Abstract
Purpose
MicroRNA plays an important role in human diseases and cancer. We seek to investigate the expression status, clinical relevance, and functional role of microRNA in non-small cell lung cancer.
Experimental Design
We performed miRNA expression profiling in matched lung adenocarcinoma and uninvolved lung using 56 pairs of fresh-frozen (FF) and 47 pairs of formalin-fixed, paraffin-embedded (FFPE) samples from never smokers. The most differentially expressed miRNA genes were evaluated by Cox analysis and Log-Rank test. Among the best candidate, miR-708 was further examined for differential expression in two independent cohorts. Functional significance of miR-708 expression in lung cancer was examined by identifying its candidate mRNA target and through manipulating its expression levels in cultured cells.
Results
Among the 20 miRNAs most differentially expressed between tested tumor and normal samples, high expression level of miR-708 in the tumors was most strongly associated with an increased risk of death after adjustments for all clinically significant factors including age, sex, and tumor stage (FF cohort: HR, 1.90; 95% CI, 1.08-3.35; P=.025 and FFPE cohort: HR, 1.93; 95% CI, 1.02-3.63; P=.042). The transcript for TMEM88 gene has a miR-708 binding site in its 3′ UTR and was significantly reduced in tumors high of miR-708. Forced miR-708 expression reduced TMEM88 transcript levels and increased the rate of cell proliferation, invasion, and migration in culture.
Conclusions
MicroRNA-708 acts as an oncogene contributing to tumor growth and disease progression by directly down regulating TMEM88, a negative regulator of the Wnt signaling pathway in lung cancer.
Keywords: NSCLC, adenocarcinoma, miR-708, never smoker, survival, TMEM88, Wnt signaling
Introduction
Lung cancer is one of the most common cancers worldwide, and smoking is a major cause of the disease. However, approximately 10% to 25% of cases are not attributable to smoking (1). Genetically, lung cancers from never smokers have a distinct gene mutation pattern compared with those of current and former smokers (2). Cumulative evidence suggests that lung cancer in never smokers (i.e., persons who smoked <100 cigarettes in their lifetime) may develop through molecular mechanisms that differ from those of lung cancer in smokers (2-5). Using gene expression profiling, several studies have (5-8) reported different pathways of genes involved in cellular transformation and tumor formation in smoker and never smoker lung adenocarcinomas (3, 9). Yanaihara et al (5) and Seike et al (7) also reported differentially expressed microRNA (miRNAs) in lung cancer from smokers and never smokers, respectively.
MiRNAs represent a new class of regulatory molecules that participate in various biological processes, such as development differentiation, cell proliferation, and apoptosis (10-13). MiRNAs can function as either oncogenes or tumor suppressor genes through regulation of their target genes (14-17). Because a miRNA can potentially target a diverse set of mRNA, it plays a critical role in lung tumorigenesis and affect patient outcomes (8, 16, 18, 19). Using miRNA profiling, recent studies have shown miR-21, miR-155, and let-7 as some of the miRNA genes commonly altered in lung cancers, affecting tumor progression or overall survival, or both (5, 7, 8). In a recent validation study, miR-21 overexpression was a consistent feature among lung adenocarcinomas and was strongly predictive for cancer-specific death or relapse-free survival in 3 independent cohorts (20). In addition, miR-21, together with several other miRNA genes, appears to be a valuable plasma biomarker for the prediction, diagnosis, and prognosis of computed tomography (CT)–detected lung cancers (21, 22).
In this study, we aim to identify miRNA genes that might play a role in never smoker lung adenocarcinomas. To test this hypothesis, we first focused on examining miRNA and mRNA expression profiles in two cohorts of never smoker lung adenocarcinoma samples. We identified miR-708 as one of the most frequently overexpressed miRNA in these tumors and validated this result in two other NSCLC cohorts. We examined the clinical impact of miR-708 and its biological function through loss- and gain-of-function experiments. We provide evidence that miR-708 contributes to lung cancer development and progression through regulating a Wnt signaling regulator gene, TMEM88.
Methods
Patients
Primary lung tumors and corresponding normal lung tissues in never smokers (<100 cigarettes lifetime) were obtained from 103 patients with adenocarcinoma diagnosed between January 1997 and September 2008. There were 56 pairs from fresh-frozen (FF) tissues and 47 pairs from formalin-fixed, paraffin-embedded (FFPE) tissues as part of a systematic study to examine the genetic and epidemiological factors contributing to never smoker lung cancer (23). This study was approved by the Mayo Clinic (Rochester, Minnesota, USA) Institutional Review Board. Written informed consent was obtained from all patients. The clinicopathologic characteristics of patients had no statistically significant difference between the FF and FFPE cohorts for all tested clinical variables (P>.02). Two independent sample sets were used for validation study. Samples from the National Cancer Institute (NCI) included available total RNA from 94 adenocarcinomas and 73 normal lung from FFPE tissues and were a part of a NSCLC cohort (24). Among the cases used, there were 45 smokers, 5 never smokers, 44 unknown. In the Korean cohort, primary lung tumors and matched normal lung tissues were obtained from 34 patients with adenocarcinoma who underwent resection with curative intent at Kyungpook National University Hospital, Daegu, South Korea, from January 2003 to July 2007. Fifteen patients were never smokers and 19 patients were smokers. Detailed clinical information and respective institutional approvals on both cohorts are as described (24, 25). Clinical information of all patients used in this study is summarized in Supplemental Table 1.
