consistent with near wild-type plasma S1P levels in Spns2- deficient mice, Spns2-deficient RBC secrete S1P normally (Fig. 2D,E).
Figure 2. Spns2 is essential to supply lymph S1P, but makes a minor contribution to plasma S1P.
(A-E) Spns2 makes a minor contribution to plasma S1P.
(A) Plasma S1P of Spns2f/fTie2-Cre+ (Δ) and littermate control mice quantified by mass spectrometry (n=6, error bars show standard deviation). (B) Surface S1PR1 on CD62LhiCD4+T cells circulating in the blood of a representative Spns2f/fTie2-Cre+ mouse (red) and its littermate control (black). Isotype control is shaded grey; note that based on staining of S1PR1 knockout animals, the isotype control staining may be artificially low (Green et al. 2011 and data not shown). (C) The ratio of surface S1PR1 MFI on CD62LhiCD4+T cells in the blood of a Spns2f/fTie2-Cre+ or Spns2tr/tr (Tr) mouse to surface S1PR1 MFI on CD62LhiCD4+ T cells in the blood of its littermate control. Circles indicate Spns2f/fTie2-Cre+ mice and controls (8 pairs analyzed in 7 experiments), and squares indicate Spns2tr/tr mice and controls (3 pairs analyzed in 3 experiments). (D-E) Spns2f/fTie2-Cre+ or Spns2tr/tr and littermate control RBC were incubated with [3-3H]sphingosine (Sph), which crosses the plasma membrane into the cytosol where it can be phosphorylated. After 90 minutes, the cell pellet (P) and supernatant (S) were collected. Extracted lipids were separated by thin layer chromatography (TLC) to assess the distribution of [3-3H]S1P. (D) A representative TLC plate visualized by Phosphorimager. (E) Data pooled from 5 experiments. % secreted S1P: 100×[S1P in supernatant]/[S1P in pellet + S1P in supernatant]. Circles indicate Spns2f/fTie2-Cre+ mice and controls, and squares indicate Spns2tr/tr mice and controls.
(F-H) Spns2 is essential to supply lymph S1P.
(F) Lymph S1P of Spns2f/fTie2-Cre+ mice and littermate controls quantified by mass spectrometry (n=2-3, error bars show standard deviation). (G) Surface S1PR1 on CD62LhiCD4+ T cells circulating in the lymph of a representative Spns2f/fTie2-Cre+ mouse (red) and its littermate control (black). Isotype control is shaded grey. (H) The ratio of surface S1PR1 MFI on CD62LhiCD4+ T cells in the lymph of a Spns2f/fTie2-Cre+ or Spns2tr/tr (Tr) mouse to surface S1PR1 MFI on CD62LhiCD4+ T cells in the lymph of its littermate control. Circles indicate Spns2f/fTie2-Cre+ mice and controls (8 pairs analyzed in 7 experiments), and squares indicate Spns2tr/tr mice and controls (3 pairs analyzed in 3 experiments). *p<0.05, **p<0.01
We next measured lymph S1P in Spns2-deficient mice. Tandem mass spectrometry measurements revealed a dramatic loss of lymph S1P in Spns2f/fTie2-Cre+ mice compared to littermate controls (Fig. 2F). Again, as a complementary measure of S1P, we examined surface S1PR1 expression on naïve T cells in lymph. T cells in the lymph of Spns2tr/tr and Spns2f/fTie2- Cre+ mice expressed robust surface S1PR1 compared to littermate controls (Fig. 2G,H), suggesting that the concentration of lymph S1P is very low in Spns2-mutant animals.
