Abstract
Background
The Integrated Relaxation Pressure (IRP) is the esophageal pressure topography (EPT) metric used for assessing the adequacy of esophagogastric junction (EGJ) relaxation in the Chicago Classification of motility disorders. However, because the IRP value is also influenced by distal esophageal contractility, we hypothesized that its normal limits should vary with different patterns of contractility.
Methods
522 selected EPT studies were used to compare the accuracy of alternative analysis paradigms to that of a motility expert (the ‘gold standard’). Chicago Classification metrics were scored manually and used as inputs for MATLAB™ programs that utilized either strict algorithm-based interpretation (fixed abnormal IRP threshold of 15 mmHg) or a classification and regression tree (CART) model that selected variable IRP thresholds depending on the associated esophageal contractility.
Results
The sensitivity of the CART model for achalasia (93%) was better than that of the algorithm-based approach (85%) on account of using variable IRP thresholds that ranged from a low value of >10 mmHg to distinguish type I achalasia from absent peristalsis to a high value of >17 mmHg to distinguish type III achalasia from distal esophageal spasm. Additionally, type II achalasia was diagnosed solely by panesophageal pressurization without the IRP entering the algorithm.
Conclusion
Automated interpretation of EPT studies more closely mimics that of a motility expert when IRP thresholds for impaired EGJ relaxation are adjusted depending on the pattern of associated esophageal contractility. The range of IRP cutoffs suggested by the CART model ranged from 10 to 17 mmHg.
Keywords: achalasia, esophageal motility disorders, classification and regression tree, esophageal manometry, esophageal pressure topography
INTRODUCTION
The adequacy of esophagogastric junction (EGJ) relaxation during swallowing is a major diagnostic criterion in the interpretation of clinical esophageal manometry studies. In the Chicago Classification of esophageal pressure topography (EPT), the Integrated Relaxation Pressure (IRP) is the primary tool used in this assessment, with normal being defined as <15 mmHg based on a study comparing criteria for detecting impaired deglutitive EGJ relaxation in a large group of patients and control subjects (1–3). The IRP is defined as the average lowest pressure through the EGJ for four contiguous or non-contiguous seconds within the relaxation window (Figure 1). By incorporating both a measure of the completeness of relaxation and the duration of time that relaxation is sustained, the IRP improves the sensitivity of detecting achalasia. This single measure of deglutitive relaxation exhibited 98% sensitivity and 96% specificity for distinguishing well-defined achalasia patients from control subjects and patients with varying esophageal diagnoses (2, 3). Thus, the IRP has become a cardinal metric in the Chicago Classification for defining esophageal motor disorders (4).
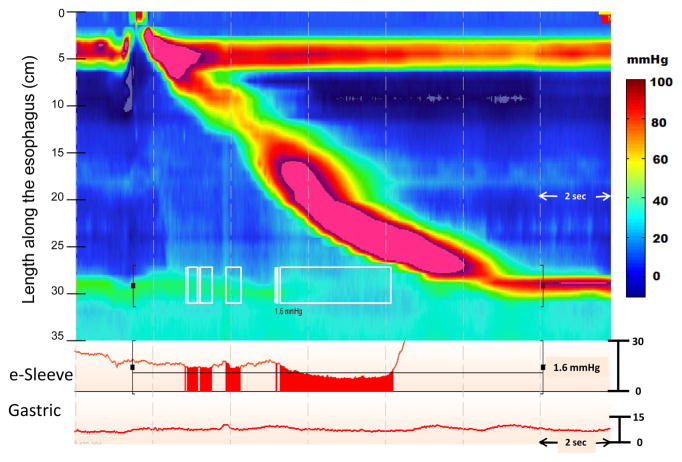
Figure 1.
IRP calculation for a normal swallow. The IRP is a complex metric as it involves accurately localizing the margins of the EGJ, demarcating the time window following deglutitive upper sphincter relaxation within which to anticipate EGJ relaxation to occur and then applying an e-sleeve measurement within that 10 second time box (highlighted by the black brackets). The e-sleeve is referenced to gastric pressure and provides a measure of the highest pressure through the axial domain at an instant in time and is plotted as a line tracing. The IRP is presented as the mean value of the four seconds during which the e-sleeve value is least. These time points are demarcated by the white boxes on the EPT plot and by the shaded red area noted on the red line tracing of the EGJ. In this example, the 4 second IRP is 1.6 mmHg which is slightly higher than intragastric pressure.