RNA Extraction and Gene Expression Profiling by Microarray
The FF tissues were extracted at the Mayo Clinic Biospecimen Accession and Processing Core using the miRNeasy kit (Qiagen Inc, Valencia, California, USA) using the manufacturer's protocols. Unstained FFPE tissues were sectioned to 10-μm thickness and 4 to 8 tissue sections were placed into a 1.5-mL tube. Xylene (1 mL) was added for deparaffinization and vortexed vigorously at room temperature for 3 minutes. After the ethanol series to remove xylenes, RNA was extracted with the miRNeasy FFPE kit (Qiagen Inc) and quantified using Nanodrop (Thermo Fisher Scientific Inc, Waltham, Massachusetts, USA). After extraction, all RNA samples were stored at −80°C until used. The Illumina MicroRNA Expression Profiling and Whole-Genome DASL (cDNA-mediated, annealing, selection, and ligation) assay V2.0 (Illumina, Inc, San Diego, California, USA) were used to evaluate miRNA and mRNA gene expression signatures, respectively. All DASL array experiments were performed at the Mayo Clinic Gene Expression Core according to manufacturer's instructions. Portions of the mRNA expression data for the 56 paired FF samples have been previously described (23). The tumor content for all Mayo samples was kept at 60% or more. Total RNA from the NCI Cohort was isolated using the RecoverAll™ Total Nucleic Acid Isolation Kit (Life Technologies, Carlsbad, CA) following manufacturer's protocol.
Microarray Data Analysis
The miRNA expression profiling was performed on all 56 FF and 47 FFPE paired tumor and normal lung samples. The mRNA expression profiles were analyzed for the same 56 pairs of FF tissues. The microarray data for both miRNA and mRNA were processed and normalized through BeadStudio software (Illumina, Inc) using the quantile normalization method. The normalized data were then log2 transformed and analyzed using the Partek Genomics Suite (Partek Inc, St. Louis, Missouri, USA). The one-way ANOVA model was applied to identify differentially expressed genes for all analyses. The multiple testing was corrected using the Benjamani-Hochberg false discovery rate (FDR) method. For miRNA or mRNA genes, having a differential fold change >±1.5 at raw P value <.01 and FDR <5% were considered as significant candidates. For miRNA analysis, we focused on genes having significant expression differences in both FF and FFPE cohorts by first selecting significantly changed miRNAs in each cohort independently and then selected the top 20 miRNA targets common between the two datasets.
We further sub-classified FF tumors into miR-708 “high” and “low” group based on the mean of expression after normalization and compared mRNA gene expression differences between the miR-708-H and miR-708-L tumors. This analysis identified 169 mRNA genes differentially expressed based on fold change > 1.5 and FDR <5%. Hierarchical clustering and principal component analysis were performed on these169 genes based on the Euclidean distance and the average linkage clustering algorithm through the Partek Genomics Suite. We also surveyed these 169 genes using TargetScan and MicroCosm databases to identify predicted mRNA targets of miR-708. The Gene Expression Omnibus (GEO) accession number for all miRNA and mRNA DASL profiling data of this study is GSE36681.
Survival Analysis
We subjected the most differentially expressed microRNA candidates (20 shared genes) for survival analysis based on their expression profiles in tumor samples from the FF and the FFPE cohorts. Hazard ratio (HR) and 95% confidence intervals (CI) were estimated using the multivariate Cox proportional hazards model with adjustment for age, sex, and pathologic stage using R package. Survival probabilities were estimated by the Kaplan-Meier method and the differences in overall survival were compared through the Log-Rank test. The expression levels of the tested miRNAs were entered as a continuous value for Cox analysis and dichotomized as positive or negative on the basis of mean after normalization. Similar analysis was carried out for mRNA candidates on the basis of mRNA expression profiles.
Real-Time Quantitative Polymerase Chain Reaction
We performed real-time quantitative PCR (RT-qPCR) using miScript PCR System (Qiagen Inc) for miR-708 and TaqMan Gene Expression Assays (Applied Biosystems, Foster City, California, USA) for TMEM88. The relative expression levels were calculated using the ΔΔCt method, and RNU6B or GUSB as the internal control. The PCRs were run using the ABI 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA).
MiR-708 Modified Cells
For transient expression, cells were transfected in a 6-well plate at 1 × 105 cells per well with the miR-708 mimic (40 or 80 nM), anti–miR-708 (40 nM), or nonspecific control (mock) oligonucleotide (40 nM) using HiPerFect transfection reagent (Qiagen Inc). At 72 hours post transfection, cells were harvested for RT-qPCR or cell counting.
For miR-708 stable expression, human lung adenocarcinoma epithelial (A549) cells and human embryonic kidney 293T (HEK293T) cells were plated at 3 × 103 cells per well in 96 wells and transduced at 5 MOI with miR-708 or nontargeting (mock) shMIMIC miRNA viral particles (Open Biosystems, Thermo Fisher Scientific, Huntsville, Alabama, USA). Infected cells were selected under puromycin and tested for stable expression of the transduced gene by RT-qPCR.
Cell Counting and Soft Agar Assay
Cell counting was done by trypsinization and counting with a hemacytometer at the end of the experiment. For the soft agar assay, A549 cells stably transduced with miR-708 or the mock control construct were plated at ∼100 cells per well in 6-well plates. Cells were cultured for 2 weeks and evaluated by counting colonies that were >90 μm in diameter using GelCount (Oxford Optronix Ltd, Milton Park, Oxford, United Kingdom).
