In these 2 population-based studies of congenital cytomegalovirus, all moderate and severe sequelae were apparent in the first year of life, with only mild problems presenting subsequently. Adverse outcomes resulted from both primary and nonprimary maternal infection in pregnancy.
Keywords: cytomegalovirus infection, congenital infection, infections in pregnancy, sensorineural hearing loss, long-term follow-up
Abstract
Background. Congenital cytomegalovirus (CMV) is an important cause of neurological problems, particularly sensorineural hearing loss, but data on long-term sequelae and the impact of nonprimary maternal infection are limited. We report updated findings on childhood outcomes from 2 large prospective studies.
Methods. Pregnant women in Malmö, Sweden, and London, United Kingdom, were included between 1977 and 1986, and newborns were screened for CMV (virus culture of urine or saliva). Cases and matched controls underwent regular, detailed developmental assessments up to at least age 5 years.
Results. One hundred seventy-six congenitally infected infants were identified among >50 000 screened (Malmö: 76 [4.6/1000 births]; London: 100 [3.2/1000 births]); 214 controls were selected. Symptoms were recorded in 11% of CMV-infected neonates (19/176) and were mostly mild; only 1 neonate had neurological symptoms. At follow-up, 7% of infants (11/154) were classified as having mild, 5% (7/154) moderate, and 6% (9/154) severe neurological sequelae. Four of 161 controls (2%) had mild impairment. Among children symptomatic at birth, 42% (8/19) had sequelae, versus 14% (19/135) of the asymptomatic infants (P = .006). All moderate/severe outcomes were identified by age 1; mild sequelae were first identified at age 2–5 years in 6 children, and age 6–7 years in 3. Among the 16 children with moderate/severe outcomes, 2 had mothers with confirmed and 7 with presumed nonprimary infection.
Conclusions. Moderate or severe outcomes were reported in 11% of children with congenital CMV identified through population screening, all by 1 year; all impairment detected after this age was mild. Nonprimary infections contributed substantially to the burden of childhood congenital CMV disease.
Cytomegalovirus (CMV) is a common congenital infection, affecting approximately 0.2%–0.5% of newborns in Europe [1]. Although most infants with congenital CMV are asymptomatic at birth, about 10%–15% present with symptoms [2]; a substantial proportion of these (approximately 40%–60%) develop long-term sequelae, such as sensorineural hearing loss (SNHL), cerebral palsy, mental retardation, and visual impairments [1]. Sequelae are most frequent among infants with neurological signs at birth. CMV-related problems, particularly SNHL, also develop in about 10%–15% of children who are asymptomatic at birth. Cases of late-onset hearing loss have been reported [3–5], but it is unclear to what extent asymptomatic children remain at risk in the long term. Transmission of CMV from mother to fetus can result from primary maternal infection in pregnancy or following reactivation of a previous infection or reinfection with a new strain (nonprimary infection). Evidence from early prospective studies demonstrated that neurological damage could occur following nonprimary infection [6–8], but it remains uncertain whether the risk differs according to type of maternal infection.
A better understanding of the natural history of congenital CMV infection is needed to inform decisions about prevention and treatment, as well as cost-effectiveness analyses. However, long-term follow up is challenging and few studies of congenital CMV have monitored children beyond 2–3 years of age [1]. In a 2007 meta-analysis, only 1 study was identified, from Sweden, which included comprehensive assessment of hearing and developmental outcomes beyond 3 years of age [1, 9]. To address some of these gaps, we report findings from another long-term prospective study of congenital CMV, in the United Kingdom, combined with the previously reported data from Sweden. Both studies, carried out in the 1970s–1980s, relied on population-based screening of newborns, with detailed follow-up of cases and matched controls until at least 5 years of age.
METHODS
Malmö Study
Pregnant women delivering between 1977 and 1985 at the only hospital in Malmö were included [8, 9]; blood samples collected in routine care at the first antenatal visit, 24 and 32 weeks’ gestation, and delivery were retrieved. For multiparous women, preconception sera were frequently available in a serum bank. All babies were tested for congenital CMV by virus culture of urine samples taken within 2 weeks of birth. When congenital CMV was diagnosed, all available maternal sera were tested for CMV immunoglobulin G (IgG) and immunoglobulin M (IgM) [10]. For mothers of children with sequelae, results were confirmed by blinded analyses with sensitive IgM tests in London (P. Griffiths) and Stockholm (M. Forsgren); sera were preabsorbed to avoid false-positive IgM reactions due to rheumatoid factors [9]. From 1978 to 1981, first or 25th consecutive births after index cases were selected as age-matched controls if negative for congenital CMV.
