Abstract
A traumatic spinal injury can destroy cells, irreparably damage axons, and trigger a cascade of biochemical responses that increase the extent of injury. Although damaged central nervous system axons do not regrow well naturally, the distributed nature of the nervous system and its capacity to adapt provide opportunities for recovery of function. It is apparent that activity-dependent plasticity plays a role in this recovery and that the endogenous response to injury heightens the capacity for recovery for at least several weeks post-injury. To restore locomotor function, researchers have investigated the use of treadmill-based training, robots, and electrical stimulation to tap into adaptive activity-dependent processes. The current challenge is to maximize the degree of functional recovery. This manuscript reviews the endogenous neural system response to injury, and reviews data and presents novel analyses of these from a rat model of contusion injury that demonstrates how a targeted intervention can accelerate recovery, presumably by engaging processes that underlie activity-dependent plasticity.
Keywords: spinal cord injury, locomotion, electrical stimulation, plasticity, kinematics, coordination
The critical role of the spinal cord in a broad range of physiological functions is clearly demonstrated by the deficits observed after spinal cord injury (SCI) and by the medical conditions that develop in the acute and chronic phases after injury.1–3 Locomotor deficits have been extensively studied, and numerous techniques designed to promote and accelerate recovery of locomotor function have been developed. 4 This emphasis on locomotor function is based on several considerations: its clinical relevance, its repetitive nature, and the fact that it can be readily observed and measured.
Locomotion is clinically relevant because mobility has a direct effect on quality of life by enabling individuals to participate in a range of activities and has an indirect effect on health by enabling exercise and physical activity. These secondary health benefits can reduce the likelihood and/or severity of conditions such as diabetes, cardiovascular disease, and soft tissue breakdown — all of which are of great concern for individuals with SCI.5 Restoration of locomotor ability can slow or reverse the downward spiral that can occur when injury leads to reduced activity.6
Since locomotion involves repetitive activation of neural circuits, it is well-suited for the study of the processes of activity-dependent plasticity and their potential role in providing functional benefits. In many spinal cord injuries7 (and in many animal models8), a critical subset of the spinal circuitry responsible for generating the oscillatory patterns that drive locomotion may retain substantial capabilities and may be particularly responsive to therapeutic strategies that activate them in a repetitive manner.9
Finally, locomotion can be directly observed, can be rated by widely-used clinical measures,1 and can be quantitatively characterized using a combination of well-established biomechanical measures10, 11 as well as some novel dynamical systems approaches.12 This observability facilitates the use of locomotion as a behavior that is a biomarker of neural function as well as deficit, and of subsequent recovery after injury.
In developing and evaluating therapies for promoting recovery after SCI, it is important to recognize that the degree of success is often highly dependent on the time (post-injury) at which they were administered. Many clinical trials are performed using subjects in the chronic post-injury phase, which provides a stable baseline from which to assess the therapeutic intervention. Furthermore, it facilitates participation in the experimental protocol, since potential subjects would have worked through many of the lifestyle adjustments brought on by the injury. While there is evidence that therapy administered even long after the injury can have positive effects,13 administering therapy early has the potential to accelerate recovery.14 This window of opportunity may primarily be the result of factors involved in the physiological response to trauma, but they may also benefit from an upward spiral effect in which capabilities enable activity, which in turn enhances capabilities.
This review provides a brief overview of spinal cord injury, its pathophysiology, and its endogenous recovery, and then describes current methods of rehabilitation with an emphasis on strategies that leverage the endogenous neural response to repetitive stimuli. We present results from a therapy that uses electrical stimulation in a rat model to accelerate recovery following contusion injury.
Physiological response to spinal cord injury
Although spinal cord injury can result from tumor growth or other disorders, it typically occurs due to mechanical trauma to the spine that breaks vertebrae and/or displaces adjacent vertebra.15 The two primary factors that determine the clinical impact of an injury are the spinal level and the severity. Most injuries are incomplete (iSCI) in that some connections across the injury site remain intact,16 thus sparing some supraspinal influence on function and, perhaps more importantly, providing a substrate for plasticity that may improve communication across the injury level, thereby affording some degree of functional recovery. In instances of complete SCI, plasticity in circuits below the level of injury may enhance function.17–19
Directly following the traumatic injury is a complex cascade of events, which, if unaltered, tends to cause additional damage to the nervous system. Knowing the details of this sequence may help determine appropriate timing for rehabilitation strategies. The following description of the stages of injury summarizes a detailed explanation provided by Bareyre and Schwab 2003.20 Beginning with a mechanical lesion that causes immediate damage to the cord, the acute phase of injury continues until a few days after injury. The mechanical trauma and initial response may result in reduced blood flow, which can cause ischemic necrosis as well as subsequent edema in the spinal cord. These events can produce changes in extracellular ion concentrations, which, over time (days in animal models, weeks in humans), can lead to neural failure and spinal shock. The secondary phase of injury includes the time period from minutes to weeks following injury. It is marked by excessive release of neurotransmitters, inflammatory reactions, and apoptosis. During this period, the size of the astrocytes increase in a process termed reactive gliosis, creating a glilal scar at the site of injury. Neutrophils, macrophages, and T-cells migrate toward the injury site. Although they perform important functions in the body’s response to injury, they can also have detrimental effects on central nervous system tissue. In the chronic phase of injury (days to years) apoptosis can continue and surviving cells can be further affected by impairments in channel and receptor functions. Finally, there can be significant scar formation along with demyelination of some fibers and Wallerian degeneration of axons that no longer have a target due to cell loss that immediately followed the injury or occurred in subsequent phases of the response.