Cell Migration, Invasion, and Proliferation Assays
In a 6-well plate, 1 × 106 A549 cells stably transduced by miR-708 or mock control construct were plated as a monolayer and were wounded with a p200 tip after 2 days. Images of the wounded area were captured at time intervals of 0, 18, and 40 hours. Cell migration into the wounded area was measured and calculated in triplicate using ImageJ version 1.44m (National Institutes of Health, Bethesda, Maryland, USA). For the invasion assay, H1299 cells were seeded at 2 × 105 cells per well and transiently transfected with 40 nM of miR-708 mimic, anti–miR-708, or control oligonucleotide (mock). At 48 hours posttransfection, cells were trypsinized and reseeded at 1 × 105 cells per well in serum-free medium added to the upper chamber of each Matrigel insert in 24-well format (BD Bioscience [New Jersey, USA], 8-μM pore size). The lower chamber held 10% serum-containing medium. Cells were incubated for 12 hours. Noninvading cells on the top chamber were removed and cells on the membrane's lower surface were stained with hematoxylin-eosin, captured under a microscope, and counted in 5 random fields.
Luciferase Reporter Assay
We used luciferase assay to investigate whether miR-708 modulates the TMEM88 expression. To do so, we generated the 368-bp fragment by PCR using primers, a forward (5′-GGGCTCGAG TGA CCC TCG AGT CAA GAA CAA-3′), a reverse (5′-GGGCGGCCGC TTA TTG ATG CGT GGA CAC TCC-3′), and the cDNA from 293T cells to amplify the TMEM88-3′UTR region. The PCR product was cloned into the XhoI/NotI 3′UTR site of the psiCHECK-2 plasmid (Promega Corp, Madison, Wisconsin, USA). The correct sequence of all the clones was verified through DNA sequencing. HEK293T cells stably transduced with miR-708 and the mock shMIMIC miRNA were plated into a 12-well plate in Dulbecco's Modified Eagle Medium supplemented with 10% heat-inactivated fetal bovine serum. The cells then were transfected with psiCHECK2-TMEM88 UTR constructs containing 3′UTR of TMEM88 using Effectene (Qiagen Inc, Hilden, Germany). The cells were harvested 48 hours after transfection and lysed in accordance with the manufacturer's instructions (Promega Corp). Renilla luciferase activity was measured with a Lumat LB 953 (EG&G Berthhold, Bad Wildbad, Germany) and the results were normalized through firefly luciferase activity. All experiments were conducted in triplicate (ie, 3 wells for each condition).
Results
MiRNA Signatures in Never Smoker Lung Adenocarcinoma
We examined miRNA expression profiles using total RNA from 56 pairs of FF and 47 pairs of FFPE primary never smoker lung adenocarcinomas and matched noninvolved (normal) lung samples (Supplemental Table 1). Using the criteria of fold change >±1.5 and P<.01, we observed 51 miRNAs in the FF cohort and 34 miRNAs in the FFPE cohort that were differentially expressed among the 858 Hsa miRNA genes examined. Among these two sets of genes, 20 miRNAs were common between the two cohorts and the fold change ranged between 2.6 to -2.1 (Table 1). Significantly, the direction and the fold of miRNA expression differences between the tumor and normal samples were very similar for both the FF and FFPE cohorts indicating the robustness of the gene selection algorithm.
Table 1. Commonly Expressed miRNAs Between FF and FFPE Cohorts in Never Smoker Lung Adenocarcinomas.
| miRNA | Fold Change (FF) | P Value (FF) | Fold Change (FFPE) | P Value (FFPE) |
|---|---|---|---|---|
| hsa-miR-144* | -2.075 | 8.1E-08 | -2.050 | 8.3E-06 |
| hsa-miR-34b | -1.942 | 1.7E-05 | -1.987 | 2.4E-06 |
| hsa-miR-486-5p | -1.918 | 1.2E-09 | -1.824 | 3.4E-08 |
| hsa-miR-592 | -1.830 | 1.7E-05 | -1.622 | 3.1E-05 |
| hsa-miR-190b | -1.761 | 1.2E-04 | -1.560 | 2.2E-03 |
| hsa-miR-139-5p | -1.744 | 9.3E-10 | -1.577 | 5.8E-04 |
| hsa-miR-30a* | -1.743 | 9.1E-12 | -1.785 | 2.5E-10 |
| hsa-miR-34c-3p | -1.708 | 1.4E-04 | -1.955 | 1.7E-07 |
| hsa-miR-1 | -1.656 | 1.3E-05 | -1.514 | 1.1E-04 |
| hsa-miR-218 | -1.606 | 1.6E-08 | -1.641 | 5.7E-08 |
| hsa-miR-182 | 1.646 | 1.1E-12 | 2.055 | 8.0E-09 |
| hsa-miR-135b | 1.691 | 5.0E-13 | 2.861 | 1.6E-14 |
| hsa-miR-9 | 1.817 | 5.5E-04 | 1.546 | 2.6E-03 |
| hsa-miR-708 | 1.958 | 1.3E-08 | 1.748 | 7.3E-05 |
| hsa-miR-183 | 2.064 | 5.4E-14 | 2.147 | 7.7E-09 |
| hsa-miR-196a | 2.071 | 2.7E-03 | 2.132 | 2.1E-04 |
| hsa-miR-31 | 2.125 | 4.8E-05 | 1.977 | 1.4E-04 |
| hsa-miR-21* | 2.377 | 2.3E-09 | 1.936 | 6.9E-09 |
| hsa-miR-210 | 2.507 | 1.1E-09 | 1.832 | 4.3E-05 |
| hsa-miR-96 | 2.577 | 3.3E-14 | 2.032 | 1.9E-09 |
Abbreviations: FF, fresh-frozen; FFPE, formalin-fixed, paraffin-embedded.