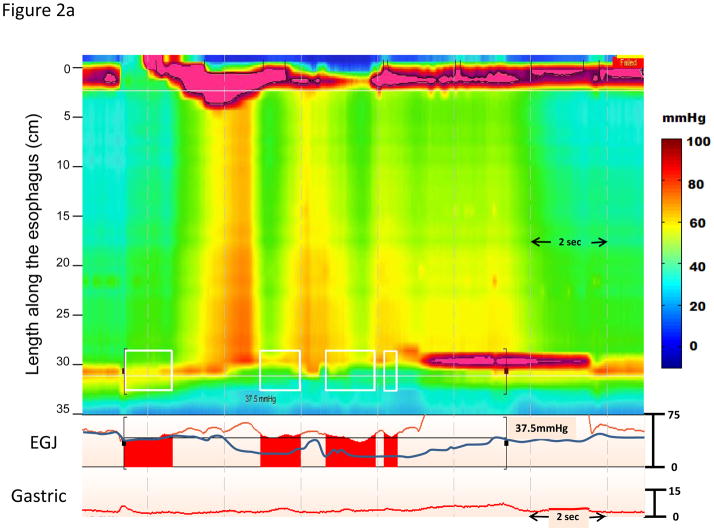
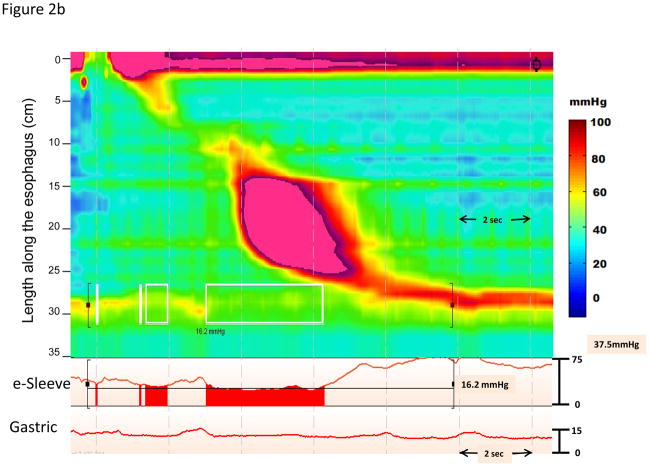
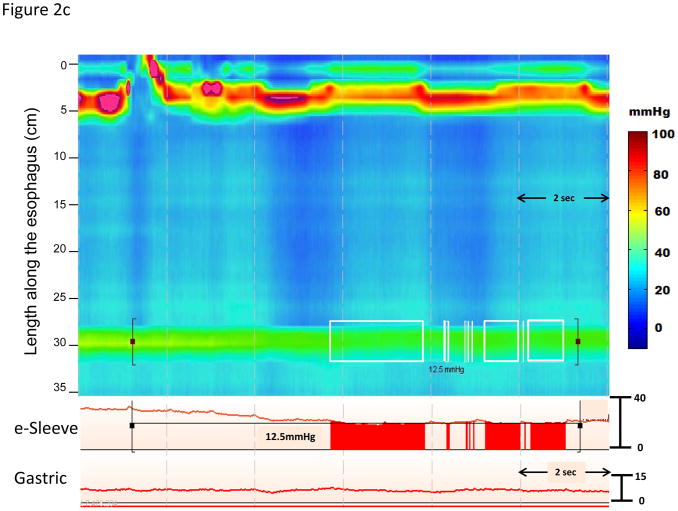
Although the IRP is substantially better validated than any previous measure of EGJ relaxation, it is a complex metric potentially dependent on not only the adequacy of lower esophageal sphincter relaxation, but also on the pattern and timing of distal esophageal contractility, and on the adequacy of EGJ opening following relaxation (Figure 2). Figure 2a represents an example where the increased IRP is partly attributable to high intraesophageal pressure during panesophageal pressurization that is also accompanied by esophageal shortening. Note that the blue line representing a conventional measure of LES relaxation exhibits pseudo-relaxation and would fail to detect the impaired EGJ relaxation. The IRP can also be influenced by other patterns of distal esophageal contractility. A premature distal contraction in essence shortens the window during which the IRP can be assessed which tends to increase the IRP measure (Figure 2b). Conversely, in the absence of any distal esophageal contractility and low intraesophageal pressure, the IRP is entirely dependent on the sphincter itself, potentially leading to ‘normal’ values despite still being obstructive to bolus flow (Figure 2c). Given that the IRP is partially dependent on these additional variables, it is logical that the IRP cutoff for defining abnormal function might vary with the contractile and or pressurization pattern in the esophagus. This might have clinical implications as to whether or not patients meet diagnostic criteria for a subtype of achalasia or EGJ outflow obstruction. For example, the patient illustrated in Figure 2c had an IRP value of 12 mmHg and would be classified as absent peristalsis using strict Chicago Classification criteria. However, the patient had an abnormal timed barium esophagram consistent with achalasia and subsequently had an excellent clinical response to a laparoscopic Heller myotomy.
