Abstract
Idiosyncratic drug-induced liver injury (DILI) is an important but relatively infrequent cause of potentially severe acute and chronic liver injury. The aim of this clinical research workshop was to review and attempt to standardize the current nomenclature and terminology used in DILI research. Because DILI is a diagnosis of exclusion, selected elements of the medical history, laboratory tests, and previous reports were proposed to improve causality assessment. Definitions and diagnostic criteria regarding the onset of DILI, evolution of liver injury, risk factors, and mandatory testing versus optional testing for competing causes were reviewed. In addition, the role of intentional and inadvertent rechallenge, liver histology, and host genetic polymorphisms in establishing the diagnosis and prognosis of DILI were reviewed. Consensus was established regarding the need to develop a web-of-knowledge database that provides concise, reliable, and updated information on cases of liver injury due to drugs and herbal and dietary supplements. In addition, the need to develop drug-specific computerized causality assessment methods that are derived from prospectively phenotyped cases was a high priority. Proposed scales for grading DILI severity and assessing the likelihood of an agent causing DILI and written criteria for improving the reliability, accuracy, and reproducibility of expert opinion were reviewed. Finally, the unique challenges of assessing causality in children, patients with underlying liver disease, and subjects taking herbal and dietary supplements were discussed. Conclusion: Workshop participants concluded that multicenter referral networks enrolling patients with suspected DILI according to standardized methodologies are needed. These networks should also collect biological samples that may provide crucial insights into the mechanism(s) of DILI with the ultimate aim of preventing future cases of DILI.
Drug-induced liver injury (DILI) is increasingly being recognized as a cause of clinically significant acute and chronic liver disease. It is the leading cause of acute liver failure (ALF) in several Western countries and the most common reason for regulatory actions regarding approved medications.1,2 The lack of objective confirmatory diagnostic tests coupled with the highly variable clinical presentation of DILI can often lead to a delay in recognition. Causality assessment instruments have been developed to help standardize the diagnosis, but currently available instruments have been derived from expert opinion rather than an objective and iterative process of identifying and weighing diagnostic features.3–5
In 2003, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established the Drug-Induced Liver Injury Network (DILIN) as a long-term initiative to promote basic and clinical research involving DILI. The network initiated a prospective study to collect bona fide cases of DILI with the intention of improving the understanding of its causes, mechanisms, and natural history.6,7 An additional goal was to develop improved diagnostic criteria, definitions, and causality assessment instruments to facilitate research. This latter aim was the impetus for organizing an international clinical research workshop on December 1–2, 2008, to review and attempt to standardize current nomenclature and terminology used in DILI research. This summary provides an overview of the major topics discussed at the workshop, an assessment of currently used definitions and causality instruments, and a list of future needs.
Epidemiology of DILI
The incidence of DILI is largely unknown because of the paucity of prospective population-based studies and the relatively low frequency of liver injury attributable to drugs. DILI represents 1.2% to 6.6% of cases of acute liver disease seen at tertiary referral centers.8–10 The incidence of DILI in the general population has been estimated to be 1 to 2 cases per 100,000 person years.11 However, the estimated incidence of DILI was 14 cases per 100,000 patient years in a prospective study from northern France, which is 10-fold higher than the rate reported to regulatory agencies.12
There is marked geographic variability in the agents responsible for causing DILI (Table 1). In Western countries, the majority of cases are associated with antibiotics, anticonvulsants, and psychotropic agents. In the United States, for instance, amoxicillin/clavulanate, isoniazid, nitrofurantoin, and fluoroquinolones are the most frequent causes of DILI.6 Implicated drugs differ slightly between European and US studies, largely because of differences in approved agents and prescribing habits.8,11–13 In Asian countries, herbal and dietary supplements rather than conventional medications are often the most common causes of DILI.14–16 Herbals and dietary supplements currently represent less than 10% of cases of DILI in Western countries, although this proportion appears to be increasing.6
Table 1.
Etiologies and Outcomes of DILI in Different Parts of the World Presented at the Workshop
| Country | Sweden | Spain* | United States (DILIN) | Korea | Japan | Singapore |
|---|---|---|---|---|---|---|
| Years | 1975–2005 | 1994–2008 | 2004–2007 | 2005–2007 | 1997–2006 | 2004–2006 |
| n | 784 | 603 | 300 | 371 | 1676 | 31 |
| Case ascertainment | Government registry | 45 centers | Prospective, 5 centers | Prospective, 17 centers | Retrospective, multicenter | Prospective, population-based |
| HC/mixed/cholestatic (%) | 52/21/29 | 55/21/25 | 56/20/24 | NA | 59/20/21 | 74/6/19 |
| Mean age (years) | 58 (42–74) | 54 (13–88) | 48 ± 18 | 49.0 ± 14.5 | 55 | 51 |
| Female (%) | 57 | 49 | 60 | 63.3 | 57 | 45 |
| Hospitalized (%) | NA | 54 | 54 | 100 | NA | |
| Died or transplanted (%) | 9.2 | 5.4 | 10.1 | 1.3 | 3.7 | 9.6 |
| Chronic DILI (%) | NA | 16.9 | 13.6 | NA | 8.4 | NA |
| Most frequent agents (%) | Antibiotics (27) Analgesics (5) Disulfiram (3.4) Carbamazepine (2.2) Lipid-lowering agents (1) |
Antibiotics (39) CNS agents (15) Analgesics (11) Lipid-lowering agents (5) H2 blockers (5) Endocrine agents (4) |
Antimicrobials (45.5) CNS agents (15) Herbals (9) Immunomodulator (5.5) Analgesics (5) Antihypertensives (5) Antineoplastics (4) Lipid-lowering agents (3.4) |
Herbal drugs (27.5) Prescription drugs (20.8) Health and dietary supplements (13.7) Medicinal herbs or plants (9.4) Folk remedies (8.6) OTC drugs (6.5) |
Antibiotics (14) CNS agents (10) Dietary supplements (10) Analgesics (9.9) Chinese herbals (7.1) |
Traditional CM (54) Prescription drug (26) Malay herb (16) |
Abbreviations: CM, Chinese medicine; CNS, central nervous system; HC, hepatocellular; NA, not available; OTC, over the counter.
Adapted from Lucena et al.50
Diagnostic Elements
DILI is a diagnosis of exclusion that relies on multiple elements in the medical history, presentation, laboratory results, and subsequent course. The key diagnostic elements for DILI include (1) the time to onset, (2) clinical features, (3) the time and course of recovery, (4) specific risk factors, (5) the exclusion of other diagnoses, and (6) previous reports on the hepatotoxicity of the implicated agent. The diagnosis can be further strengthened by (7) rechallenge and, in some cases, (8) liver biopsy, although these latter elements are not always available or even appropriate. Each of these elements is important in assigning causality in DILI, and standardization is needed for all eight.