P values were calculated using 1-way ANOVA.
MiR-708 Is Associated with Overall Patient Survival in Never Smoker Lung Adenocarcinoma
To investigate the potential biological significance of these 20 consistently altered miRNAs, we performed Cox proportional hazards model analyses for each cohort. Increased expression of miR-708 was associated with an increased risk of death by both univariate analysis (FF cohort, P=.016; FFPE cohort, P=.003) and after adjustment for patient's age at diagnosis, sex, and tumor stage (FF cohort: hazard ratio, 1.90; 95% confidence interval, 1.08-3.35; P=.025 and FFPE cohort: HR, 1.93; 95% CI, 1.02-3.63; P=.042) (Figure 1).
Figure 1.
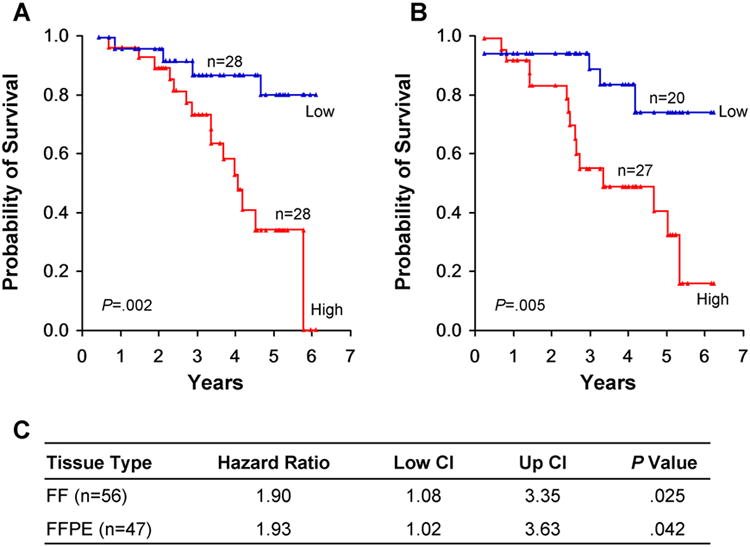
MiR-708 Status and Survival From Lung Adenocarcinoma in Never Smokers. A and B, Tumor samples from the FF cohort (A) and FFPE cohort (B) were analyzed for survival by Kaplan-Meier method. The number of samples (n) in each arm are as indicated. C, Cox proportional hazard model analysis for miR-708. CI indicates confidence interval.
Validation of miR-708 Expression in Lung Cancer
We first confirmed the miR-708 expression status by quantitative PCR in 30 paired FF samples used for expression profiling (Figure 2A) and in a Korean cohort of 15 paired FF samples (Figure 2B). We also examined miR-708 expression in smoker lung adenocarcinomas including 19 paired FF samples from Korea (Figure 2C) and in a NCI cohort of mostly smokers (Figure 2D). Consistently, miR-708 expression level was significantly increased in tumor compared to the matched normal lung (Figure 2A-C, P<.05-.001) and in the NCI cohort of nonmatched tumor and normal lung samples (Figure 2D, P<.001). In addition, we examined 85 lung squamous cell carcinoma samples from the same NCI cohort (24) and observed a similarly high miR-708 expression in the tumors (Supplemental Figure 1). However, no survival significance was observed for the NCI cohort (P = .239) and the sample size of the Korean cohort was too small for survival analysis.
Figure 2.
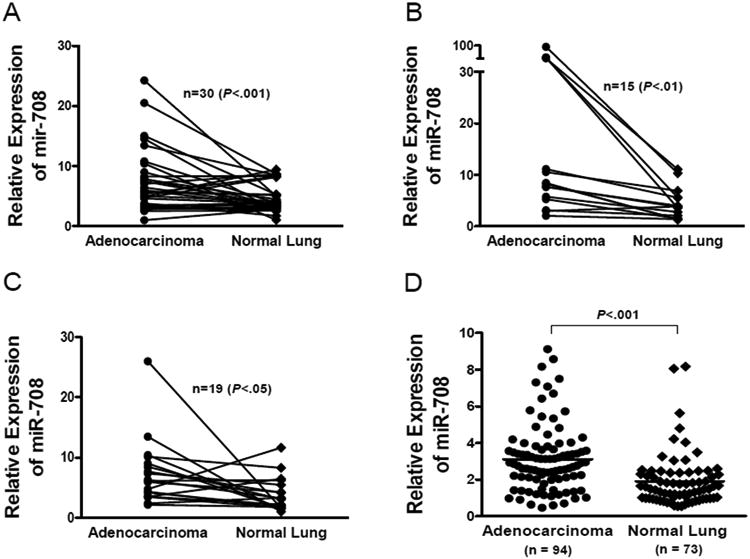
Validation of miR-708 Overexpression by Real-Time qPCR in Lung Adenocarcinoma. Paired FF tumor and normal tissues from the Mayo Clinic (A) and Korea cohorts (B). And, lung adenocarcinoma from smokers using paired FF samples from Korea cohort (C) and non-paired tumor and normal FFPE tissues from the National Cancer Institute cohort (D). Sample numbers (n) and tissue types are indicated on each graph. P values are as shown paired 2 tailed t test (A-C) and by unpaired 2-tailed t test (D).