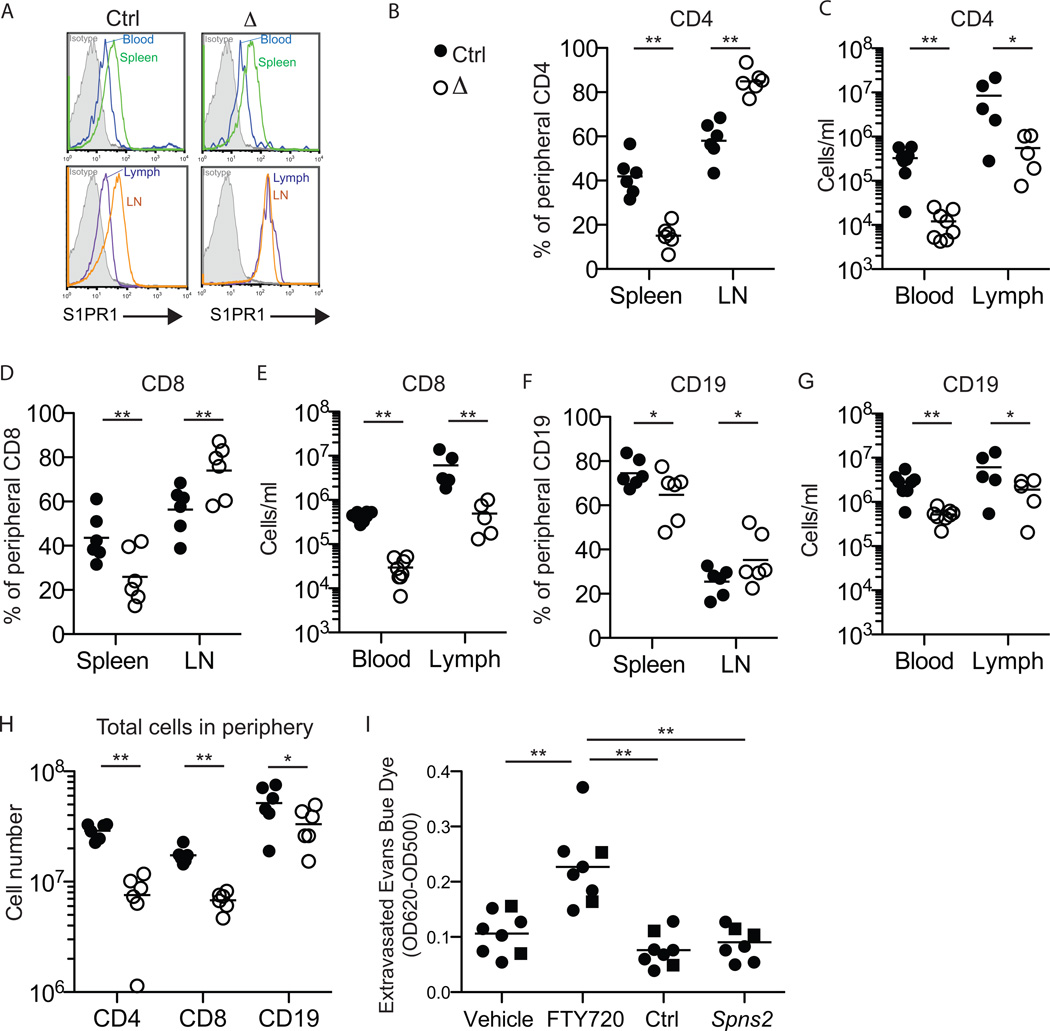
We then turned to the effects of Spns2 deletion on lymphocyte trafficking. Naïve lymphocytes normally circulate among secondary lymphoid organs surveying for antigen. Exit from the spleen is guided by the high concentration of S1P in plasma compared to the spleen. One indication of this gradient is that surface S1PR1 is higher on lymphocytes in the spleen than in blood; this difference is maintained in Spns2f/fTie2Cre+ mice (Fig. 3A). Exit from lymph nodes is guided by the high concentration of S1P in lymph compared to lymph nodes. Again, one indication of this gradient is that surface S1PR1 is higher on lymphocytes in lymph nodes than in lymph; this difference is abolished in Spns2f/fTie2Cre+ mice (Fig. 3A).
Figure 3. Spns2 is required for peripheral lymphocyte circulation.
(A) Surface S1PR1 on CD62LhiCD4+ T cells in the blood and spleen (top panels) and in the lymph and lymph nodes (bottom panels) of a representative Spns2f/fTie2Cre+ mouse (Δ) and its littermate control (Ctrl). Isotype control is shaded grey. Data are representative of 8 pairs of mice analyzed in 7 experiments.