Figure 2.
Three representative swallows of instances where the IRP is influenced by the timing and pattern of the distal esophageal contraction. Panel A represents an example patient with type II achalasia. In the example, the IRP is noted to be 37.5 mmHg and this value suggests a significant obstruction is occurring through the EGJ. The blue line tracing through the EGJ represents a simulation of a conventional single sensor technique placed through the EGJ. The measurement from this single sensor is subject to significant movement artifact and in this case the sensor records normal relaxation (pseudorelaxation) and the diagnosis would have been missed. Panel B represents an example where the IRP threshold may be too low to distinguish distal esophageal spasm from type III achalasia. This patient had a mean IRP of 16.2 mmHg and premature contractions with a distal latency < 4.5 seconds on multiple swallows. Although, there are periods where the EGJ relaxation pressure is below the threshold of 15 mmHg, the deglutitive window is limited by the premature contraction and thus, the 4 second time demand for the IRP measurement may be exaggerated by the small time window allotted for measurement. This could potentially alter the diagnosis of DES and this patient would have been characterized as type III achalasia despite evidence of intact relaxation. Panel C represents another instance where the IRP threshold of 15 mmHg may be misleading. This patient had a mean IRP value of 12.0 mmHg with absent peristalsis. This single swallow example illustrates how a lower IRP value may be associated with EGJ obstruction and poor bolus transit when the swallow is failed and associated with minimal esophageal pressurization. This patient had a timed barium esophagram exhibiting a sustained column at 5 minutes and was treated as achalasia with a successful outcome.
Given the interdependence between the IRP and features of distal esophageal contractility, we hypothesized that the optimal cutoff value defining impaired EGJ relaxation may vary with contractile pattern. Hence, the aim of this study was to utilize a classification and regression tree (CART) analysis to determine diagnostic IRP cutoffs based on the different contractile patterns. CART analysis is a nonparametric decision tree methodology that can segment populations into meaningful subgroups (5). Since its first development by Breiman and colleagues (6), CART modeling has increasingly been applied to health science and clinical research to improve diagnosis and management decisions (5, 7). We hypothesized that the IRP cutoff values for diagnosing impaired EGJ relaxation would be modified by CART analysis and that this will improve classification accuracy within the Chicago Classification scheme.
METHODS
Subjects
Clinical EPT studies from a database of 2000 studies spanning from January 19, 2007 to May 12, 2010 were utilized for this study; 522 patients (196 males, mean age 51 years, range 18–91 years) from this data set were selected for this analysis. Technically limited studies and patients with previous surgery were excluded. Given the focus on assessing optimal IRP cut-off values, the population was enriched with patients with an abnormal IRP. A chart review was performed to determine the original clinical diagnosis (JEP). The study protocol was approved by the Northwestern University Institutional Review Board.
Manometry
High resolution manometry studies were done with a 4.2 mm outer diameter solid-state assembly with 36 circumferential sensors spaced at 1-cm intervals (Given Imaging, Los Angeles, CA). Before recording, transducers were calibrated at 0 and 300 mmHg using externally applied pressure. Studies were done in a supine position after at least a 6-hr fast. The manometry assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with about 3 intra-gastric sensors. The catheter was fixed in place by taping it to the nose. The manometric protocol included a 5 minute period to assess basal sphincter pressure and ten 5-ml water swallows.