MiR-708 and Cell Proliferation, Migration, and Invasion
The biological role of miR-708 in cell growth was investigated in several human cell lines through loss- or gain-of-function experiments. We first tested the H1299 lung cancer cells which have a relatively high level of endogenous miR-708 expression and in minimally transformed BEAS-2B normal lung cells which have undetectable level of the gene (Supplemental Figure 2). We modulated cellular miR-708 levels by transiently transfecting these cells with either miR-708 oligomers or anti–miR-708 oligomers (Figure 3). Knockdown of endogenous miR-708 reduced cell proliferation in H1299 cells (P<.05) but had no effect on BEAS-2B cells (Figure 3A). However, overexpression of miR-708 in these cells increased proliferation by 60% (P<.001) in both H1299 and BEAS-2B cells compared with the mock transfected cells. Similarly, transiently overexpressing miR-708 in H1299 cells increased cell invasion by 2-fold compared with the mock control. In contrast, those transfected by anti–miR-708 oligomer showed a decreased rate of cell invasion (Figure 3B) (P<.01). Furthermore, when we stably expressed miR-708 in A549 lung cancer cell lines, there was a nearly 3-fold increase in colony growth of the infected cells compared with the mock control and a 1.6-fold increase in cell migration measured by wound healing test (Figure 3C and 3D) (P<.01).
Figure 3.
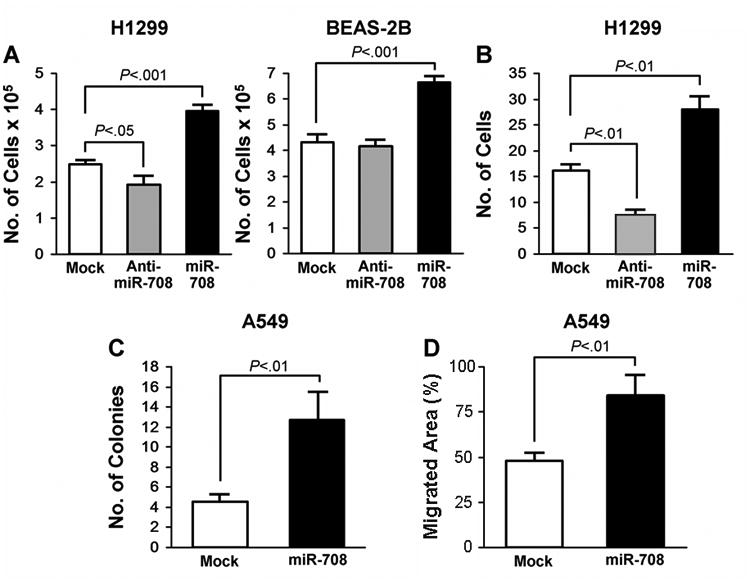
MiR-708 Overexpression Promotes Cell Growth, Proliferation, and Migration. A, Increased growth of H1299 and BEAS2B cells after transfection with indicated oligomers (40 nM each) and B, No. of similarly transfected H1299 cells invading through Matrigel membrane. C, Soft agar assay in A549 cells stably infected with mock or miR-708 shMIMIC miRNA viral particles. D, Wound healing/scratch assay for stably infected A549 cells. P values, when significant, are shown and calculated using unpaired 2-tailed t test. Error bars represent mean±SEM.
Mir-708 Status and Gene Expression Signature in Lung Adenocarcinoma from Never Smokers
To determine the gene expression changes associated with miR-708, we selected 169 genes most differentially expressed on the basis of its expression status in the 56 FF tumor samples. Tumors clustered separately when dichotomized on the basis of expression status of miR-708 (Figure 4A). Furthermore, patients whose tumors had high expression of miR-708 (miR-708-H) were more likely to have an overall less favorable survival than those with low miR-708 (miR-708-L) expression in their tumors (Figure 4B). Among these genes, KRT6A and MMP1, 10, 11, and 13, as well as TFF1 and TOP2A were highly expressed while TMEM88 was reduced in miR-708-H tumors (Supplemental Table 2).
Figure 4.
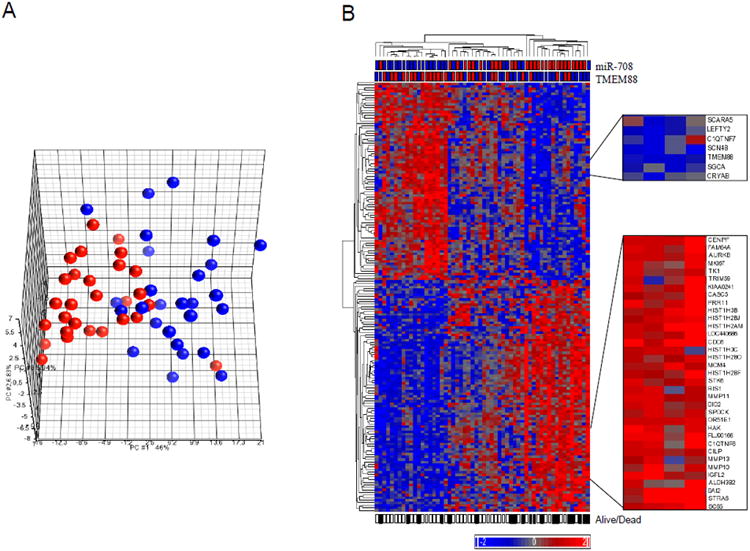
Principal Component Analysis Plot and Hierarchal Clustering of 169 Genes According to miR-708 Expression Status in Tumor Tissues. A, Multidimensional scaling of 56 tumors based on 169 differentially expressed genes. B, Genes are listed from top to bottom; samples are listed from left to right. Gene expression levels are represented by heat map, with relative intensities indicated by the heat scale. The miR-708 and TMEM88 status in the tumors are indicated above the heat map. The filled squares underneath the heat map indicate deceased patients; open squares indicate patients still alive at the last follow-up. Selected clusters of genes differentially expressed in miR-708-H and miR-708-L groups are shown. Colors denote tumor that were either miR-708-H (Red) or miR-708-L (Blue). The complete list of all 169 genes is shown in order in Supplemental Table 2.