(B-G) Lymphocyte distribution in Spns2f/fTie2Cre+ mice and littermate controls.
(B,D,F) Percent of total peripheral CD62LhiCD4+ T cells (B), CD62LhiCD8+ T cells (D), and CD62LhiCD19+ B cells (F) in the spleen and lymph nodes (LN). Total peripheral lymphocytes are defined as those in spleen and a subset of LN (brachial, axillary, inguinal, and mesenteric); blood and lymph make a negligible contribution. [For example, (B, spleen) shows 100×(# CD62LhiCD4+ T cells in spleen)/(# CD62LhiCD4+ T cells in spleen + # CD62LhiCD4+ T cells in LN).] Data pool 6 pairs of mice analyzed in 5 experiments. (C,E,G) Total number of CD62LhiCD4+ T cells (C), CD62LhiCD8+ T cells (E), and CD62LhiCD19+ B cells (G) in blood and lymph. Data pool 8 pairs of mice analyzed in 7 experiments for blood, and 5 pairs of mice analyzed in 4 experiments for lymph. (H) Total number of CD62LhiCD4+ T cells, CD62LhiCD8+ T cells, and CD62LhiCD19+ B cells in the periphery. Data pool 6 pairs of mice analyzed in 5 experiments.
(I) Vascular permeability in Spns2f/fTie2Cre+ mice and littermate controls.
Spns2-deficient mice and littermate controls, and C57BL6 mice treated with FTY720 or vehicle, were injected intravenously with Evans Blue dye. After 90 minutes, mice were perfused with PBS. Lungs were removed and extravasated Evans Blue dye was quantified by spectrophotometry. Circles indicate Spns2f/fTie2-Cre+ mice and controls (6 groups analyzed in 6 experiments), and squares indicate Spns2tr/tr mice and controls (2 groups analyzed in 2 experiments).
*p<0.05, **p<0.01. See also Fig. S2.
We predicted that in the presence of normal plasma S1P, egress from the spleen would proceed as usual, but in the absence of lymph S1P, egress from lymph nodes would be blocked. Over time, this should lead to a redistribution of lymphocytes from spleen to lymph nodes, as any cell that left the spleen and entered a lymph node would be trapped in the lymph node. This would also lead to a loss of circulating lymphocytes in blood and lymph. Spns2f/fTie2-Cre+ mice had a reduced percentage of total peripheral naïve CD4 T cells in the spleen compared to littermate controls (15% vs. 42%), and reciprocally an increased percentage of total peripheral CD4 T cells in the lymph nodes (85% vs 58%) (Fig. 3B). Furthermore, Spns2f/fTie2-Cre+ mice had a 24-fold reduction in CD4 T cells circulating in blood, and a 10-fold reduction in CD4 T cells circulating in lymph (Fig. 3C). CD8 T cell redistribution in Spns2f/fTie2-Cre+ mice followed the same pattern (Fig. 3D,E). Consistent with previous findings that B cells have higher tolerance for disruption of S1P gradients(Pham et al., 2010; Schwab et al., 2005), the effect on B cells was less dramatic, although it showed the same trend (Fig. 3F,G). Layered on top of the lymphocyte redistribution in Spns2f/fTie2-Cre+mice, we saw an overall reduction in lymphocyte numbers in the periphery (Fig. 3H). Part of this reduction may be due to inefficient egress of mature T cells from the thymus in Spns2-deficient animals (Fig. S2 and Fukuhara et al., 2012; Hisano et al., 2012; Nijnik et al., 2012).