During all phases following SCI, the central nervous system undergoes substantial reorganization. These changes include synaptic plasticity, axonal sprouting and cellular proliferation throughout the central nervous system, not just caudal to the injury.21 Some of the changes may involve the formation of new pathways to bypass the damaged regions, while other changes may involve reorganization in undamaged regions to take on new or modified functions, as occurs in the reorganization of cortical maps.22 Although expression of neurotrophic factors increases in the days following injury, neurotransmitter receptors and transporters and ion channels are downregulated.20 This is likely due to decreased firing of the neurons both from the lack of supraspinal input following the injury as well as the typical immobilization following injury. Investigations of the dendrites caudal to the injury demonstrate a significant loss of dendritic branching23 as well as changes at the synaptic level that result in substantial alterations in electrophysiological properties.24 Of particular clinical importance are changes in electrophysiological properties and connectivity that result in hyperreflexia and autonomic dysreflexia. 25–27
Despite the loss of cells due to the initial trauma and the potentially detrimental effects of the physiological response, there is clinical evidence that at least some degree of functional recovery is possible. Most importantly, there is growing clinical evidence that some interventions can enhance and/or accelerate functional recovery and the scientific community is developing an improved understanding of the mechanisms that mediate functional recovery.
From our perspective, a combination of four critical observations motivate the investigation of interventions delivered at specific times post-injury that use electrical stimulation: (1)the downregulation in the production of proteins that form neurotransmitter receptors, transporters and ion channels may be due to reduced activity levels, which would suggest that the downregulation could be reversed by enhancing neural activity; (2) many of the processes involved in plasticity at the subcellular, cellular and circuit levels are upregulated after injury, thus making the system highly responsive to inputs that could help to direct plasticity towards functional reorganization; (3) the complex interactions amongst the various components of the physiological response to injury are time-dependent and they are likely to affect the efficacy of interventions designed to promote functional recovery; (4) and electrical stimulation provides a means to directly activate specific neurons and/or to indirectly activate diverse sets of neurons in a coordinated manner by producing movements through activation of motoneurons to generate muscle contractions. Cyclic movements, such as locomotion, can elicit repetitive patterns of activation that may maximize the benefits to be derived from activity-dependent plasticity.
By delivering an appropriately timed intervention that utilizes electrical stimulation to produce cyclic movements, it may be possible to maximize the therapeutic benefits by reversing the downregulation of receptors and other proteins, and directing plasticity to produce functional reorganization.
Assessment of locomotor recovery
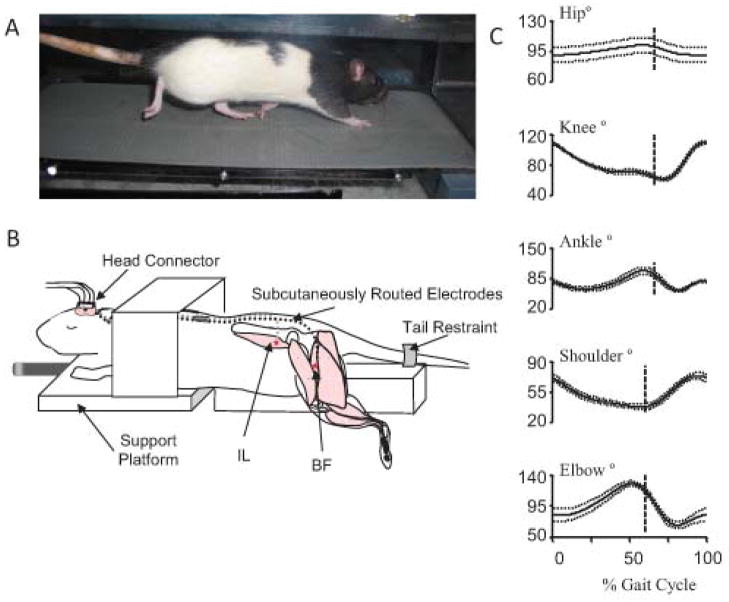
When evaluating new therapies, animal models are often used for SCI research since techniques have been developed to induce injuries with less variability than is observed in human patients. In both animal models and humans, sensitive and reliable assessment tools are needed to determine the effectiveness of SCI therapies on any timescale. Assessments of movement utilize outcome measures that reflect the varying extent to which recovery can occur, from increasing range of motion of a joint to increasing speed. Locomotion is often used, as it is a repetitive, reproducible movement that relies on coordination of multiple neurological and biomechanical levels. Locomotor assessments range from clinical measures,1 to 3D kinematic analysis.10 Locomotion can be tested over ground or on a treadmill (Fig. 1A). While the clinical measures do capture the level of recovery, they are not very sensitive28 and rely on subjective assessment by individual experimenters. However, these types of measures are relatively easy to assess, so theyare included in most experiments. In the rat model, the Basso Beatie and Breshnehan locomotor score29 fulfills a similar role. However, to view smaller changes in recovery without relying on experimenter subjectivity, 3D gait analysis is often used (e.g., Fig. 1C).