Analysis Protocol
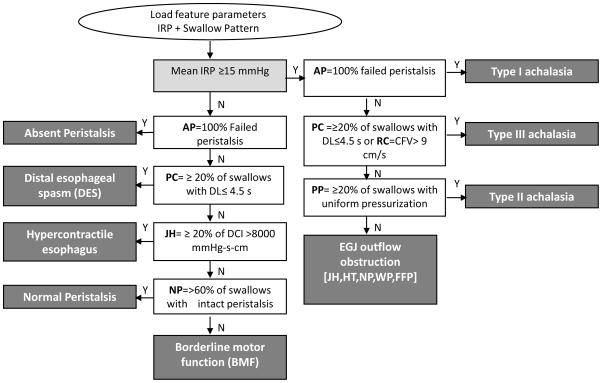
Pressure topography metrics utilized in the Chicago Classification including the Integrated Relaxation Pressure (IRP), peristaltic integrity (or breaks), Distal Contractile Integral (DCI), Contractile Front Velocity (CFV), and Distal Latency (DL) were measured for each swallow. Each swallow was scored by a blinded expert (SR) using Manoview™ software (Given Imaging, Los Angeles, CA). These data were then uploaded into a MATLAB™ program that utilized a strict cut-off value for the IRP (15 mmHg) and other criteria of the Chicago Classification to generate the algorithmic diagnosis, consistent with the 2012 iteration of the Chicago Classification of esophageal motility (Figure 3) (4).
Figure 3.
Flowchart of algorithm-based approach for automated diagnosis of esophageal motility disorders from EPT studies. The input feature parameters included the integrated relaxation pressure (IRP) and utilized peristaltic integrity, distal esophageal latency (DL), contractile front velocity (CFV), and Distal Contractile Integral (DCI) to define contractile patterns. These values were applied to formulate the final Chicago Classification diagnosis (dark gray boxes).
The same mean IRP values and Chicago Classification metrics from all 522 studies that were input into the algorithmic diagnosis program were also used as inputs to train and test a CART model using another MATLAB™ program. The CART model utilized the clinical diagnoses of another expert in EPT interpretation (JEP) as the ‘gold standard’ and allowed that the optimal IRP cutoff could be different for different esophageal contractile patterns. To facilitate this, each study was categorized as one of nine contractile patterns (Table 1) utilized in the Chicago Classification as a defining feature for the final diagnosis, which could potentially then be modified depending on the IRP. Modification based on the IRP could alter the final diagnosis to one of the achalasia subtypes or to EGJ outflow obstruction. In essence, the CART model learned to modify the IRP for specific contractility patterns from a systematic analysis of observed discrepancies between the strictly algorithmic diagnoses and those of the expert in clinical interpretation.
Table 1.
Esophageal contractile patterns, their defining characteristics, and their codes after analysis of the ten component test swallows for input to the CART model. These differ from the final Chicago Classification diagnoses in that in some cases they could be modified to an achalasia subtype or EGJ outflow obstruction depending on the corresponding IRP value. For instance, absent peristalsis could be modified to type I achalasia.
| Contractile pattern | Code | Definition |
|---|---|---|
| Absent Peristalsis | AP | 100% failed peristalsis with minimal (<3 cm) integrity of the 20 mmHg IBC† distal to the proximal pressure trough (P) |
| Frequent Failed Peristalsis | FFP | Greater than 3 but less than 10 swallows with failed peristalsis |
| Panesophageal Pressurization | PP | ≥20% of swallows with uniform pressurization of 30 mmHg from the UES to the EGJ |
| Premature Contraction | PC | ≥20% of swallows with DL ≤4.5 s |
| Jackhammer | JH | Swallow with DL >4.5 s and DCI >8000 mmHg-s-cm |
| Rapid Contraction | RC | ≥20% of swallows with contractile front velocity (CFV) >9 cm/s and DL > 4.5 seconds |
| Hypertensive | HT | Mean DCI greater than 5000 but no swallow with value >8,000 mmHg-s-cm |
| Weak Peristalsis | WP | > 20% swallows with large breaks in the 20 mmHg IBC (>5 cm in length) or >30% swallows with small breaks in the 20 mmHg IBC (2–5 cm in length) |
| Normal Peristalsis | NP | ≥60% of swallows with an intact 20 mmHg IBC (or no break >2 cm) not meeting any other code |
IBC = Isobaric contour
Classification and Regression Tree (CART) Model
The basic principle of the CART model was to sequentially divide the entry population of 522 EPT studies into subpopulations grouped by the variables in Table 1 such that the resulting subpopulations were maximally homogeneous with respect to the final Chicago Classification diagnoses. At each node of the analysis, the CART algorithm identified the most robust candidate variable (or group of variables) to divide the nodal population into two sub-populations that were then progressively subdivided until each subpopulation was comprised of the maximal homogeneity for a single Chicago Classification diagnosis. If the variable selected at a node was the IRP, the program also found the best cutoff value pertinent to that node to facilitate accurate classification of the remaining population. When a terminal node (final diagnosis) was reached, the program calculated the accuracy with which subjects were so classified using the expert diagnosis as the ‘gold standard’. In practice, the minimum node size is usually set at 10% of the overall learning sample to avoid potentially over-fitting the model potentially making the final decision tree not generalizable to other candidate populations.