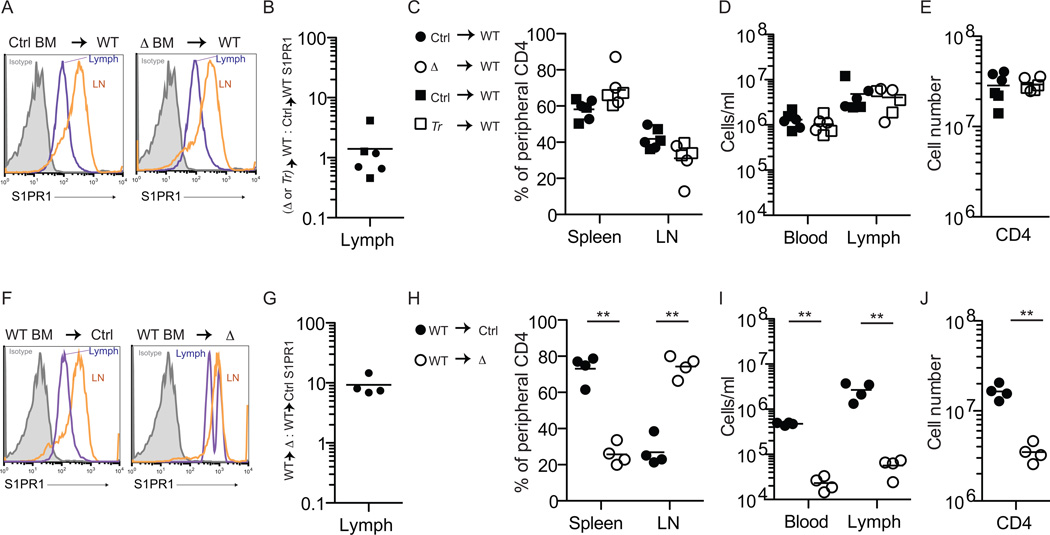
We next asked whether the requirement for Spns2 to supply lymph S1P and enable egress was intrinsic to endothelial cells. Tie2-Cre deletes in both hematopoietic cells and endothelial cells, so to distinguish between the two we made bone marrow (BM) chimeras. First, lethally irradiated wild-type (WT) mice were reconstituted with Spns2-deficient or littermate control BM. We found no reduction in lymph S1P or defect in lymphocyte trafficking in WT mice with Spns2-deficient BM (Fig. 4A-E, Fig. S3, and Fig. S4). In complementary experiments, Spns2f/fTie2Cre+ mice and littermate controls were reconstituted with WT BM. In Spns2f/fTie2Cre+ mice with WT BM, lymph S1P remained low and lymphocyte circulation impaired (Fig. 4F-J, Fig. S3, and Fig. S4). These results are consistent with the requirement for the sphingosine kinases in lymphatic endothelial cells rather than hematopoietic cells to supply lymph S1P (Pham et al. 2010), and with weak-to-undetectable expression of Spns2 in lymphocytes (Fig. 1A,B).
Figure 4. Spns2 is required in endothelial cells to supply lymph S1P and support lymphocyte circulation.
(A-E) Ubiquitin C:GFP+ mice were lethally irradiated and reconstituted with BM from Spns2-deficient mice or littermate controls (GFP+ mice were used to allow assessment of RBC chimerism). Mice were analyzed >6 weeks after transplantation, when RBC and circulating lymphocytes were 97–99% donor-derived. Deletion of Spns2 in Spns2f/fTie2Cre+ (Δ) donor hematopoietic stem and progenitor cells (HSPC) was confirmed by PCR. Data compile 6 pairs of recipients, with 2 pairs of Spns2f/fTie2-Cre+/control BM donors (circles) and 1 pair of Spns2tr/tr (Tr)/control BM donors (squares), analyzed in 6 experiments. (A) Representative histogram of surface S1PR1 on CD62LhiCD4+ T cells in the lymph and lymph nodes of the indicated chimeras. Isotype control is shaded grey. (B) The ratio of surface S1PR1 MFI on CD62LhiCD4+ T cells in the lymph of a WT mouse with Spns2-deficient BM to surface S1PR1 MFI on CD62LhiCD4+ T cells in the lymph of a WT mouse with littermate control BM. (C) Percent of total donor-derived peripheral CD62LhiCD4+ T cells in the spleen and lymph nodes. (D) Total number of donor-derived CD62LhiCD4+ T cells in blood and lymph. (E) Total number of donor-derived CD62LhiCD4+ T cells in the periphery.