Medication Time to Onset
The time to onset or latency of DILI is typically measured from the first day on which the suspected agent was taken to the day of onset of symptoms, jaundice, or laboratory test abnormalities, whichever is first. The latency may be difficult to define because the time of starting the medication is unclear, the initial symptoms were vague and are poorly remembered, or laboratory tests results were obtained at an arbitrary time, perhaps days or weeks after the onset of injury. The time to onset may also be difficult to assess because the medication was stopped and started or given in several courses or in various doses. Finally, the onset of injury can occur days or weeks after the medication is stopped (as is typical for amoxicillin/clavulanate hepatotoxicity). Nevertheless, the time to onset, even if not precise, is important in making a diagnosis and in deciding which of several medications, herbals, or dietary supplements being taken is the most likely cause of the injury.3,6 To facilitate research and standardize reporting, it is important to define whether the latency is measured to the date of onset of symptoms, jaundice, or first identified objective laboratory abnormalities.
Clinical and Laboratory Features
The clinical course of DILI is typically categorized as hepatocellular, cholestatic, or mixed hepatocellular-cholestatic on the basis of clinical symptoms, laboratory profiles, and/or histological features. These categorizations can aid in diagnosis. Symptoms of DILI are highly variable and can include the usual symptoms of any acute liver injury, such as fatigue, nausea, abdominal pain, fever, dark urine, jaundice, and pruritis. The types of symptoms and pattern of onset of symptoms can help in distinguishing hepatocellular injury from cholestatic injury. For example, pruritis typically occurs early in cholestatic cases of DILI but occurs late, if at all, in hepatocellular injury. Symptoms of hypersensitivity can also help in the diagnosis of DILI. Rash, fever, facial edema, and lymphadenopathy, along with eosinophilia or atypical lymphocytosis, are important early features that, when present, point to hypersensitivity as a cause of the injury and are typical for specific agents such as aromatic anticonvulsants, sulfonamides, and allopurinol.
The diagnosis of DILI usually rests on finding abnormalities of biochemical liver tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (Alk P), gamma-glutamyl transpeptidase (GGT), and bilirubin. These same tests are typically used to categorize liver injury as either hepatocellular or cholestatic. The distinction is usually based on the ratio of serum ALT results to Alk P results with respect to their upper limits of normal (ULNs): the R ratio.3 Thus, the R ratio is equal to (ALT/ULN)/(Alk P/ULN). An R ratio ≥ 5 denotes hepatocellular injury, and an R ratio ≤ 2 denotes cholestatic injury. Ratios between 2 and 5 are categorized as mixed. In situations in which ALT or Alk P was elevated before the medication was started, baseline values can be used instead of ULN in the calculations. The categorization of liver injury based on R ratios has been used in the diagnosis and assessment of causality in DILI, although the reliability of these cut points has not been assessed prospectively.
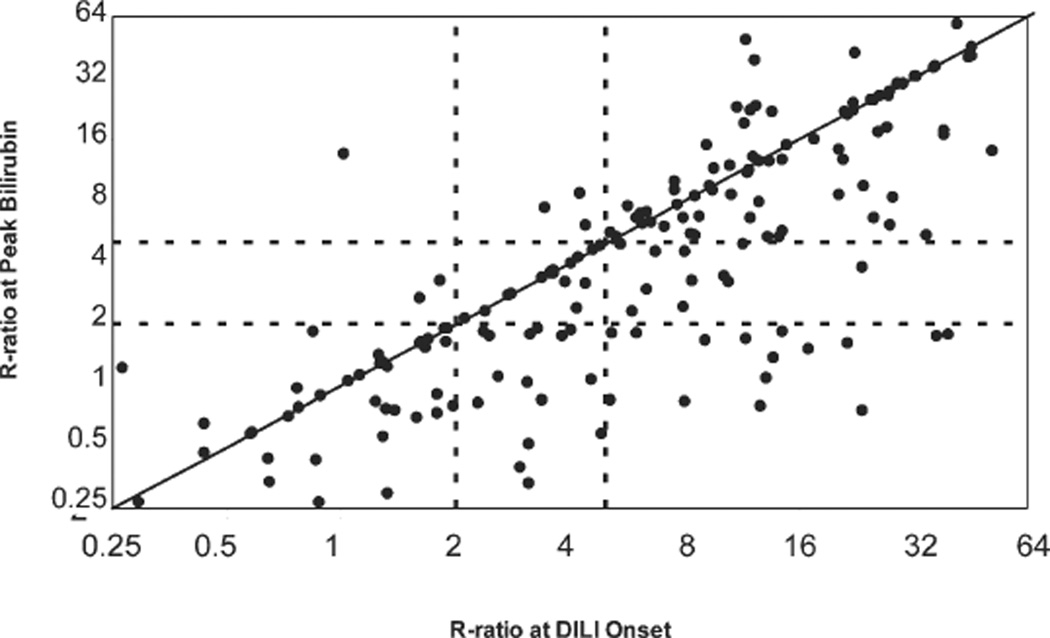
An important issue in calculating the R ratio is which values to use during the course of illness. In most reports, the R ratio is calculated with the initial laboratory results (preferably taken on the same day), although this is not always stated, and in some instances, patients may be misclassified. In an analysis of 192 patients with DILI attributed to a single agent who were enrolled in the DILIN prospective study, the calculation of the R ratio from initial values indicated that 57% had hepatocellular injury, 21% had mixed injury, and 22% had cholestatic liver injury. The same calculation made with values taken at the peak of serum bilirubin elevations showed that only 45% of cases were hepatocellular, whereas cholestatic (37%) and mixed cases (17%) were more frequent (Fig. 1). Thus, a shift in the pattern of relative ALT or Alk P elevations often occurs during the course of DILI, and cases are more likely to be designated as mixed or cholestatic if values are taken at a later time point or if the patient is first seen relatively late in the course of illness.
Fig. 1.
Comparison of the R ratio at onset and at the time of peak bilirubin elevation in 192 subjects enrolled in the prospective DILIN study. Only patients with at least two determinations of ALT, Alk P, and bilirubin in whom DILI was attributed to a single agent and given a causality score of at least “probable” were included. Although there was a close correlation between the two scores (r = 0.80, P < 0.0001), there was a general shift to lower R values between onset and peak bilirubin. The dotted lines indicate levels of the R ratio used to separate cholestatic (≤2), mixed (2–5), and hepatocellular injury (≥5).
Liver histology is considered the gold standard of defining whether liver injury is cholestatic or hepatocellular. However, liver histology is not always available and may be subject to sampling error, and results may vary with the timing of biopsy: hepatocellular injury is more prominent in the initial few days or weeks of injury, whereas cholestatic features are more prominent later. The reliability of clinical symptoms versus biochemical results (R value) and the importance of timing in assessing histological features of DILI have yet to be defined and deserve prospective analysis.