Statistical Analysis
Sensitivity, specificity and the associated number of misclassified cases were used to compare the performance of the CART model and algorithm based analysis for predicting nine diagnostic categories of the Chicago Classification were assessed: achalasia Type I, II, or III, EGJ outflow obstruction, distal esophageal spasm, absent peristalsis, hypercontractile esophagus, borderline motor function (BMF), and normal. The BMF group consisted of weak peristalsis with large or small peristaltic defects, frequent failed peristalsis, rapid contractions with normal latency, and hypertensive peristalsis.
RESULTS
The expert diagnoses of the 522 EPT studies were: 110 normal, 71 achalasia (14 type I, 39 type II and 18 type III), 56 EGJ outflow obstruction, 28 absent peristalsis, 11 distal esophageal spasm (DES), 21 hypercontractile esophagus, and 225 BMF (110 weak peristalsis, 72 frequent failed peristalsis, 11 rapid contractions, and 32 hypertensive peristalsis).
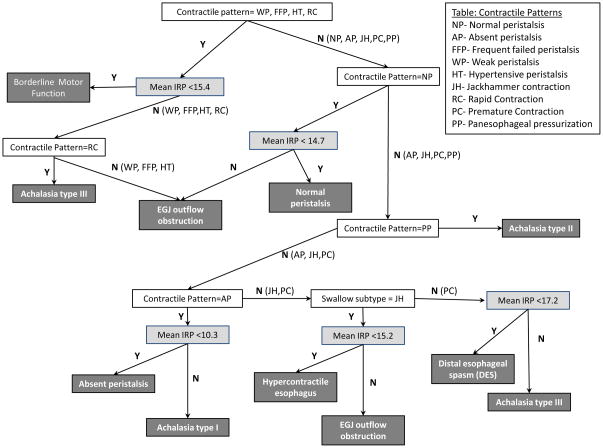
The CART model derived from reconciling the algorithmic approach to classification with that of the expert by tailoring the IRP cutoffs to specific nodal populations in the algorithm is diagrammed in Figure 4. Several interesting observations emerged from the CART model. First and foremost, the IRP was not the variable selected in the first node of the model, in essence confirming the hypothesis that different IRP cutoff values were applicable to subpopulations based on contractile patterns. Secondly, the IRP cutoff values in the model ranged from a low of >10 mmHg to best distinguish type I achalasia from absent peristalsis to >17 mmHg that best distinguished type III achalasia from DES. Finally, the diagnosis of type II achalasia could be established without the IRP entering the algorithm. In this case, the observed contractile pattern in the esophageal body in essence trumped any potential IRP value observed because that contractile pattern (panesophageal pressurization) does not occur without outflow obstruction.
Figure 4.
The hierarchy of the Classification and Regression Tree (CART) model starts from the top with terminal nodes at the bottom. Codes for contractile patterns are listed in the top text box and described in Table 1. The CART model utilized contractile patterns (white boxes) and best-fit IRP cut-off values (light gray boxes) to best distinguish the Chicago Classification diagnoses (dark gray boxes). Note that the achalasia type II pattern did not require a distinct IRP cut-off as the swallow pattern was pathognomonic for this achalasia subtype.
Table 2 compares the sensitivity, specificity, and number of misclassified cases of the CART model and the algorithmic approach compared to the expert diagnosis. The CART model achieved 94% agreement with the expert compared to 92% for the algorithm-based approach. Overall, the sensitivity using the CART model for achalasia was 93 % (66/71) compared to 85% (60/71) using the standard criterion of a single cutoff value of ≥15 mmHg (Table 2). Both schemes were associated with excellent specificity (>99%). As might be expected, the discrepancy in accuracy was greatest in distinguishing diagnoses heavily based upon the IRP cutoff such as distinguishing type I achalasia from absent peristalsis (4 cases) and instances where an IRP was below 15mmHg in patients with panesophageal pressurization and type II achalasia (6 cases).