(F-J) Spns2f/fTie2-Cre+ mice and littermate controls were lethally irradiated and reconstituted with BM from Ubiquitin C:GFP+ mice. Mice were analyzed >6 weeks after transplantation, when RBC were 88–99% donor-derived and lymphocytes were 85–98% donor-derived. Data compile 4 pairs of mice analyzed in 4 experiments. (F) Representative histogram of surface S1PR1 on CD62LhiCD4+ T cells in the lymph and lymph nodes of the indicated chimeras. Isotype control is shaded grey. (G) The ratio of surface S1PR1 MFI on CD62LhiCD4+ T cells in the lymph of a Spns2f/fTie2-Cre+ mouse with WT BM to surface S1PR1 MFI on CD62LhiCD4+ T cells in the lymph of its littermate control with WT BM. (H) Percent of total donor-derived peripheral CD62LhiCD4+ T cells in the spleen and lymph nodes. (I) Total number of donor-derived CD62LhiCD4+ T cells in blood and lymph. (J) Total number of donor-derived CD62LhiCD4+ T cells in the periphery. *p<0.05, **p<0.01. See also Fig. S3 and Fig. S4.
Taken together, these data demonstrate that Spns2 expression by endothelial cells is essential for secretion of lymph S1P, while Spns2 is dispensable for RBC secretion of plasma S1P. A major goal in improving therapies that disrupt S1P signaling is to achieve greater receptor and tissue specificity. Although FTY720 is remarkably effective in multiple sclerosis, at least in part because it inhibits exit of activated lymphocytes from lymphoid organs and hence prevents them from reaching the brain, FTY720 has serious vascular and cardiac side-effects. As the first differential requirement for lymph and blood S1P to our knowledge, Spns2 may be an attractive target for immune suppressive drugs that inhibit lymphocyte egress while minimizing effects on vascular stability. In fact, we found that there is little difference in lung vascular permeability between Spns2-deficient and control animals, and Spns2-deficient mice show decreased lung vascular permeability compared to mice treated with FTY720 (Fig. 3I). Future work will assess whether Spns2 plays a similarly minor role in other tissues and in inflammation.
As has been previously reported, we find that exit of mature T cells from the thymus of Spns2f/fTie2-Cre+animals is inefficient (Fukuhara et al., 2012; Hisano et al., 2012; Nijnik et al., 2012). Although we cannot exclude a role of other Spns2 substrates in promoting thymic egress, the defect is likely due to disruption of the S1P gradient at the exit site, an interpretation supported by the slight increase in S1PR1 levels on thymocytes of Spns2-deficient mice (Fig. S2). And although we cannot know whether Spns2 plays as dominant a role in S1P export from blood vessel endothelial cells in vivo as it does in lymphatic endothelial cells, it is likely a significant contributor. Endothelial cells have been reported to express several of the ABC transporters implicated in S1P export by cultured cells, but how this expression varies among subsets remains to be determined.
One fascinating question is why there is a loss of peripheral lymphocytes in Spns2-deficient mice. The thymic egress block may be a relatively small part of the explanation. Mice with a similar accumulation of mature T cells in the thymus due to disruption of S1P gradients, such as mice lacking lipid phosphate phosphatase 3 in thymic epithelial cells or mice lacking sphingosine kinases in neural crest-derived thymic pericytes, have a smaller reduction in peripheral T cell numbers than Spns2-deficient mice(Breart et al., 2011; Zachariah and Cyster, 2010). By contrast, mice that lack lymph S1P due to ablation of sphingosine kinases in lymphatic endothelial cells, which does not affect thymic egress because mature T cells exit the thymus into blood, have a similar reduction in peripheral lymphocyte numbers to Spns2-deficient mice(Pham et al., 2010; Zachariah and Cyster, 2010). Future studies will address whether the decrease in overall lymphocyte number in the absence of lymph S1P may reflect a role of S1P itself, or another factor that cells receive during circulation, in promoting survival or homeostatic proliferation (Oskouian and Saba, 2010).
Much work remains to be done to identify the transporters that supply S1P in different tissues and disease states; they may be excellent candidates for local regulation of S1P. As the first known differential requirement for lymph and blood S1P, Spns2 is an attractive target for specific spatial modulation of S1P signaling.