TMEM88 Is a Potential Downstream Target of miR-708
We further examined these 169 genes to identify likely targets of miR-708. Seven genes were predicted on the basis of the TargetScan and MicroCosm databases (Supplemental Table 3). One of these genes, Target transmembrane protein (TMEM) 88, was down regulated by 1.6-fold in tumors with high miR-708 expression levels compared with those that had low miR-708 expression levels and was associated with overall survival. Overall, TMEM88 expression level was low by RT-qPCR in the FF cohort compared with the matched noninvolved lung (Figure 5A) (P<.001). Patients whose tumors had relatively higher expression levels of TMEM88 had more favorable overall survival (Figure 5B) (P=.039 log-rank). In Cox analysis, the hazard ratio for TMEM88 expression was 0.41 (95% CI, 0.19-0.86; P=.018). In the luciferase assay, miR-708 expression resulted in a 2.5-fold reduction of luciferase activity in the construct containing TMEM88-3′UTR (Figure 5C) (P<.01). Furthermore, the mRNA level of endogenous TMEM88 decreased in a dose-dependent manner in cells overexpressing miR-708 (Figure 5D) (P<.001).
Figure 5.
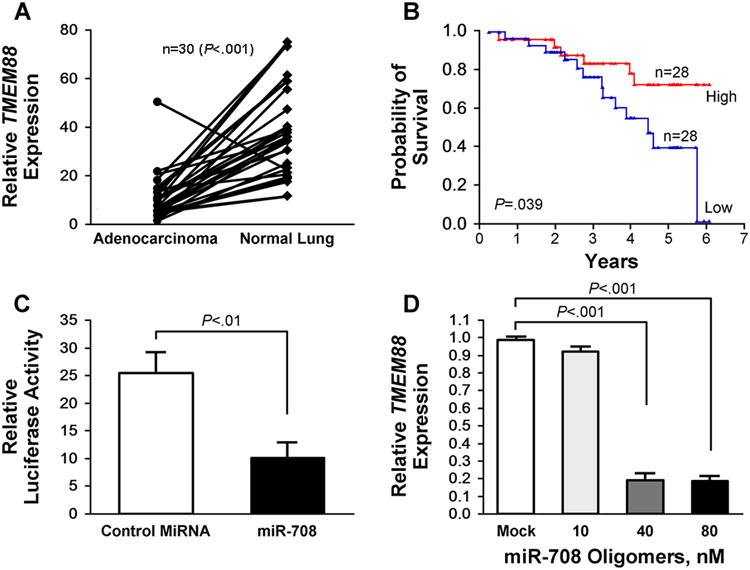
TMEM88 is a Potential Downstream Target of miR-708. A, Expression status of TMEM88 in paired FF adenocarcinoma and normal lung. B, TMEM88 expression and survival from lung adenocarcinoma in never smokers. C, Exogenous miR-708 expression reduced luciferase activity when the gene was downstream of TMEM88-3′UTR. D, MiR-708 directly down regulates TMEM88 in H1299 cells in a dose-dependent manner. P values, when significant, are as shown and calculated using unpaired 2-tailed t test. Error bars represent mean±SEM.
Discussion
The critical role of miRNA in cancer has become increasingly apparent. Studies have shown that these small regulatory RNA molecules participate in a diverse set of cell signaling processes, such as apoptosis, cell proliferation, and epithelial-to-mesenchymal transition (26-29). In lung cancer, miR-21 has been shown to be associated with overall survival or to participate in regulating cell proliferative signaling pathways, or both (5, 7, 20, 30). Differential gene expression studies have also identified various miRNA genes that predict lung cancer tumor type, as well as tumor development (21, 31, 32). In the present study, we identified 20 most significantly altered miRNA genes using 2 independent cohorts of lung adenocarcinoma samples from never smokers. Four of the 20 genes—miR-9, miR-210, miR-218, and miR-708, have been reported in lung cancer from smoker patients with lung squamous cell carcinomas (5, 7, 31). miR-182 and miR-183 were reported to be overexpressed in lung adenocarcinoma from nonsmokers in a Chinese cohort (30). MiR-183 has been shown to function as an oncogene by targeting the transcription factor EGR1 and promotes tumor cell migration (33). Jointly, these results suggest that a significant fraction of miRNA alteration observed in lung cancer of never smokers could also occur in smokers and lung tumor of other histological subtype.
Among the genes significantly overexpressed in the tumors in our study, miR-708 was most significantly associated with survival in the lung adenocarcinoma from never smoker patients by both univariate and multivariate analysis (Figure 1). Patients whose lung adenocarcinomas were high in miR-708 expression had a shorter survival while those with low miR-708 expression tended to have a better survival (Figure 4). In our validation analysis, miR-708 was high in a majority of the tumors tested including those from lung adenocarcinoma of smoker and never smokers (Korean Cohort), as well as in squamous cell carcinomas (NCI cohort). Functionally, miR-708 overexpression resulted in increased cell proliferation, migration, and invasion, whereas cells that were knocked down for miR-708 showed a decrease in these phenotypes in lung cancer cells (Figure 3).