Figure 1.
Rat locomotor testing and therapy. (A) Rat walking on a treadmill, reflective markers are placed on bony prominences for 3D visualization to collect kinematic data. (B) Stimulation therapy setup (figure modified from64). Electrodes for neuromuscular stimulation are implanted in the hip flexors (IL-iliacus) and extensors (BFh- biceps remoralis anterior head). Electrode leads are routed to a head connector for connection to a stimulator. The rat is mounted on a platform for stimulation training. (C) Sham injured kinematic data from a rat walking on a treadmill (data from the study described in71). Note the biphasic nature of the knee and ankle trajectories.
Quantitative measures available from 3D gait analysis include phase lag, symmetry, complexity, and range of motion. Left–right symmetry is perhaps the most easily identifiable measurement. Uninjured individuals tend to have gait patterns that exhibit left–right symmetry.10 Following injury, this symmetry can be disrupted, although it is often regained to some extent during recovery. Intralimb joint angle phase relationships help identify coordination within the limb. As the phase values approach 1, the joints are moving in sync with one another, as can be seen following various injuries. Following SCI, range of motion in the ankle increases while it decreases within the knee. Additionally, the ankle trajectory loses a pre-stance extension phase. Complexity measures may characterize the changes in neural control following iSCI.
Although characterizing overground locomotion can be informative, more challenging locomotor activities may provide a more sensitive assessment of locomotor capabilities. One possibility is to use a more complex task that requires a higher level of supraspinal input. The horizontal ladder task assesses more fine motor control than the gross locomotor skill needed for overground walking; fine motor control recovery is much more minimal than gross locomotor recovery following injury.30 Accordingly, fine motor rehabilitative tasks may be more efficacious. For instance, in a horizontal ladder task (for training and assessment), SCI rats showed significantly more improvement when the training was administered immediately (3–4 days) following injury as opposed to three months later.31 The early time period may be very specific.
In animals trained on a ladder starting one week post injury, performance was not improved.32 Determining the correct window of opportunity and measurement technique for therapy may enhance rehabilitation following SCI. Some other specific tasks may also suffer when training is applied early. When rats are trained to swim, early training (three days post injury) is actually worse that delayed training (two weeks post injury) in the swimming assessment and neither time point provides overground locomotor improvement.33 Finally, when using reaching training following cervical injury, early training has a negative effect on untrained tasks (horizontal ladder).34 This may point to overground locomotion (including ladder locomotion) as one of the few tasks that should be trained early. These differential responses may be due to the different tracts involved in these tasks.35 The corticospinal tract is involved with skilled forelimb movements in rats36 while treadmill locomotion can persist even following complete transection in neonatal rats.37 As described in the example later on, even short term therapy applied at the right time point has the potential to significantly accelerate locomotor recovery.
Accelerating locomotor recovery
While the body does undergo some recovery on its own, targeted interventions have the possibility of accelerating or enhancing recovery following iSCI. Many existing therapies utilize the body’s own adaptive processes. The degree of recovery is dependent on a large number of factors including location of injury, severity of injury, and immediate and long-term care.
In addition to the repetitive motion therapies discussed below, a large number of different types of therapy have been attempted for spinal cord injury. These include the application of transplants and neurotrophic factors,38 viral vectors,39 neural bypasses,40 and combinations of treatments.41, 42
Passive exercise
One of the most basic types of rehabilitation following iSCI is passive exercise. Limbs are moved with the help of physical therapists, motorized bikes, or robots. Much of the control of movement is organized around reflex circuits. These circuits coordinate movement around a joint and between joints.43 Thus, when reflexes are disrupted, as seen following iSCI, movement coordination may suffer as well. Passive movement therapy has been shown to normalize some of these improper reflex actions44 and enhance general reflex modulation following SCI.45 For instance, passive cycling exercise has been shown to affect a pathway involved in regulation of protein synthesis, cell growth, and proliferation,46 and upregulate neurotrophic factors while minimizing muscle mass loss following spinal cord injury47. The primary limitation of passive exercise is that it does not directly activate motoneurons, thus possibly limiting (though not eliminating) the activation of processes that would promote activity-dependent plasticity.