Table 2.
Comparison of sensitivity, specificity, and number of misclassified cases relative to the expert reading using the CART model or an algorithmic-based approach to diagnosis.
| CART model | Algorithm-based approach | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Misclassified cases N (%) | Sensitivity | Specificity | Misclassified cases N (%) | |
| Achalasia type I | 92.9% | 99.8% | 1 (7.1) | 71.4% | 100.0% | 4 (28.6) |
| Achalasia type II | 94.9% | 100.0% | 2 (5.1) | 84.6% | 100.0% | 6 (15.4) |
| Achalasia type III | 88.9% | 99.8% | 2 (11.1) | 94.4% | 99.8% | 1 (5.6) |
| EGJ outflow obstruction | 96.4% | 99.8% | 2 (3.6) | 98.2% | 98.7% | 1 (1.8) |
| Distal esophageal spasm | 100.0% | 99.8% | 0 (0) | 100% | 99.8% | 0 (0) |
| Absent peristalsis | 96.4% | 100.0% | 1 (3.6) | 96.4% | 99.2% | 1 (3.6) |
| Hypercontractile esophagus | 95.2% | 99.6% | 1 (4.8) | 90.5% | 100% | 2 (9.5) |
| Borderline motor function† | 93.3% | 97.0% | 15 (6.7) | 92.4% | 97.0% | 17 (7.6) |
| Normal | 95.5% | 96.6% | 5 (4.5) | 95.5% | 96.1% | 5 (4.5) |
Encompasses rapid contractions with normal latency, hypertensive peristalsis, weak peristalsis with large or small peristaltic defects, and frequent failed peristalsis
DISCUSSION
The aim of this study was to apply a rigorous CART model to define optimal IRP cutoff values for defining abnormal EGJ relaxation in the context of the Chicago Classification of clinical EPT studies. The major advantage of the CART model is in the use of an adaptable threshold for the optimal IRP cutoff value based on the distal esophageal contractile pattern rather than using a strict 15 mmHg cutoff as is done in the algorithmic analysis method. The diagnostic accuracy of the two interpretive methodologies was tested against the clinical classification established by an expert utilizing all available clinical data. Key distinguishing features of the CART model were: 1) modifying the IRP cutoff value for distinguishing between type I achalasia and absent peristalsis to >10 mmHg, 2) modifying the IRP cutoff value for distinguishinged between type III achalasia and DES to >17 mmHg, and 3) accepting that the contractile pattern of panesophageal pressurization in and of itself was diagnostic of type II achalasia. Analytical results from 522 patients demonstrated that the classification sensitivity for achalasia using the CART model was improved to 93% compared to 85% using the algorithmic approach with both schemes having greater than 99% specificity.
The novelty of this study lies in the application of a CART model to define flexible IRP cutoff values for achalasia subtypes. Previous studies of diagnostic accuracy in achalasia have utilized a single cut-off value derived by determining the upper limit of a normal population, typically the 95th percentile. However, defining abnormality based on an extreme among normal subjects ignores two important facts: 1) patients are more heterogeneous than control populations and 2) the physiology defining the 95th percentile in normal subjects may not adequately characterize the patient population. In the case of achalasia, since there are abnormalities of EGJ relaxation and esophageal contractility, an analysis that can concurrently explore the effect of multiple variables is logical. The CART model used in this study is a nonparametric statistical procedure that identifies mutually exclusive and exhaustive subgroups of a population whose members share common characteristics that influence the dependent variable of interest, in this case the final Chicago Classification diagnosis. Classification and regression tree modeling is considered the best decision tree analytical method because it is designed to sequentially select the independent variables that are most different with respect to the target variable(s) (8).