Course of Liver Injury After Cessation of the Drug
The course of the liver injury after the suspect medication, herbal, or dietary supplement is stopped is considered the dechallenge and is an important element in assessing the likelihood of DILI. If a medication is causing liver injury, its withdrawal should be followed by clinical improvement. However, liver injury may continue to worsen for a few days or as long as a few weeks after a hepatotoxic agent is stopped. Furthermore, the speed of improvement is variable. A general belief is that improvement is slower among patients with mixed or cholestatic injury versus patients with hepatocellular injury.3,4 Yet, among the first 300 patients enrolled in the DILIN prospective study, the mean time to the resolution of jaundice was similar in patients with hepatocellular injury (30 days) and in patients with cholestatic or mixed liver injury (38 days; P = 0.6).6
Improvement when a medication is stopped is not always a reliable indication of causality. The liver injury may simply have reached its maximum intensity when the medication was stopped, and the subsequent improvement was merely coincidental. Furthermore, causality assessment is often needed early in the course of illness before there is full recovery. Finally, patients who develop chronic liver injury due to DILI or ALF may improve minimally if at all with the cessation of the medication.17–19 Thus, improvement with the cessation of the suspect medication supports causality but is not always available or reliable even when present. Thus, reliable criteria are needed for assessing improvement in liver injury following drug discontinuation that can be readily applied to cases and also account for situations of ALF or evolution to chronic injury.
Risk Factors
There is much literature on risk factors for the development of DILI, such as age, sex, race, alcohol use, smoking, concomitant medications, underlying liver disease, and, of course, genetics. One difficulty with these analyses is that DILI is not a single disease but represents a large variety of hepatic reactions to a large variety of chemical agents. Thus, older age appears to be an important risk factor for the development of hepatic injury in response to isoniazid,20 but younger age appears important for valproate-induced liver injury21 and aspirin-induced Reye’s syndrome.22 Women may be more likely to develop hepatic injury from medications,6,16 but they are also more likely to take them. In large, population-based studies, women have not been found to be at greater risk of hepatotoxicity for most drugs,11–13 but they may be more susceptible to severe outcomes and ALF.13 Black or African American subjects appear to be more likely to develop anticonvulsant hypersensitivity and liver injury,23 whereas Caucasian whites appear to be at increased risk for developing abacavir and flucloxacillin-related liver injury.24,25 More importantly, these factors account for a relative increase in risk. In the individual case, risk factors are not helpful in assessing the likelihood of DILI.
Genetics are believed to play an important role in DILI in response to specific medications.2 Indeed, in recent years, several genetic markers have been tightly linked to an increased risk of developing DILI. These markers, however, are largely medication-specific. Thus, hypersensitivity reactions to abacavir and flucloxacillin are closely linked to human leukocyte antigen B*5701,24,25 estrogen-induced cholestasis is closely linked to variants in adenosine triphosphate–binding cassette B11 (bile salt export pump),26 and valproate toxicity is closely linked to variants of mitochondrial polymerase gamma (POLG1).27 None of these factors is, however, absolute, and hepatotoxicity can occur in patients without these specific markers. The identification of genetic markers associated with DILI is likely to lead to a better understanding of the mechanisms of injury and potentially preventative strategies rather than diagnostic tools.
Exclusion of Other Causes of Liver Injury
Most causality instruments for assessing DILI and published reports in the literature include results of virological, serological, and radiological testing.3 However, there is little standardization of what is considered necessary, and in some but not all instances, specialized testing may be appropriate to exclude Wilson disease, sclerosing cholangitis, hepatitis E, or graft-versus-host disease. Testing for hepatitis A, B, and C as well as common autoantibodies is probably appropriate in all cases, as is hepatic and biliary imaging to exclude pancreaticobiliary disease and to help exclude nonalcoholic fatty liver disease.
Elements in the clinical history that are also important in making this diagnosis of exclusion include a careful history of alcohol use and whether there is a preceding period of heart failure, hypotension, hyperthermia, or hypoxia that might cause ischemic hepatitis or complications of sepsis and parenteral nutrition that might lead to cholestatic hepatic injury. In retrospective analyses of the published literature, however, many of these critical elements are lacking in reports of DILI, and this makes it difficult or impossible to fully assess causality. A checklist of minimal necessary elements in the diagnosis of DILI would be clinically helpful, particularly in reporting cases either to the US Food and Drug Administration (FDA; through Med-Watch) or in case reports in the literature (Tables 2 and 3). This checklist should include not just diagnostic tests needed but also what clinical information is necessary to assess the likelihood of the agent being causative.
Table 2.
Elements Necessary for Reporting Cases of DILI
|
Abbreviations: ANA, antinuclear antibody; anti-HBc, antibody to hepatitis B core antigen; HAV, hepatitis A virus; HBsAg, hepatitis B surface antigen; IgM, immunoglobulin M; SmAb, smooth muscle antibody.
Table 3.
Elements Not Always Necessary but Supportive of Assessment and Helpful in Reporting Some Cases of DILI
|
Abbreviations: AMA, antimitochondrial antibody; CMV, cytomegalovirus; CPK, creatinine phosphokinase; EBV, Epstein-Barr virus; ERCP, endoscopic retrograde cholangiopancreatography; HBsAg, hepatitis B surface antigen; HDV, hepatitis D virus; HEV, hepatitis E virus; IgG, immunoglobulin G; IgM, immunoglobulin M; LDH, lactate dehydrogenase; LKM, liver-kidney microsomal; MRCP, magnetic resonance cholangiopancreatography; PCR, polymerase chain reaction.
Previous Reports of DILI
An important element in assessing DILI is whether the medication, herbal, or dietary supplement has been previously implicated in liver injury and whether the pattern of injury (signature) is similar to that of reported cases. This information, however, may not be reliable or accessible. First, not all medications have a characteristic signature, in that the time to onset, pattern of enzyme elevations (R ratio), clinical course, severity, and speed of recovery may vary greatly. Second, in the case of a new or rarely used medication, there will be little or no previous experience with or information about the clinical features of its hepatotoxicity. Even when there is previous information, the literature, being spread over many years, journals, and languages, may be difficult to obtain. Information on liver toxicity for herbal and dietary supplements is even less available. Finally, many published cases have missing key elements or have not definitively excluded other common causes of liver injury that can be mistaken for DILI. For these reasons, except for agents with a very characteristic signature and a wide experience in the literature, the signature of hepatic injury is not often useful.
There is currently no standardized system for classifying the potential of specific drugs and/or herbal and dietary supplements to cause liver injury. Ideally, drugs could be characterized by the likelihood of causing DILI based on the number of persons who develop liver injury with respect to the number of persons exposed. Except for the most common causes of hepatotoxicity, such as isoniazid, however, this information is largely unknown. For most agents, DILI is rare and occurs in 1:10,000 to 1:1,000,000 persons exposed.11 Furthermore, DILI cases are not regularly reported. The MedWatch system (a part of the Adverse Event Reporting System), sponsored by the FDA, captures thousands of reports of adverse events yearly, but reporting is voluntary, and cases of DILI are greatly underreported.28 In addition, cases that are reported are often inadequately described, and it is difficult to assign causality.