Active exercise
Active exercise in which the individual generates volitional contractions to perform specific, repetitive movements may be the most intuitive rehabilitative strategy following iSCI. One of the most common is treadmill-based locomotor retraining therapy in which the individual walks on a treadmill with a harness providing some body weight support and physical therapists helping to move the legs. Newer versions of this therapy include robot-assisted movements.48 The addition of volitional drive to the passive exercise regime may help to activate a network of supraspinal and spinal circuits that may engage activity-dependent processes. If the volitional drive is successful in producing muscle contractions, it may enhance sensory feedback by activating muscle spindles and Golgi tendon organs. Active locomotor training following SCI in rodent models has led to an increase in expression of neurotrophic factors49 mitigating the effects of some negative adaptations. The effect of active exercise on increased expression of neuotrophic factors and synaptic plasticity in the spinal cord is particularly strong.50, 51
Repetitive active training has also been shown to promote cortical remapping52–54 and improve corticospinal drive.55, 56 The primary limitation of active exercise is that it requires some level of function in the individual to work. The results achieved by the individual are often relative to the amount of function they have before starting therapy.
Electrical Stimulation
A number of clinical and basic science studies suggest that therapy is most likely to efficiently promote functional reorganization if it produces rich, appropriately-timed patterns of activity that are associated with functional activities.57, 58 While performing passive movements or active (volitional) activities, the patterns of activity produced in the spinal cord and supraspinal circuits are likely to be deficient relative to the patterns in an uninjured individual. Most notably, paralyzed or paretic muscles may not receive sufficient excitation to generate contractions of sufficient strength. This insufficiency in motor drive would reduce the activity of sensors in the muscle and tendon and would limit or alter the activity in other somatic sensors by impairing the production of a suitable movement pattern. If delivered appropriately, it may be possible to use electrical stimulation of motoneurons to produce muscle contractions in an appropriately-timed sequence to activate sensors in the muscles and to generate functional movements that activate somatic sensors. This rich pattern of activity in sensorimotor circuits may prove to be more effective than passive or volitional exercise in activating activity-dependent processes to provide functional gains.6 Furthermore, it may be possible to initiate an electrical stimulation intervention earlier in the post-injury phase than protocols that depend upon volitional activation. In a number of studies, it has been demonstrated that electrical stimulation can affect the properties of the spinal pattern generator:59 it can increase neurotransmitter expression 60, and it has been shown to partially restore spinal reflex action.61 While the goal of electrical stimulation is generally to elicit appropriate movement, even stimulation of the sensory afferents alone may increase recovery following iSCI.62 Thus, electrical stimulation addresses much of the pathophysiology of SCI as well as the sites of neural plasticity identified during the recovery phase. The properties described above apply to electrical stimulation in general (including open-loop stimulation, as described in our example below), but further gains may be obtained with more advanced controllers. One important challenge is to deliver patterns of electrical stimulation that are appropriately timed to generate the desired movement pattern in a specific individual. Given the complex, time-dependent interactions of the processes of the endogenous response to injury, a second key challenge is to identify the appropriate time-window after injury to deliver electrical stimulation-based therapy.
Adaptive control of electrical stimulation
For any given movement, although there is some consistency in muscle activity patterns across individuals, there is substantial variability due to individual differences in neural and musculoskeletal properties, and such differences are likely to be exacerbated after injury.28,63 Pre-defined, or open-loop, stimulation patterns are therefore limited in their ability to produce desired movement patterns and the limitations are magnified by the high rate of fatigue of electrically-stimulated muscle64–66. Adaptive control strategies have been developed to help account for the variability observed across individuals and for muscle fatigue. 65, 67–70 If successful, this type of control accurately produces the desired motion in a highly repeatable manner66 and may therefore maximize the therapeutic benefits of electrical stimulation.