The results derived from the CART model utilized in this study are consistent with the physiology of the pressure dynamic through the EGJ during swallowing, a delicate interplay of factors either promoting and impeding bolus transit. The IRP was developed to account for this complexity by incorporating both a measure of the completeness of relaxation and the persistence of that relaxation. Depending on the pattern of associated esophageal contractility, intrabolus pressure ins the distal esophageal body can influence the IRP measurement to varying degrees. However, in the absence of any esophageal contractility, the IRP is entirely dependent on EGJ relaxation and it is not surprising that the CART model suggested using a lower threshold for abnormal EGJ relaxation (>10 mmHg) in distinguishing type I achalasia from absent peristalsis. Our previous data suggested that patients with type I achalasia tended to respond less well to pneumatic dilation or Botox injection than to laparoscopic Heller myotomy and it is tempting to speculate that this is because the target EGJ pressure is lower in the absence of any esophageal contractility. Conversely, using the CART model there was no specifiic IRP cutoff for type II achalasia because the panesophageal pressurization pattern was pathognomonic for this achalasia subtype. This is logical in that panesophageal pressurization requires that the intrabolus pressure exceed 30 mmHg which implies EGJ outflow obstruction (9). Yet another interesting feature of the CART model was that the distinction between DES and Type III achalasia was made with the slightly greater IRP threshold of 17 mmHg. This is also likely related to the associated esophageal contractility, in this case premature distal esophageal contractions (distal latency <4.5s). With such reductions in latency, part of the 4 second IRP measurement may incorporate the premature spastic contraction thereby increasing the IRP value.
There are several practical issues to consider when using a CART model. Feature selection is critical in building a classification system. Choosing the optimal input feature variables greatly influences the performance of the CART model. We used the same feature variables in the CART model as were used in the algorithm-based approach that were, in essence, the building blocks of the Chicago Classification (3, 4). However, while the mean IRP value is easily obtained using ManoView™ software, the pattern of associated esophageal contractility must be deduced by the interpreter using a three-step scoring process (4, 10). Hence, the accuracy of the classification is dependent on the skill of the user, which will always be a variable. The eventual solution to this is to automate the measurement process and this is the focus of ongoing studies.
In conclusion, this study demonstrated that the automated analysis of EPT studies was better reconciled with expert analysis using a CART model that varied the IRP threshold value for abnormal EGJ relaxation depending on the pattern of associated esophageal contractility. The optimal IRP cutoff value ranged from a low of >10 mmHg for distinguishing type I achalasia from absent peristalsis to a high of >17 mmHg for distinguishing type III achalasia from DES. Although the study methodology was based on a statistical model rather than clinical outcome data, it does suggest that the IRP cutoff value of 15 mmHg for judging impaired EGJ relaxation should be assessed in the context of the associated esophageal contractility. Future work will need to focus on determining whether these proposed revised thresholds have predictive value in patient outcome before and after intervention.
Acknowledgments
Funding: Supported by R01 DK56033 (PJK) and R01 DK079902 (JEP) from the public Health Service
Abbreviations
- CART
classification and regression tree
- EPT
esophageal pressure topography
- HRM
high-resolution manometry
- EGJ
esophagogastric junction
- IRP
integrated relaxation pressure
- DCI
distal contractile integral
Footnotes
AUTHOR CONTRIBUTIONS
Zhiyue Lin: Analysis and interpretation of data, drafting of the manuscript, approval of the final version
Peter J. Kahrilas: Study concept and design, revising of the manuscript critically, approval of the final version
Sabine Roman: Analysis and interpretation of data, revising the manuscript critically, approval of the final version
Lubomyr Boris and Dustin Carlson: Acquisition and analysis of data
John E. Pandolfino: Study concept and design, analysis and interpretation of data, revising of the manuscript critically, approval of the final version
COMPETING INTERESTS
ZL, PJK, LB and DC have no competing interest; SR and JEP have served as consultant for Given Imaging.
References
- 1.Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying OGJ morphoogy and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1033–40. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Impaired deglutitive OGJ relaxation in clinical esophageal relaxation: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–85. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 3.JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJPM. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(S1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemon SC, Roy J, Clark MA, Friedmann PD, Racowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172–81. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 6.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. 2. Pacific Grove, CA: Wads-wirth; 1984. [Google Scholar]
- 7.Hou Q, Lin Z, Dusing R, McCallum RW. Optimizing the diagnostic power with gastric emptying scintigraphy at multiple time points. BMC Medical Research Methodology. 2011;11:84. doi: 10.1186/1471-2288-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg D, Colla P. CART: Tree-structured non-parametric data analysis. San Diego, CA: Salford Systems; 1995. [Google Scholar]
- 9.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: A heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–25. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson D, Bidari K, Roman S, Kahrilas PJ, Pandolfino JE. Interpreting esophageal pressure topography (EPT) utilizing a scheme based on individual swallow pattern categorization and formula driven dianosis. Gastroenterology. 2011;140(5 Suppl 1):S-300. [Google Scholar]