As a component of the DILIN initiative, a proposal was made to classify drugs with respect to their likelihood of causing liver injury (Table 4). Thus, agents were placed in five categories based on the number of published cases and case series in the literature: (A) >50 cases, (B) 11 to 50 cases, (C) 3 to 10 cases, (D) <3 cases, and (E) not convincingly linked to cases of hepatotoxicity. A final category (X) is necessary for those agents that have not been adequately assessed (i.e., new drugs) or are too rarely used to judge their potential for hepatotoxicity.
Table 4.
Classification of Agents by Likelihood of Causing Liver Injury
| Score | Descriptor | Definition |
|---|---|---|
| A | Known | Agent is well-established cause of hepatotoxicity; at least 50 cases are reported in the literature, some in case series (e.g., phenytoin, isoniazid, and amiodarone). |
| B | Rare | Agent has been implicated in causing hepatotoxicity; there are at least 10 but fewer than 50 cases in the literature, some in small case series (e.g., celecoxib, doxycycline, and atorvastatin). |
| C | Very rare | Agent has been rarely implicated in causing hepatotoxicity, with fewer than 10 but at least 3 convincing cases in the literature (e.g., metformin and metronidazole). |
| D | Unproven | Agent has been implicated in isolated case reports as causing liver injury, but there are fewer than 3 convincing cases in the literature (e.g., vancomycin and theophylline). |
| E | Not implicated | Agent has not been convincingly implicated in cases of liver injury (e.g., folic acid and hydrochlorothiazide). |
| X | Insufficient information | Agent has not been available for long enough or used in enough patients to judge its hepatotoxic potential (e.g., febuxostat, etravirine, and iloperidone). |
Rechallenge
Rechallenge is defined as the intentional or inadvertent re-exposure of an individual to a drug believed to have caused either current or past liver injury. In practice, intentional rechallenge is now rarely done because re-exposure can cause severe recurrence that can be fatal or lead to permanent disability.29 Rechallenge may be attempted if the initial liver injury did not have features of hypersensitivity and was not severe and if the agent is considered essential or the optimal approach to management. Thus, careful rechallenge might be appropriate for some cancer chemotherapeutic agents, antiretrovirals, or antituberculosis medications. A positive rechallenge consists of recurrence of the liver injury, usually with shorter latency and greater severity.3,4 In rare instances, rechallenge does not result in recurrent liver injury, possibly because of differences in dose, concomitant medications, or host and/or environmental risk factors at the time of rechallenge.
Liver Biopsy
Liver biopsy has been considered an important and at times essential element in diagnosis, particularly when it is initially demonstrated that a medication can cause liver injury. Nevertheless, the role that liver histology plays in the diagnosis and causality assessment of DILI is unclear.2,6 Although some histological features such as prominence of eosinophils, granulomas, zonal or massive necrosis, and cholestasis with hepatitis may increase the index of suspicion of DILI, there are no unique histological features that unequivocally confirm the diagnosis.30 Clearly, objective and prospective studies of the role of liver histology in improving the diagnosis and management of DILI are necessary.
Causality Assessment in DILI
Without specific biomarkers or diagnostic tests for DILI, formalized approaches and scoring systems have been developed to assess the likelihood that liver injury is due to a medication.3,4,31–40 Naranjo and co-workers31 from Toronto proposed a scoring system (now called the Naranjo or Adverse Drug Reaction Probability Scale) to assign attribution for adverse drug reactions that appear during clinical trials. Scores range from −4 to +13 and are based on points ranging from −1 to +2 in response to 10 questions. This scale is simple to apply and is widely used but is not specific for liver reactions and is designed for use in clinical trials rather than clinical practice; these factors limit its sensitivity and applicability.32
Regulatory authorities in France have developed a combination of chronological (C) and clinical criteria (S) to assess causality, and this is called the French pharmacovigilance methodology.33 The chronological criteria include latency, dechallenge, and rechallenge, which are categorized as being C3 (suggestive), C2 (possible), C1 (unlikely), or C0 (incompatible). The clinical criteria include the exclusion of competing causes liver injury, the presence of hypersensitivity features, findings on liver biopsy, and the presence of detectable autoantibodies for specific drugs, and they yield classifications of S1 to S3. A three-by-four table of clinical criteria versus chronology is constructed, and an overall causality score is provided; it ranges from “excluded” to “highly probable.” Advantages of this method include its simplicity and ability to be completed by regulatory authorities. Disadvantages, however, include the inability to distinguish between the lack of information and reasonable alternative causes in unlikely cases. In addition, this method tends to underestimate causality in the most severe cases because of the lack of improvement with dechallenge.
The first causality assessment instrument developed to address the unique aspects of liver injury due to medications was the Roussel Uclaf Causality Assessment Method (RUCAM).3,4 Because RUCAM is intended for broad use, there is no requirement for a minimal data set to attribute causality; instead, a lower overall score is assigned when there are missing data. The data fields of drug latency and dechallenge are stratified by hepatocellular injury versus mixed or cholestatic injury. The summed points produce a score ranging from −9 to +14, and these are then grouped into categories varying from “excluded” (score < 0) to “highly probable” (score > 8).
The test-retest reliability of RUCAM was only 0.54 when it was used by three expert hepatologists in the DILIN study, with the two measurements taking place over an interval greater than 5 months.5 In addition, the inter-rater reliability was poor at 0.45 in a comparison of the numeric RUCAM scores. For example, alcohol is a cited risk factor, but no details are given on what quantity and timing of alcohol consumption should be considered adequate to assign a score. In addition, labeling a concomitant drug as a known hepatotoxin is left to user discretion, and substantial variability could be encountered if the user sought information from a medication package insert, a textbook, or the Internet or based his assessment on personal knowledge.34 Furthermore, it is unclear how to complete the dechallenge section of RUCAM if the duration of follow-up is limited. Lastly, RUCAM does not exclude other common competing causes of liver injury such as acute hepatitis C, and criteria for a competing diagnosis of hepatic ischemia are not provided.
Dr. Maria and Dr. Victorino introduced a modification of RUCAM [the Maria and Victorino (M&V) scale] including additional elements in the scoring system, such as the presence of immunoallergic features (fever, rash, or cytopenias). The authors also excluded elements that they thought were not helpful, such as the risk factors of age, alcohol, and pregnancy as well as competing drugs and the separation of cases into hepatocellular injury versus cholestatic injury.35 The M&V scale had an excellent kappa score of 0.9 when it was applied to 50 suspected DILI cases in comparison with the consensus expert opinion of three hepatologists.36 However, in a larger independent series, the M&V scale was inferior to RUCAM and assigned lower attribution scores to most cases.37 Therefore, although the M&V scale is more stringent and specific than RUCAM, it is less sensitive, and this will likely limit its future use.