Early application of neuromuscular electrical stimulation
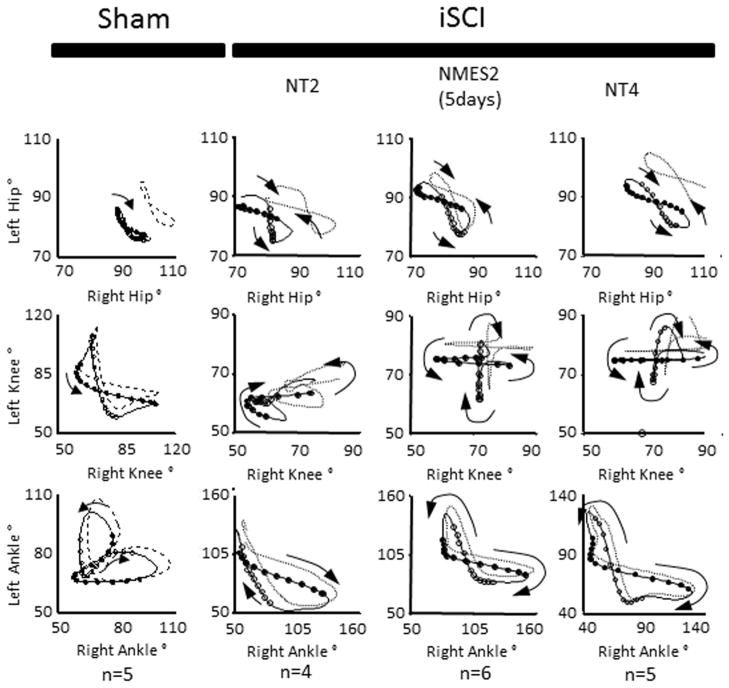
Neuromuscular electrical stimulation (NMES) (open-loop) applied during the second week post-injury may accelerate recovery following iSCI. As previously mentioned, the timing of rehabilitation techniques may contribute to their effectiveness. Rats reach the chronic phase of their recovery approximately 4–6 weeks following iSCI, so immediate intervention is needed to assess effectiveness of therapies within the window of recovery. In Jung et al 2009,64 five days of electrical stimulation to hip flexors and extensors (Fig. 1B) was applied one week following a mild-moderate T9 spinal contusion injury (tested two weeks following the injury). Animals with the same level of injury but without therapy were assessed at two weeks and four weeks71 post injury. Animals were tested using 3D gait analysis while walking on a treadmill.10 This analysis yielded trajectories of individual joint angles of the hip, knee and ankle over multiple cycles of movement. Inter- and intralimb angle–angle trajectories reflect the coordination among the different joints and limbs. Quantitative measures of symmetry between left and right joint angles can be obtained. Similarly, phase delays between particular events during the gait cycles (e.g., touch-down of each hind limb or maximum joint angle during the gait cycle) can be determined. The stimulation training protocol caused the animals to improve on a number of measures and adopt gait characteristics similar to those of animals four weeks post injury. This is evident in the left–right interlimb coordination plots (Fig. 2), particularly at the knee. When the right joint angle trajectory is plotted versus that of the left, symmetry about the diagonal indicates symmetry in locomotion. The pattern is clearly more symmetric in the two-week trained and four-week animals as compared to the two-week untrained animals. It, however, is still very different from the normal (sham) animals. Quantitative symmetry analysis also indicated that the NMES trained animals have more symmetric gait than the untrained animals.64 Both two-week NMES animals and four-week untrained animals showed a cruciform pattern in the interlimb coordination plots. This pattern indicates that one joint moves while the other is held constant. While this pattern is different from normal, it represents a further time point in the natural recovery. Since the pattern for the two-week trained animals is similar to that of the four-week untrained animals, the data suggest that NMES accelerated recovery following injury. This pattern of accelerated recovery is also reflected by the phase delay analysis (Fig. 3). Again, while not the same as the sham animals, the phase delay for the two-week stimulation trained animals is similar to the four-week untrained animals. While the left–right phase in the two-week untrained animals is different from the four-week untrained animals, it is not different from the two-week NMES animals. Both intralimb phase delay measures (hip–knee and hip–ankle) show differences following injury, with a trend toward recovery seen. Particularly, the NMES trained animals have values between the untrained two-week and untrained four-week animals. One method for quantitative analysis of gait parameters is permutation entropy.72 Permutation entropy quantifies the likelihood of a signal to change direction. This new analysis of the data from Jung and colleagues64 uses the code for PE from Olofsen et. al. 200873 for tau = 1. The level of complexity at the hip, as measured by permutation entropy, decreased due to the stimulation (Fig. 4), presumably because the intervention was a simple alternating flexor/extensor pattern of stimulation at the hip. Most notably, the ankle lost significant complexity, mostly due to the loss of the second local maxima (pre-stance extension) in the ankle trajectory. Overall, there was a trend for increasing complexity from untrained two-week to NMES two-week to untrained four-week.
Figure 2.
Sham and incomplete spinal cord injury (iSCI) intralimb coordination patterns. Averaged traces from 4–6 animals, 5–12 step cycles each. Sham: sham injured rats, NT2 and NT4: iSCI rats with no training tested two weeks and four weeks post injury respectively, NMES2: iSCI rats with five days of electrical stimulation therapy tested two weeks post injury. Sham and NT4 data derived from,71 NT2 and NMES2 derived from.64 Left joint angle plotted versus right joint angle. Symmetry in movement is indicated by symmetry about the diagonal. Symmetry is lost in the knee and ankle two weeks following injury. Symmetry is maintained if NMES therapy is added or an additional two weeks have elapsed. Note the simplification of the ankle–ankle pattern. In both the NMES and the NT4 animals, the knee–knee pattern shows a cruciform shape, with one joint moving while the opposite one remains stationary. Though the NMES2 and NT4 animals do not show the same trajectories as the sham animals, both groups were able to walk proficiently.
Figure 3.