Experimental Procedures
Mice and bone marrow chimeras
Spns2-mutant mice were obtained from the NIH Knockout Mouse Project. β-actin-FLPe (Rodriguez et al., 2000), Tie2-Cre (Kisanuki et al., 2001), R26R-EYFP (Srinivas et al., 2001), and Ubiquitin C-GFP mice (Schaefer et al., 2001) were from Jackson laboratories. All mice were on a C57BL6 background. For bone marrow chimeras, recipients were irradiated with two 6.5 Gray doses of γ irradiation from a cesium source separated by 3 hours, and received 2–10 × 106 bone marrow cells by intravenous injection. Chimeras were analyzed at least 6 weeks after transplantation. Mice were housed in specific pathogen-free conditions at the Skirball Institute animal facility. All animal experiments were performed in accordance with protocols approved by the New York University Institutional Animal Care and Use Committee.
Cell preparation and analysis
Cells were enriched either by magnetic beads (Stem Cell Technologies, biotin selection kit, used according to the manufacturer’s instructions), flow cytometry (Beckman Coulter MoFlo or BD Biosciences FACSAria), or differential centrifugation. S1P export assay was adapted from(Olivera and Spiegel, 1998), vascular permeability assay was adapted from(Camerer et al., 2009), and genomic PCR, RT-qPCR, Western blot, mass spectrometry, confocal microscopy, and flow cytometry were performed using standard procedures. (Please see Extended Experimental Procedures for detailed protocols.)
Statistics
All comparisons are by Student’s 2-tailed paired t-test. When data is plotted on a log-scale, log-transformed data are compared.
Supplementary Material
Highlights.
The transporter Spns2 is required to supply lymph but not plasma S1P
Spns2-deficient mice have disrupted peripheral lymphocyte circulation
Spns2 is required in endothelial cells to secrete lymph S1P and support trafficking
Acknowledgements
This work was supported by NIH grant R01AI085166 and an NYU Whitehead Fellowship to SRS, NIH grants R01GM50388 and P20RR021954 to AJM, NIH training grant T32-HD007520 to AM, a Human Frontier Science Program Long-Term Fellowship to BB, and NIH training grant T32-CA009161 (Levy) to WDRP. We thank Ken Cadwell, Jason Cyster, David Fooksman, Jason Hall, Sergei Koralov, MacLean Sellars, and Leslie Summers deLuca for critical comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breart B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y, Adams RH, Lafaille JJ, Escalante-Alcalde D, Morris AJ, et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208:1267–1278. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell stem cell. 2008;2:484–496. doi: 10.1016/j.stem.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CDC, Schmidt TH, Xu Y, Proia RL, Coughlin SR, et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunology. 2011;12:672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 Functions as a Sphingosine-1-Phosphate Transporter in Vascular Endothelial Cells. PloS one. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis B, Luth A, Chun J, Huwiler A, Pfeilschifter J, Schafer-Korting M, Kleuser B. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P(3) in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. Journal of molecular medicine. 2009;87:645–657. doi: 10.1007/s00109-009-0468-x. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Clare S, Hale C, Chen J, Raisen C, Mottram L, Lucas M, Estabel J, Ryder E, Adissu H, et al. The role of sphingosine-1-phosphate transporter spns2 in immune system function. Journal of immunology. 2012;189:102–111. doi: 10.4049/jimmunol.1200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine kinase. Assay and product analysis. Methods in molecular biology. 1998;105:233–242. doi: 10.1385/0-89603-491-7:233. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Saba JD. Cancer treatment strategies targeting sphingolipid metabolism. Advances in experimental medicine and biology. 2010;688:185–205. doi: 10.1007/978-1-4419-6741-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. S11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nature genetics. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. 2007;103:2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cellular immunology. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes & development. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. The Journal of biological chemistry. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanfin Z, Serrano-Sanchez M, Leiber D. ATP-binding cassette ABCC1 is involved in the release of sphingosine 1-phosphate from rat uterine leiomyoma ELT3 cells and late pregnant rat myometrium. Cellular signalling. 2011;23:1997–2004. doi: 10.1016/j.cellsig.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.