In an effort to improve RUCAM, Japanese investigators added the results of lymphocyte stimulation tests and testing for eosinophilia to the calculation.38,39 With these changes, the causality scores in 287 Japanese DILI patients shifted to higher levels of certainty in comparison with the standard RUCAM. In a recent retrospective study of 1676 Japanese DILI patients, despite the absence of elements in many of the data fields, 87% of cases were graded as highly possible, and 11% were called possible.16 A comparison of the Japanese RUCAM to expert opinion has yet to be undertaken, and the requirement for lymphocyte stimulation test results limits its usefulness when this test is not available.
Expert Opinion in Causality Assessment
Expert opinion is widely regarded as the gold standard for diagnosis when confirmatory or specific laboratory tests are not available to classify a disease entity or stage. However, there are no established criteria for what constitutes an expert or how an expert should integrate and synthesize information when making a clinical diagnosis of an adverse drug reaction such as DILI.35
The DILIN study group has attempted to standardize expert opinion for assessing causality.7,34 Individual biases are minimized by three hepatologists reviewing a prospectively collected set of laboratory and clinical data supported by a clinical narrative. Each reviewer independently assigns a percentage likelihood of attribution on a scale from 1 (definite) to 5 (unlikely; Table 5). If more than one drug or herbal product is implicated, the reviewers award an overall score as well as individual scores for up to three drugs. If the three independent reviewers cannot achieve consensus, the case is discussed by the full committee, and a final score is reached by majority vote.
Table 5.
Likelihood of Causality Used in the DILIN Prospective Study
| Score | Causality | Likelihood (%) | Textual Definition |
|---|---|---|---|
| 1 | Definite | ≥95 | Causality is “beyond a reasonable doubt.” |
| 2 | Highly likely | 75–94 | Causality is supported by “clear and convincing evidence.” |
| 3 | Probable | 50–74 | Causality is supported by “the preponderance of the evidence.” |
| 4 | Possible | 25–49 | Less than the preponderance of the evidence supports causality, but it is nevertheless possible. |
| 5 | Unlikely | <25 | Causality is unlikely or excluded. |
Some cases have been scored as “inadequate documentation to assign causality” (adapted from Fontana et al.7).
Strengths of the DILIN expert opinion process are the availability and input of the site investigator who took the history, performed the physical examination, and supervised the data collection. Also, a prospective evaluation of competing causes of liver injury and serial laboratory data through at least 6 months of follow-up are provided. An evaluation at the 6-month point also provides the opportunity to determine whether another etiology may have been responsible for the liver injury and whether rechallenge occurred. Nevertheless, the DILIN expert opinion method has its limitations, which include the lack of generalizability and the low weighted kappa score for complete agreement among all three reviewers (0.23–0.38).
Special Issues in Causality Assessment
There are important differences in assessing the causality of DILI in children versus adults. Children use medications less frequently than adults and also appear to be less susceptible to DILI. Notable exceptions include the increased susceptibility of children to Reye’s syndrome after aspirin use for febrile illnesses and their increased risk of developing liver injury after valproate; this may be related to age-related differences in cytochrome P-450 gene expression.21,22 Although there is a paucity of data regarding DILI in children, anti-epileptic drugs and psychotropic drugs appear to be most commonly implicated.41 Because the competing causes of liver injury in children and particularly infants differ from those in adults, separate causality assessment instruments may need to be developed for children with suspected DILI.
Assessing causality in patients with underlying liver disease is intrinsically more difficult because of the difficulty of excluding a flare or complication of the underlying liver disease from the DILI episode. Patients with chronic hepatitis C virus (HCV) or hepatitis B virus (HBV) infection are believed to be at increased risk of developing drug hepatotoxicity because of altered pharmacokinetics, up-regulated intrahepatic cytokine expression, and alterations in drug-metabolizing pathways.42,43 In contrast, patients with obesity and nonalcoholic fatty liver disease do not appear to be at increased risk of developing DILI, with the possible exception of methotrexate-induced and tamoxifen-induced liver injury, but they may be at risk for more severe outcomes.44,45 Multiple studies have demonstrated that subjects with human immunodeficiency virus (HIV) and HCV or HBV coinfection are at greater risk of developing ALT elevations during antiretroviral therapy than HIV-monoinfected individuals.43 However, whether this increased risk of developing liver biochemistry abnormalities during drug therapy represents hepatotoxicity versus exacerbation of the underlying hepatitis (perhaps induced by the medication) remains unclear.
The role of alcohol in susceptibility to DILI and the outcome of DILI remains controversial. Chronic consumption of alcohol can lead to cytochrome P450 2E1 induction as well as depletion of glutathione and other micronutrients, but it has been difficult to document that alcohol use increases susceptibility to hepatotoxicity.44,45 Furthermore, it is unclear whether the increased risk is related to alcoholic liver disease or alcohol intake per se. If alcohol intake predisposes to drug-induced liver disease, it remains unclear what level of intake is critical and whether current or previous alcohol intake is important.
Autoimmunity is a special problem when causality in DILI is being assessed. Some medications—most notably minocycline, nitrofurantoin, and methyldopa— typically induce an autoimmune hepatitis–like syndrome. Other medications appear to trigger the onset of autoimmune hepatitis (alpha-interferon and anti–cytotoxic T lymphocyte antigen 4). On the other hand, the onset of spontaneous autoimmune hepatitis is a major differential diagnosis in judging causality in cases of DILI with autoimmune features. Complicating the issue is the fact that some medications can induce autoantibodies even in the absence of liver injury (isoniazid, procainamide, and methyldopa).
There are special challenges for causality assessment when one is dealing with herbal medications and dietary supplements (Table 1). Many consumers take multiple formulations simultaneously, and their use may not be reported. In addition, reliably identifying the names and known active ingredients of these products is challenging and sometimes impossible. Furthermore, the potential for contamination or adulteration of these largely unregulated products with anabolic steroids and other drugs provides further complexity.14 Finally, there is a paucity of authoritative, scientific data regarding the potential hepatotoxicity of these products, which are not required to undergo efficacy and safety testing prior to marketing in many countries.
Finally, the use of multiple potentially hepatotoxic medications can make it impossible to definitely assign causality to one medication versus another. This problem is particularly encountered with the use of combination therapy in the treatment of HIV infection and tuberculosis, for which many if not most of the commonly used agents are potentially hepatotoxic.
Grading Severity in DILI
Severity in DILI can be graded as mild, moderate, or severe or more formally with severity scales used to grade adverse events, such as the AIDS Clinical Trial Group criteria (http/rcc.tech-res.com/safetyandpharmacovigilance/). Severity is a complex, subjective concept. Ideally, the grading of severity should be based on the duration of the liver injury and the degree to which it interfered with daily activities and living. This information, however, is difficult to capture, and most severity scales are based on the presence and number of symptoms, the height of liver biochemistry or bilirubin elevations, the presence of signs of hepatic failure, and the ultimate outcome, such as recovery, chronicity, or death. In developing a prospective database of cases, DILIN established a five-point system for grading severity based on symptoms, jaundice, need for hospitalization, signs of hepatic failure, and death or need for liver transplantation (Table 6). The accuracy of this simple system needs to be assessed in a prospective manner with more objective measures of severity of DILI.