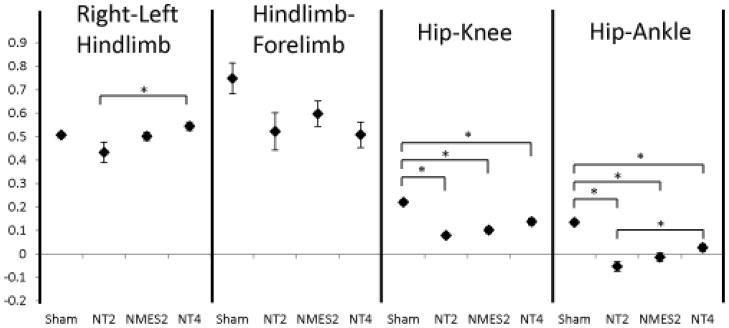
Phase delays for kinematic events. Sham and NT4 data derived from study described in,71 NT2 and NMES2 data derived from.64 Hind limb fore limb and Left–right hind limb phase delay: relative phase of each limb touchdown with respect to another within a gait cycle. Phase is defined at discrete moments within a gait cycle and varies between 0 and 1 (two consecutive touchdown events for each limb). Hip knee and hip–ankle: relative phase calculated from time between maximum joint angles for each joint during a gait cycle, within a limb. Injury causes a change in forelimb-hindlimb coordination, shifting it from approximately 0.75 to approximately 0.5, or completely out of phase. Left–right phase delay is altered following injury, however, NMES training or an additional two-week recovery period returns left right coordination to symmetry. Intralimb phase delays (hip–knee and hip–ankle) are significantly decreased following injury, with a progressive increase from stimulation therapy or additional time for recovery. Sham: sham injured rats, NT2 and NT4: iSCI rats with no training tested two weeks and four weeks post injury respectively, NMES2: iSCI rats with five days of electrical stimulation therapy tested two weeks post injury.
Figure 4.
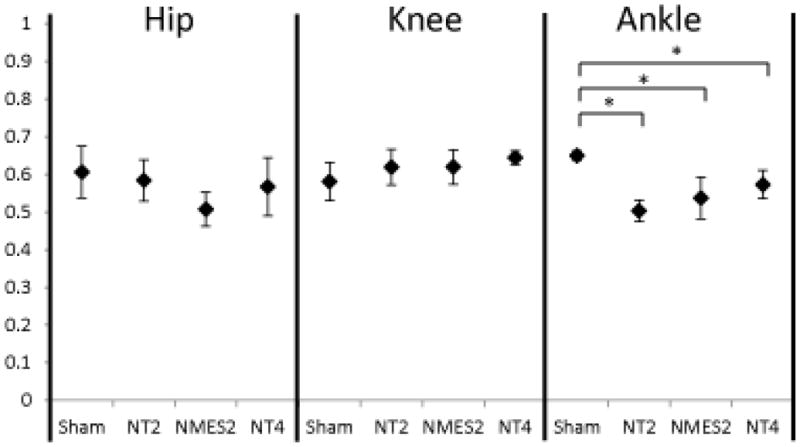
Permutation entropy complexity measures. Sham and NT4 data derived from study described in,71 and new analysis based on data of NT2 and NMES2 data from.64 Permutation entropy quantifies the complexity of a time series signal, indicating how often it changes direction. Higher values indicate higher complexity. Following stimulation therapy, hip complexity decreased due to entrainment to the simple stimulation pattern. Following injury, ankle complexity decreased with a concomitant increase in knee complexity. Sham: sham injured rats, NT2 and NT4: iSCI rats with no training tested two weeks and four weeks post injury respectively, NMES2: iSCI rats with five days of electrical stimulation therapy tested two weeks post injury.
In this example, applying a very small dose of stimulation to a small number of muscles very soon following injury was able to accelerate recovery. Although the intervention had an effect, a more complex stimulation pattern may be able to increase the effect. The course of recovery was not followed beyond this time point, however, so future studies will be needed to determine if accelerated recovery leads to improved outcomes that are persistently improved following therapy. An adaptive stimulation paradigm that can produce a movement pattern similar to that in intact animals66 may be able to provide a greater degree of recovery of the complexity of movement at each joint. It is also important to note that accelerating recovery may have a positive feedback effect on clinical outcomes because faster recovery can lead to higher activity levels, which would in turn speed recovery.
Conclusions
The complex time course of the various components of the endogenous response to injury provides the substrate for rehabilitation interventions. Results from clinical and basic science studies indicate that repetitive movements may promote functional recovery by tapping into the processes that underlie activity-dependent plasticity. The efficacy of any rehabilitation strategy may be determined, or at least strongly influenced, by the extent to which it can produce functional, coordinated patterns of activity across spinal and supraspinal circuits. Electrical stimulation therapy can accelerate recovery of function and can be readily implemented in the clinic. It can be incorporated into movement therapies of varying complexity that meet clinical constraints and are appropriate at the various stages of progression after injury. Current goals are to utilize the technique for producing complex movement patterns and to determine the appropriate windows of opportunity to maximize functional benefits. If successful, electrical stimulation may play a critical role in slowing or reversing the downward spiral that can occur when injury leads to reduction in activity levels.
Acknowledgments
This work was supported by NIH R01-NS054282.