Genetically, the miR-708 is located on chromosome 11q14.1 and is encoded in the intron 1 of the Odd Oz/ten-m homolog 4 (ODZ4) gene, a gene regulated by CCAAT enhancer-binding homologous protein (CHOP) in vertebrates (34). It is cotranscribed with its host gene and is most highly expressed in the brain and eyes and appears to have a role in endoplasmic reticulum stress of the eye (35). By quantitative polymerase chain reaction (qPCR), miR-708 has the highest expression level in the brain and eyes and appears to have a role in endoplasmic reticulum stress in the eye (35). Although the exact mechanism remains unknown, miR-708 overexpression has been reported in lymphoblastic leukemia (36) and it is one of the frequently identified microRNAs in lung cancer (31, 32). Xing et al (32) showed that miR-708 was among the 3 most predictive markers of squamous cell carcinoma using sputum while Patnaik et al (37) reported that miR-708 was 1 of 9 prognostic markers in surgically resected stage I non–small cell lung cancers. Our study is the first to demonstrate an association between miR-708 expression and overall survival in subgroup of lung cancer.
Using miRNA and mRNA gene expression profiling and integrated bioinformatics analysis, we identified the transcript TMEM88 as one of the candidate targets of miR-708 (Supplemental Figure 3). TMEM88 is located in chromosome 17p13.1 near the TP53 genes and contains two transmembrane domains and a PDZ-binding domain at its C-terminal. Recently, Lee et al (37) reported that TMEM88 interacts with the PDZ domain of disheveled (Dvl) gene and the C-terminal tail of TMEM88 sublocalizes at the cell membrane. This binding inhibits Wnt signaling induced by Xdsh but not β-catenin in Xenopus. Knockdown of TMEM88 results in an increase of Wnt activity by >2 fold (27). In our integrative analysis based on mRNA and miRNA expression profiles, TMEM88 expression was inversely associated with miR-708 expression, and the increased expression of TMEM88 correlated with a reduced overall risk of death in lung adenocarcinoma from never smokers. Furthermore, miR-708 forced expression directly inhibited TMEM88 messenger RNA level in transduced cells and via its 3′UTR binding site dependent manner (Figure 5C and 5D).
Our results suggest that miR-708 plays a critical role in lung cancer development based on the following observations:
MiR-708 is one of the most commonly overexpressed miRNAs in NSCLC and its high level expression is associated with a reduced survival in lung adenocarcinoma from never smokers.
Among the transcripts most differentially expressed between miR-708-H and miR-708-L tumors, the miR-708-H cluster included several of the metalloproteinases (MMPs), which are capable of degrading the extracellular matrix macromolecules and promote tumor cell migration and metastasis (Figure 4) such as, MMP1, 10, 11, and 13 (38-42). These genes could be indirectly regulated upon miR-708 overexpression (17, 33).
In functional studies, overexpression of miR-708 in lung cancer cells induced phenotypes consistent with increased cellular proliferation and migration.
Among the genes that were down regulated in miR-708-H tumors, TMEM88 is a negative regulator of Wnt signaling pathway (37) and appears to be a direct target of miR-708. These observations provide a tantalizing link that miR-708 promotes lung cancer progression, at least in part, through regulating Wnt signaling pathway.
It is important to note that because of the ability for each miRNA gene to target and regulate a diverse set of mRNA transcripts, it is highly likely that the role of miR-708 depends on the tissue type and cellular context. In our study, although miR-708 overexpression has been observed in smokers and never smokers of lung adenocarcinoma as well as squamous cell carcinoma, a survival difference was only observed in the adenocarcinoma of the never smoker group based on miR-708 status. This could be due to statistical chance and sampling variations among the different cohorts. It may also indicate that the significance of miR-708 on patient survival from lung cancer is dependent on the context of the cellular environment and the presences of other coexisting genetic changes in the tumor. In lung cancer from smokers, widespread genetic alterations and inactivation of a large number of different genes (43, 44) involving distinct tumorigenic processes as a result of cigarette smoking could have precluded a direct association between miR-708 status and patient survival. However, the commonly observed overexpression of miR-708 in NSCLC by our group and others jointly implicates a role of this miRNA as an important player in lung cancer development and progression.
Supplementary Material
Translational Relevance.
MicroRNA participates in a variety of biological processes and can function as either oncogene or tumor suppressor through regulating its target genes. Here, we demonstrate that miR-708 is one of the most highly overexpressed miRNAs in non-small cell lung cancer. High level of miR-708 in tumor is also associated with a reduced overall survival in lung adenocarcinomas from never smokers. Functionally, miR-708 overexpression increases the proliferation, migration, and invasion in cultured cells and down regulates TMEM88, a negative regulator of Wnt signaling. Jointly, our results support an oncogenic role of miR-708 by activating Wnt signaling pathway to promote lung cancer progression.
Acknowledgments
We thank Dr. Doo-Sub Choi and members of his laboratory for technical assistance; Dr. Eric Wieben for support. We thank Dr. Wilma Lingle and Ms. Karla Kopp from the Mayo Tissue and Cell Molecular Analysis (TACMA) and Gene Expression Core (GEC) for technical support.
Grant Support: This work was supported by Funding from the Mayo Cancer Center, the Center for Individualized Medicine and the National Institutes of Health (CA77118, CA80127 and CA84354) to Dr. Ping Yang for sample and data collection.