Table 6.
Disease Severity Scales Used in the DILIN Prospective Study
| Score | Grade | Definition |
|---|---|---|
| 1 | Mild | There are elevations in serum ALT and/or Alk P levels, but the total serum bilirubin level is <2.5 mg/dL, and INR is <1.5. |
| 2 | Moderate | There are elevations in serum ALT and/or Alk P levels, and the serum bilirubin level is ≥2.5 mg/dL, or INR is ≥1.5. |
| 3 | Moderate-severe | There are elevations in serum ALT, Alk P, and bilirubin or INR levels, and hospitalization or ongoing hospitalization is prolonged because of a DILI episode. |
| 4 | Severe | There are elevations in serum ALT and/or Alk P levels, and the total serum bilirubin level is ≥2.5 mg/dL, and there is at least one of the following:
|
| 5 | Fatal | Death or liver transplantation from a DILI event |
Cases are also rated for the presence or absence of symptoms (A = asymptomatic; S = symptomatic). Symptoms include fatigue, nausea, vomiting, right upper quadrant pain, itching, skin rash, jaundice, weakness, anorexia, and weight loss, which in the opinion of the investigator are due to the DILI (adapted from Fontana et al.7).
Cases of DILI are sometimes categorized as serious with what is colloquially known as Hy’s law, which is named after Hyman J. Zimmerman, a leader in DILI research of the 20th century. Zimmerman made the observation that cases of hepatocellular DILI with jaundice had a fatality rate of at least 10%, in contrast to acute viral hepatitis, in which the fatality rate is usually 1% or less. This observation has been confirmed in many studies, but in the initial 300 cases enrolled in DILIN, the fatality rate was similar in hepatocellular (13.4%) and cholestatic or mixed (15%) instances of hepatotoxicity with bilirubin levels greater than 2.5 mg/dL.6 The definition of what constitutes a case of hepatocellular injury with jaundice that would qualify for Hy’s law has not been firmly set, but recent recommendations from the FDA are that cases with jaundice, serum ALT levels elevated more than 10-fold, and Alk P levels elevated less than 2-fold should be considered to meet the criteria of Hy’s law.46,47
Phenotypes of DILI
Clinically, DILI can resemble almost any form of acute or chronic liver disease. In many instances, drugs cause a recognizable and reliable clinical phenotype. Thus, anabolic steroids usually induce a bland cholestasis, estrogenic steroids cause cholestasis with mild hepatocellular injury, sulfonamides lead to an abrupt immunoallergic hepatitis with a short incubation period, and isoniazid causes an acute viral hepatitis–like clinical syndrome 1 to 6 months after therapy is started. Some medications share a clinical phenotype even though they have little similarity in their structure or mechanism of action. Thus, allopurinol can induce an acute liver injury that is accompanied by fever, rash, and eosinophilia and resembles the typical DRESS (drug rash, eosinophilia, and systemic symptoms) syndrome caused by aromatic anticonvulsants such as phenytoin and carbamazepine, even though these drugs share no structural homology.
The phenotypic characterization of DILI deserves better definition and standardization of diagnostic criteria. Phenotypes that might be defined include bland cholestasis (androgenic steroids), acute cholestatic hepatitis (sulfonylureas), acute hepatic necrosis (acetaminophen and halothane), acute viral hepatitis–like syndrome (isoniazid and flutamide), immunoallergic hepatitis (sulfonamides, penicillins, macrolide antibiotics, and aromatic anticonvulsants), typical cholestatic hepatitis due to medications (amoxicillin/clavulanate), autoimmune hepatitis–like injury (minocycline, nitrofurantoin, and methyldopa), sinusoidal obstruction syndrome (busulfan and melphalan), microvesicular steatosis with lactic acidosis and hepatic failure (aspirin, tetracycline, and antiviral nucleoside analogues), fatty liver disease (amiodarone, valproic acid, and methotrexate), chronic hepatitis (methyldopa and hydralazine), and vanishing bile duct syndrome (phenothiazines, amoxicillin, and ibuprofen; Table 7). Attempts are needed to standardize the definition of the major phenotypes of DILI. Thus, the diagnosis of chronic DILI is usually based on the finding of laboratory or radiological evidence of persistent liver injury at least 6 months after onset. However, other possibilities are that the abnormalities merely represent slow recovery or that they represent a chronic but preexisting form of liver disease. Criteria for excluding these other possibilities and for significant clinical evidence for persistent injury are needed.
Table 7.
Phenotypes or Clinical Patterns of DILI Deserving Diagnostic Criteria
| Immunoallergic hepatitis |
| Autoimmune hepatitis–like |
| Acute hepatic necrosis |
| Acute viral hepatitis–like |
| ALF |
| Cholestatic hepatitis |
| Bland cholestasis |
| Acute fatty liver with lactic acidosis |
| Nonalcoholic fatty liver |
| Sinusoidal obstruction syndrome |
| Chronic hepatitis |
| Nodular regeneration |
| Vanishing bile duct syndrome |
| Cirrhosis |
Treatment of DILI
The obvious first step in treating DILI is to discontinue the implicated drug as soon as the diagnosis is suspected. Many patients start to improve within hours or days of stopping therapy, but many have prolonged illness, some develop chronic injury, and an important minority suffer ALF and die or require emergency liver transplantation.1,41 Developing mechanism-based treatments for severe DILI is an important but challenging goal of research. Corticosteroids are often used in DILI patients with severe or progressive liver injury, but data supporting their safety and efficacy are lacking.48 Similarly, ursodiol and antioxidant therapy are often applied in patients with severe or prolonged DILI, but their efficacy has not been shown in controlled studies.
In a prospective, randomized controlled trial of N-acetylcysteine for ALF not due to acetaminophen, spontaneous survival was found to be greater in treated subjects.49 Post hoc analyses showed a major effect of intravenous N-acetylcysteine in 45 cases of ALF due to medications (i.e., spontaneous survival of 58% versus 27%). These findings suggest that N-acetylcysteine should be offered to patients with ALF due to DILI, but further studies are needed to determine its efficacy in subjects with less severe injury.
Future Needs in DILI Research
Several needs in the standardization of nomenclature and the development of causality instruments were identified at this workshop that could aid in the clinical diagnosis and management of DILI and benefit future research on the genetics and mechanisms of DILI as well as potential improvements in prevention and treatment. Diagnostic criteria and definitions would be helpful for the phenotypes of liver injury and for defining features such as the time to onset and hepatocellular DILI versus cholestatic DILI, as would minimal definitions for factors used in assessing DILI, such as eosinophilia, fever, rash, and alcohol use. Widely used systems for grading the severity of DILI and assessing the potential of medications to cause liver injury would help in collaborative research and in the assessment of secular trends in DILI. Agreement on minimal elements to include in case reports can help to improve the literature on DILI and improve the quality of liver adverse event reports.