References
- 1.Marino RJ, Graves DE. Metric properties of the ASIA motor score: subscales improve correlation with functional activities. Archives of physical medicine and rehabilitation. 2004;85:1804–1810. doi: 10.1016/j.apmr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 2.McKinley WO, et al. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Archives of physical medicine and rehabilitation. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- 3.Weaver LC, et al. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res. 2006;152:245–263. doi: 10.1016/S0079-6123(05)52016-8. [DOI] [PubMed] [Google Scholar]
- 4.Ramer MS, Harper GP, Bradbury EJ. Progress in spinal cord research - a refined strategy for the International Spinal Research Trust. Spinal Cord. 2000;38:449–472. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- 5.Bajotto G, Shimomura Y. Determinants of disuse-induced skeletal muscle atrophy: exercise and nutrition countermeasures to prevent protein loss. J Nutr Sci Vitaminol (Tokyo) 2006;52:233–247. doi: 10.3177/jnsv.52.233. [DOI] [PubMed] [Google Scholar]
- 6.Baldi JC, et al. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–469. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- 7.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 8.Ichiyama RM, et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minassian K, et al. Neuromodulation of lower limb motor control in restorative neurology. Clinical neurology and neurosurgery. 2012;114:489–497. doi: 10.1016/j.clineuro.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thota AK, et al. Neuromechanical control of locomotion in the rat. J Neurotrauma. 2005;22:442–465. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- 11.Johnson WL, et al. Quantitative metrics of spinal cord injury recovery in the rat using motion capture, electromyography and ground reaction force measurement. J Neurosci Methods. 2012;206:65–72. doi: 10.1016/j.jneumeth.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Spardy LE, et al. A dynamical systems analysis of afferent control in a neuromechanical model of locomotion: I. Rhythm generation. J Neural Eng. 2011;8:065003. doi: 10.1088/1741-2560/8/6/065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harkema SJ, et al. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Archives of physical medicine and rehabilitation. 2012;93:1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Wernig A, et al. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 15.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 16.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson AR, et al. Central nociceptive sensitization vs. spinal cord training: opposing forms of plasticity that dictate function after complete spinal cord injury. Frontiers in physiology. 2012;3:396. doi: 10.3389/fphys.2012.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 19.Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Archives of physical medicine and rehabilitation. 2012;93:1487–1497. doi: 10.1016/j.apmr.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Bareyre FM, Schwab ME. Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 2003;26:555–563. doi: 10.1016/j.tins.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008;45:229–240. doi: 10.1682/JRRD.2007.03.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 23.Bose P, et al. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp Neurol. 2005;191:13–23. doi: 10.1016/j.expneurol.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Thompson FJ, Parmer R, Reier PJ. Alteration in rate modulation of reflexes to lumbar motoneurons after midthoracic spinal cord injury in the rat. I. Contusion injury. J Neurotrauma. 1998;15:495–508. doi: 10.1089/neu.1998.15.495. [DOI] [PubMed] [Google Scholar]
- 25.Weaver LC, et al. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- 26.Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- 27.Wong ST, Atkinson BA, Weaver LC. Confocal microscopic analysis reveals sprouting of primary afferent fibres in rat dorsal horn after spinal cord injury. Neuroscience letters. 2000;296:65–68. doi: 10.1016/s0304-3940(00)01601-3. [DOI] [PubMed] [Google Scholar]
- 28.McKay WB, et al. Neurophysiological characterization of motor recovery in acute spinal cord injury. Spinal Cord. 2011;49:421–429. doi: 10.1038/sc.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 30.McEwen ML, Springer JE. Quantification of locomotor recovery following spinal cord contusion in adult rats. J Neurotrauma. 2006;23:1632–1653. doi: 10.1089/neu.2006.23.1632. [DOI] [PubMed] [Google Scholar]
- 31.Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J Neurophysiol. 2005;94:255–264. doi: 10.1152/jn.00970.2004. [DOI] [PubMed] [Google Scholar]
- 32.Onifer SM, et al. Horizontal ladder task-specific re-training in adult rats with contusive thoracic spinal cord injury. Restorative neurology and neuroscience. 2011;29:275–286. doi: 10.3233/RNN-2011-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RR, et al. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krajacic A, et al. Advantages of delaying the onset of rehabilitative reaching training in rats with incomplete spinal cord injury. Eur J Neurosci. 2009;29:641–651. doi: 10.1111/j.1460-9568.2008.06600.x. [DOI] [PubMed] [Google Scholar]
- 35.Kanagal SG, Muir GD. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp Neurol. 2009;216:193–206. doi: 10.1016/j.expneurol.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Anderson KD, Gunawan A, Steward O. Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp Neurol. 2005;194:161–174. doi: 10.1016/j.expneurol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Giszter SF, et al. How spinalized rats can walk: biomechanics, cortex, and hindlimb muscle scaling--implications for rehabilitation. Annals of the New York Academy of Sciences. 2010;1198:279–293. doi: 10.1111/j.1749-6632.2010.05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bregman BS, et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- 39.Wu HF, et al. The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials. 2013;34:1686–1700. doi: 10.1016/j.biomaterials.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Campos LW, et al. Regenerating motor bridge axons refine connections and synapse on lumbar motoneurons to bypass chronic spinal cord injury. J Comp Neurol. 2008;506:838–850. doi: 10.1002/cne.21579. [DOI] [PubMed] [Google Scholar]