Footnotes
Conflict of interest: None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 3.Powell CA, Spira A, Derti A, DeLisi C, Liu G, Borczuk A, et al. Gene expression in lung adenocarcinomas of smokers and nonsmokers. Am J Respir Cell Mol Biol. 2003;29:157–62. doi: 10.1165/rcmb.2002-0183RC. [DOI] [PubMed] [Google Scholar]
- 4.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101:10143–8. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 7.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A. 2009;106:12085–90. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–51. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 10.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–7. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karp X, Ambros V. Developmental biology. Encountering microRNAs in cell fate signaling. Science. 2005;310:1288–9. doi: 10.1126/science.1121566. [DOI] [PubMed] [Google Scholar]
- 12.Sempere LF, Sokol NS, Dubrovsky EB, Berger EM, Ambros V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity. Dev Biol. 2003;259:9–18. doi: 10.1016/s0012-1606(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 13.Xu P, Guo M, Hay BA. MicroRNAs and the regulation of cell death. Trends Genet. 2004;20:617–24. doi: 10.1016/j.tig.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–10. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–9. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 20.Saito M, Schetter AJ, Mollerup S, Kohno T, Skaug V, Bowman ED, et al. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res. 2011;17:1875–82. doi: 10.1158/1078-0432.CCR-10-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–8. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Liu Z, Todd NW, Zhang H, Liao J, Yu L, et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011;11:374. doi: 10.1186/1471-2407-11-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Sheu CC, Ye Y, de Andrade M, Wang L, Chang SC, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010;11:321–30. doi: 10.1016/S1470-2045(10)70042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10:4314–24. doi: 10.1158/1078-0432.CCR-03-0489. [DOI] [PubMed] [Google Scholar]
- 25.Lee SY, Kim MJ, Jin G, Yoo SS, Park JY, Choi JE, et al. Somatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancers. J Thorac Oncol. 2010;5:1734–40. doi: 10.1097/JTO.0b013e3181f0beca. [DOI] [PubMed] [Google Scholar]
- 26.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 27.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–40. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osada H, Takahashi T. let-7 and miR-17-92: small-sized major players in lung cancer development. Cancer Sci. 2011;102:9–17. doi: 10.1111/j.1349-7006.2010.01707.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411:846–52. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 30.Cho WC, Chow AS, Au JS. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer. 2009;45:2197–206. doi: 10.1016/j.ejca.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Patnaik SK, Kannisto E, Knudsen S, Yendamuri S. Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res. 2010;70:36–45. doi: 10.1158/0008-5472.CAN-09-3153. [DOI] [PubMed] [Google Scholar]
- 32.Xing L, Todd NW, Yu L, Fang H, Jiang F. Early detection of squamous cell lung cancer in sputum by a panel of microRNA markers. Mod Pathol. 2010;23:1157–64. doi: 10.1038/modpathol.2010.111. [DOI] [PubMed] [Google Scholar]
- 33.Sarver AL, Li L, Subramanian S. MicroRNA miR-183 functions as an oncogene by targeting the transcription factor EGR1 and promoting tumor cell migration. Cancer Res. 2010;70:9570–80. doi: 10.1158/0008-5472.CAN-10-2074. [DOI] [PubMed] [Google Scholar]
- 34.Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, et al. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–30. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behrman S, Acosta-Alvear D, Walter P. A CHOP-regulated microRNA controls rhodopsin expression. J Cell Biol. 2011 doi: 10.1083/jcb.201010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schotte D, Chau JC, Sylvester G, Liu G, Chen C, van der Velden VH, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23:313–22. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Finkelstein D, Li X, Wu D, Shi DL, Zheng JJ. Identification of transmembrane protein 88 (TMEM88) as a dishevelled-binding protein. J Biol Chem. 2010;285:41549–56. doi: 10.1074/jbc.M110.193383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehan E, Ben-Dor A, Liao W, Lipson D, Frimer H, Rienstein S, et al. Chromosomal aberrations and gene expression profiles in non-small cell lung cancer. Lung Cancer. 2007;56:175–84. doi: 10.1016/j.lungcan.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Hsu CP, Shen GH, Ko JL. Matrix metalloproteinase-13 expression is associated with bone marrow microinvolvement and prognosis in non-small cell lung cancer. Lung Cancer. 2006;52:349–57. doi: 10.1016/j.lungcan.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Kettunen E, Anttila S, Seppanen JK, Karjalainen A, Edgren H, Lindstrom I, et al. Differentially expressed genes in nonsmall cell lung cancer: expression profiling of cancer-related genes in squamous cell lung cancer. Cancer Genet Cytogenet. 2004;149:98–106. doi: 10.1016/S0165-4608(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 41.Mari BP, Anderson IC, Mari SE, Ning Y, Lutz Y, Kobzik L, et al. Stromelysin-3 is induced in tumor/stroma cocultures and inactivated via a tumor-specific and basic fibroblast growth factor-dependent mechanism. J Biol Chem. 1998;273:618–26. doi: 10.1074/jbc.273.1.618. [DOI] [PubMed] [Google Scholar]
- 42.Sun T, Gao Y, Tan W, Ma S, Zhang X, Wang Y, et al. Haplotypes in matrix metalloproteinase gene cluster on chromosome 11q22 contribute to the risk of lung cancer development and progression. Clin Cancer Res. 2006;12:7009–17. doi: 10.1158/1078-0432.CCR-06-0464. [DOI] [PubMed] [Google Scholar]
- 43.Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465:473–7. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 44.Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–90. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