Consensus agreement on how to complete causality instruments such as the RUCAM and M&V scales would be beneficial and bring some degree of consistency to these instruments. Most helpful, however, would be the creation of an improved causality assessment instrument that is reliable, reproducible, and easy to use. Such an instrument would ideally be computer-based, use readily available clinical information only, and require no or minimal subjective determinations. A computer-based system could adjust scores according to the phenotype of injury and permit the use of more specific clinical features, such as results of liver biopsy and genetic tests. Prototype models of a computer-based causality instrument need to be developed with the essential components and item scaling. Refinement of the data fields and associated instructions is best carried out via testing on subsets of well-characterized, definite cases of DILI. Finally, applying the causality method to a wider array of cases with lower attribution scores is required to determine the discriminating capabilities of the instrument.
In summary, progress in the understanding and control of hepatotoxicity has been hindered by its unpredictable and variable clinical presentation and course. If advances are to be made in this field, collaborative efforts using consensus-based diagnostic criteria and standardized nomenclature and instruments will be essential.
Acknowledgment
The authors recognize the important contributions of all the speakers and participants at this meeting for their valuable insights, questions, and discussion. Cited speakers and participants include the following: Jay Hoofnagle, M.D. (NIDDK, Bethesda, MD); Donald Lindberg, M.D. (National Library of Medicine, Bethesda, MD); Leonard Seeff, M.D. (NIDDK, Bethesda, MD); Neil Kaplowitz, M.D. (University of Southern California Keck School of Medicine, Los Angeles, CA); Mark Avigan, M.D. (FDA, Silver Spring, MD); Jose Serrano, M.D., Ph.D. (NIDDK, Bethesda, MD); Raul J. Andrade, M.D., Ph.D. (University Hospital of Malaga, Malaga, Spain); Christopher Day, M.D., Ph.D. (Newcastle University, Newcastle upon Tyne, United Kingdom); Einar Bjornsson, M.D., Ph.D. (Sahlgrenska University Hospital, Gothenburg, Sweden); Dominique Larrey, M.D., Ph.D. (St. Eloi Hospital, Montpellier School of Medicine, Montpellier, France); Hajime Takikawa, M.D., Ph.D. (Teikyo University School of Medicine, Tokyo, Japan); Seng Gee Lim, M.D. (National University of Singapore, Singapore); Byung-Min Ahn, M.D. (Gumi CHA Hospital, South Korea); M. Isabel Lucena, M.D. (Servico de Farmacologia Clinica, Malaga, Spain); James Rochon, Ph.D. (Duke Clinical Research Institute, Durham, NC); Don Rockey, M.D. (University of Texas Southwestern, Dallas, TX); Naga Chalasani, M.D. (Indiana University School of Medicine, Indianapolis, IN); Robert Fontana, M.D. (University of Michigan School of Medicine, Ann Arbor, MI); Timothy Davern, M.D. (University of Southern California at San Francisco, San Francisco, CA); William Balistreri, M.D. (Children’s Hospital Medical Center, Cincinnati, OH); David Kleiner, M.D., Ph.D. (National Cancer Institute, Bethesda, MD); Paul Watkins, M.D. (University of North Carolina School of Medicine, Chapel Hill, NC); Paul Hayashi, M.D. (University of North Carolina School of Medicine, Chapel Hill, NC); Herbert Bonkovsky, M.D. (Carolinas Health Care System, Charlotte, NC); Guruprasad Aithal, M.D., Ph.D. (University Hospital, Nottingham, United Kingdom); James Knoben, Pharm.D., M.P.H. (National Library of Medicine, Bethesda, MD); and John McHutchison, M.D. (Duke University Medical Center, Durham, NC).
The clinical research workshop “Standardization of Nomenclature and Causality Assessment in Drug Induced Liver Injury” was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Library of Medicine of the National Institutes of Health. Funding for the Drug-Induced Liver Injury Network is provided by the National Institute of Diabetes and Digestive and Kidney Diseases under cooperative agreements 1U01DK065201, 1U01DK065193, 1U01DK065184, 1U01DK065211, 1U01DK065238, and 1U01DK065176. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas is funded by Instituto de Salud Carlos III. The Spanish DILI Registry is partially supported by the Spanish Medicine Agency, and the work presented in this workshop was supported by Fondo de Investigación Sanitaria (PI 07/0980).
Abbreviations
- ALF
acute liver failure
- Alk P
alkaline phosphatase
- ALT
alanine aminotransferase
- AMA
antimitochondrial antibody
- ANA
antinuclear antibody
- anti-HBc
antibody to hepatitis B core antigen
- AST
aspartate aminotransferase
- CM
Chinese medicine
- CMV
cytomegalovirus
- CNS
central nervous system
- CPK
creatinine phosphokinase
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- EBV
Epstein-Barr virus
- ERCP
endoscopic retrograde cholangiopancreatography
- FDA
US Food and Drug Administration
- GGT
gamma-glutamyl transpeptidase
- HAV
hepatitis A virus
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HC
hepatocellular
- HCV
hepatitis C virus
- HDV
hepatitis D virus
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- INR
international normalized ratio
- LDH
lactate dehydrogenase
- LKM
liver-kidney microsomal
- M&V
Maria and Victorino
- MRCP
magnetic resonance cholangiopancreatography
- NA
not available
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- OTC
over the counter
- PCR
polymerase chain reaction
- RUCAM
Roussel Uclaf Causality Assessment Method
- SmAb
smooth muscle antibody
- ULN
upper limit of normal
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Watkins P, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–631. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 3.Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 4.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 5.Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB, et al. Reliability of the RUCAM for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Rationale, design and conduct of the Drug Induced Liver Injury Network prospective study. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Valle MB, Klinteberg AV, Alem N, Olsson R, Björnsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–1195. doi: 10.1111/j.1365-2036.2006.03117.x. [DOI] [PubMed] [Google Scholar]
- 9.Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute non-fulminant drug-induced hepatitis in a United States tertiary referral center. J Clin Gastroenterol. 2005;39:64–67. [PubMed] [Google Scholar]
- 10.Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007;102:558–562. doi: 10.1111/j.1572-0241.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 11.de Abajo FJ, Montero D, Madurga M, Garcia Rodriguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58:71–80. doi: 10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sgro C, Clinard F, Quazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 13.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Tan BH, Chan CL, Sutedja DS, Lee YM, Khor C, et al. Drug-induced liver injury at an Asian center: a prospective study. Liver Int. 2007;27:465–474. doi: 10.1111/j.1478-3231.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim JB, Sohn JH, Lee HL, Kim JP, Han DS, Hahm JS, et al. Clinical characteristics of acute toxic liver injury. Korean J Hepatol. 2004;10:125–134. [PubMed] [Google Scholar]