- 41.van den Brand R, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 42.Dose F, Taccola G. Coapplication of noisy patterned electrical stimuli and NMDA plus serotonin facilitates fictive locomotion in the rat spinal cord. J Neurophysiol. 2012;108:2977–2990. doi: 10.1152/jn.00554.2012. [DOI] [PubMed] [Google Scholar]
- 43.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. McGraw-Hill, Health Professions Division; New York: 2000. [Google Scholar]
- 44.Skinner RD, et al. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain research. 1996;729:127–131. [PubMed] [Google Scholar]
- 45.Cote MP, et al. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu G, et al. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupont-Versteegden EE, et al. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- 48.Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85:52–66. [PubMed] [Google Scholar]
- 49.Hutchinson KJ, et al. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 50.Ying Z, et al. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Ying Z, et al. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beekhuizen KS, Field-Fote EC. Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabilitation and neural repair. 2005;19:33–45. doi: 10.1177/1545968305274517. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther. 2007;87:208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- 54.Winchester P, et al. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabilitation and neural repair. 2005;19:313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- 55.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 56.Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–2589. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- 57.Perez MA, Field-Fote EC, Floeter MK. Patterned sensory stimulation induces plasticity in reciprocal ia inhibition in humans. J Neurosci. 2003;23:2014–2018. doi: 10.1523/JNEUROSCI.23-06-02014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin Neurophysiol. 2010;121:1655–1668. doi: 10.1016/j.clinph.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 59.Lavrov I, et al. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- 60.Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cellular and molecular neurobiology. 2004;24:379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knikou M, Conway BA. Effects of electrically induced muscle contraction on flexion reflex in human spinal cord injury. Spinal Cord. 2005;43:640–648. doi: 10.1038/sj.sc.3101772. [DOI] [PubMed] [Google Scholar]
- 62.Dobkin BH. Do electrically stimulated sensory inputs and movements lead to long-term plasticity and rehabilitation gains? Current opinion in neurology. 2003;16:685–691. doi: 10.1097/01.wco.0000102622.38669.ac. [DOI] [PubMed] [Google Scholar]
- 63.Leroux A, Fung J, Barbeau H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp Brain Res. 1999;126:359–368. doi: 10.1007/s002210050743. [DOI] [PubMed] [Google Scholar]
- 64.Jung R, et al. Neuromuscular stimulation therapy after incomplete spinal cord injury promotes recovery of interlimb coordination during locomotion. J Neural Eng. 2009;6:55010. doi: 10.1088/1741-2560/6/5/055010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SJ, et al. Adaptive control of movement for neuromuscular stimulation-assisted therapy in a rodent model. IEEE Trans Biomed Eng. 2009;56:452–461. doi: 10.1109/TBME.2008.2008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fairchild MD, et al. Repetetive hindlimb movement using intermittent adaptive neuromuscular electrical stimulation in an incomplete spinal cord injury rodent model. Exp Neurol. 2010;223:623–633. doi: 10.1016/j.expneurol.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abbas JJ, Chizeck HJ. Neural network control of functional neuromuscular stimulation systems: computer simulation studies. IEEE Trans Biomed Eng. 1995;42:1117–1127. doi: 10.1109/10.469379. [DOI] [PubMed] [Google Scholar]
- 68.Basu I, et al. Adaptive control of deep brain stimulator for essential tremor: entropy-based tremor prediction using surface-EMG. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2011;2011:7711–7714. doi: 10.1109/IEMBS.2011.6091900. [DOI] [PubMed] [Google Scholar]
- 69.Emborg J, et al. Design and test of a novel closed-loop system that exploits the nociceptive withdrawal reflex for swing-phase support of the hemiparetic gait. IEEE Trans Biomed Eng. 2011;58:960–970. doi: 10.1109/TBME.2010.2096507. [DOI] [PubMed] [Google Scholar]
- 70.Ferrante S, et al. Functional electrical stimulation controlled by artificial neural networks: pilot experiments with simple movements are promising for rehabilitation applications. Functional neurology. 2004;19:243–252. [PubMed] [Google Scholar]
- 71.Hillen BH, Jung R. Joint-specific changes in locomotor complexity in the absence of muscle atrophy following incomplete spinal cord injury in the rat. J Neuroeng Rehabil. 2012 doi: 10.1186/1743-0003-10-97. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bandt C, Pompe B. Permutation entropy: a natural complexity measure for time series. Physical review letters. 2002;88:174102. doi: 10.1103/PhysRevLett.88.174102. [DOI] [PubMed] [Google Scholar]
- 73.Olofsen E, Sleigh JW, Dahan A. Permutation entropy of the electroencephalogram: a measure of anaesthetic drug effect. British journal of anaesthesia. 2008;101:810–821. doi: 10.1093/bja/aen290. [DOI] [PubMed] [Google Scholar]