- 16.Takikawa H, Murata Y, Horiike N, Fukui H, Onji M. Drug-induced liver injury in Japan: an analysis of 1676 cases between 1997 and 2006. Hepatol Res. 2009;39:427–431. doi: 10.1111/j.1872-034X.2008.00486.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharp JR, Ishak KG, Zimmerman HJ. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann Intern Med. 1980;92:14–19. doi: 10.7326/0003-4819-92-1-14. [DOI] [PubMed] [Google Scholar]
- 18.Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44:731–735. doi: 10.1136/gut.44.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50:511–517. doi: 10.1016/j.jhep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Pande JN, Singh SP, Khilnani GC, Khilnani S, Tandon RK. Risk factors for hepatotoxicity from antituberculosis drugs: a case-control study. Thorax. 1996;51:132–136. doi: 10.1136/thx.51.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreifuss FE, Santilli N, Langer DH, Sweeney KP, Moline KA, Menander KB. Valproic acid hepatic fatalities: a retrospective review. Neurology. 1987;37:379–385. doi: 10.1212/wnl.37.3.379. [DOI] [PubMed] [Google Scholar]
- 22.Heubi JE, Partin JC, Partin JS, Schubert WK. Reye’s syndrome: current concepts. Hepatology. 1987;7:155–164. doi: 10.1002/hep.1840070130. [DOI] [PubMed] [Google Scholar]
- 23.Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–245. doi: 10.3949/ccjm.66.4.239. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CA, Foisy MM, Dewhurst N, Higgins N, Robinson L, Kelly DV, et al. Abacavir hypersensitivity reaction: an update. Ann Pharmacother. 2008;42:387–396. doi: 10.1345/aph.1K522. [DOI] [PubMed] [Google Scholar]
- 25.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 26.Meier Y, Zodan T, Lang C, Zimmermann R, Kullak-Ublick GA, Meier PJ, et al. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol. 2008;14:38–45. doi: 10.3748/wjg.14.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFarland R, Hudson G, Taylor RW, Green SH, Hodges S, McKiernan PJ, et al. Reversible valproate hepatotoxicity due to mutations in mitochondrial DNA polymerase gamma (POLG1) Arch Dis Child. 2008;93:151–153. doi: 10.1136/adc.2007.122911. [DOI] [PubMed] [Google Scholar]
- 28.Tsong Y. Comparing reporting rates of adverse events between drugs with adjustment for year of marketing and secular trends in total reporting. J Biopharm Stat. 1995;5:95–114. doi: 10.1080/10543409508835100. [DOI] [PubMed] [Google Scholar]
- 29.Papay JI, Clines D, Rafi R, Yuen N, Britt SD, Walsh JS, et al. Drug-induced liver injury following positive drug rechallenge. Regul Toxicol Pharmacol. 2009;54:84–90. doi: 10.1016/j.yrtph.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner DE. The pathology of drug-induced liver disease. Semin Liver Dis. 2009;29:364–372. doi: 10.1055/s-0029-1240005. [DOI] [PubMed] [Google Scholar]
- 31.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Cortes M, Lucena MI, Pachkoria K, Borraz Y, Hidalgo R, Andrade RJ. Evaluation of Naranjo adverse drug reactions probability scale in causality assessment of drug-induced liver injury. Aliment Pharmacol Ther. 2008;27:780–789. doi: 10.1111/j.1365-2036.2008.03655.x. [DOI] [PubMed] [Google Scholar]
- 33.Begaud B, Evreux JC, Jouglard J, Lagier G. Unexpected or toxic drug reaction assessment (imputation): actualization of the method used in France. Therapie. 1985;40:111–114. [PubMed] [Google Scholar]
- 34.Rockey DC, Seeff LB, Rochon J, Chalasani N, Bonacini M, Fontana RJ, et al. Comparison between expert opinion and RUCAM for assignment of causality in drug-induced liver injury. Hepatology. 2010 doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 36.Aithal GP, Rawlins MD, Day CP. Clinical diagnostic scale: a useful tool in the evaluation of suspected hepatotoxic adverse drug reactions. J Hepatol. 2000;33:949–952. doi: 10.1016/s0168-8278(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 37.Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De, La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33:123–130. doi: 10.1053/jhep.2001.20645. [DOI] [PubMed] [Google Scholar]
- 38.Masumoto T, Horiike N, Abe M, Kumaki T, Matsubara H, Akbar SM, et al. Diagnosis of drug-induced liver injury in Japanese patients by criteria of consensus meetings in Europe. Hepatol Res. 2003;25:1–7. doi: 10.1016/s1386-6346(02)00148-1. [DOI] [PubMed] [Google Scholar]
- 39.Takikawa H, Takamori Y, Kumagi T, Onji M, Watanabe M, Shibuya A, et al. Assessment of 387 Japanese cases of drug induced liver injury by the diagnostic scale of the international consensus meeting. Hepatol Res. 2003;27:192–195. doi: 10.1016/s1386-6346(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 40.Arimone Y, Begaud B, Miremont-Salame G, Fourrier-Réglat A, Moore N, Molimard M, et al. Agreement of expert judgment in causality assessment of adverse drug reactions. Eur J Clin Pharmacol. 2005;61:169–173. doi: 10.1007/s00228-004-0869-2. [DOI] [PubMed] [Google Scholar]
- 41.Murray KF, Hadzic N, Wirth S, Bassett M, Kelly D. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47:395–405. doi: 10.1097/MPG.0b013e3181709464. [DOI] [PubMed] [Google Scholar]
- 42.Wong WM, Wu PC, Yuen MF, Cheng CC, Yew WW, Wong PC, et al. Antituberculosis drug-related liver dysfunction in chronic hepatitis B infection. Hepatology. 2000;31:201–206. doi: 10.1002/hep.510310129. [DOI] [PubMed] [Google Scholar]
- 43.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 44.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62–65. doi: 10.1097/00000441-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Whiting-O’Keefe QE, Fyfe KH, Sack KD. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am J Med. 1991;90:711. [PubMed] [Google Scholar]
- 46.Temple R. Predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 47.Center for Drug Evaluation and Research. [Accessed March 2010];Guidance for industry: drug-induced liver injury: premarketing clinical evaluation. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090. Published July 2009.
- 48.Furhoff AK. Adverse reactions with methyldopa—a decade’s reports. Acta Med Scand. 1978;203:425–428. doi: 10.1111/j.0954-6820.1978.tb14900.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee WM, Hynan L, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous n-acetylcysteine improves spontaneous survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucena MI, Andrade RJ, Kaplowitz N, Garcia-Cortes M, Fernandez MC, Romero-Gomez M, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and gender. Hepatology. 2009;49:2001–2009. doi: 10.1002/hep.22895. [DOI] [PubMed] [Google Scholar]