All cases and controls were examined by a pediatrician at birth and on multiple occasions in early life and up to age 10 years; assessments included neurologic, audiologic, and ophthalmologic status and/or development [8–11]. Hearing was assessed using age-appropriate tests, including auropalpebral reflex, observation tests (pure tones, informal sounds, BOEL test), peep-show audiometry, and play audiometry [11]. Neurodevelopmental examination at 2 years of age was based on the Griffiths developmental scale adapted to Swedish standards [8, 10, 12]. At 7 years, gross and fine motor functions were assessed using the Stott test (scores of 0 to −2, normal; −3 to −10, abnormal) and intellectual development was assessed using the Wechsler Intelligence Scale for Children [10].
West London Study
Women were recruited between 1979 and 1986 from antenatal clinics in 3 hospitals serving local populations with a broad mix of sociodemographic characteristics [7, 13]. Blood samples, collected at first antenatal visit and after 27 and 35 weeks’ gestation, were tested for CMV IgG and IgM. Throat swabs were taken from all babies in the first week of life; if CMV was detected by virus culture, congenital infection was confirmed on urine samples collected within 2 weeks of birth. Urine samples were also taken from infants of mothers with confirmed primary infection in pregnancy. At one hospital where women were recruited for the first year only, infant screening continued for another 5 years; all those with congenital infection were included in the study and maternal booking and postnatal bloods were retrieved where possible to ascertain type of maternal infection. Because of the diverse sociodemographic profile of the London population, controls were matched for maternal ethnic origin (white, black, Asian), country of birth, age at booking, parity, marital status, social class, and infant's month of birth and sex [7]. Information was collected on infant condition at birth as part of the routine newborn examination. Cases and controls were assessed for vision, speech, hearing, and general development by the study pediatrician on up to 7 occasions between 6 weeks and 5 years. At 9 months and 3 years full audiological assessment was carried out; at 3 years this included pure-tone audiometry, or, if not possible, a distraction hearing test. Neurodevelopmental examination at 2 years was based on the Griffiths developmental scale [12, 14]. The 5-year assessment included audiological and neurodevelopmental examinations, and for those with normal development or mild sequelae, measurement of intelligence quotient (IQ) using the Wechsler Pre-School Primary Scale of Intelligence [15].
Ethical Considerations
The Malmö study was approved by the Ethics Committee of the Medical Faculty of the University of Lund. The West London Study was approved by the local ethics committees at the 3 hospitals.
Definitions
Type of maternal infection was classified as confirmed or presumed primary or nonprimary (Table 1). In both studies, any symptoms noted at the routine newborn examination were recorded, and in this analysis, infants were classified as symptomatic at birth based on presence of 1 or more of the following: petechiae, tachypnea, hepatomegaly, splenomegaly, thrombocytopenia, seizures, microcephaly, and hypotonia. Growth retardation was not classified as a symptom. Delivery at <37 weeks’ gestation was considered preterm.
Table 1.
Classification of Maternal Cytomegalovirus Infection in Pregnancy
| Classification | Summary |
|---|---|
| Confirmed primary | First blood sample in pregnancy CMV IgG negative and subsequent sample CMV IgG positive (seroconversion confirmed by retesting of samples in pair) (n = 48); or IgG negative on single antenatal blood sample and infant born with confirmed congenital infection (n = 11); |
| Presumed primary | First antenatal blood sample CMV IgM positive (n = 23) |
| Confirmed nonprimary | CMV IgG positive on a blood sample collected before conception (eg, in a previous pregnancy) (n = 21) |
| Presumed nonprimary | CMV IgG positive/IgM negative on initial blood sample taken in the first trimester of pregnancy (n = 23); or a 4-fold or greater rise in CMV IgG titer in paired samples without the appearance of CMV IgM (n = 1) |
| Unclassified | Test results not fulfilling above criteria, or no maternal samples available (n = 49) |
Abbreviations: CMV, cytomegalovirus; IgG, immunoglobulin G; IgM, immunoglobulin M.
Children were classified as having normal development, or mild, moderate, or severe impairment. Development was considered normal following a satisfactory neurodevelopmental examination, including audiology, with no other problems identified. Unilateral SNHL of any severity, mild bilateral SNHL (20–39 dB hearing loss in the better ear), mild motor impairment with minimal implications, or clinically recognized developmental or language delay in the absence of hearing loss or other problems were classified as mild impairment. Moderate impairment included moderate (40–69 dB) or severe (70 dB or more in the better ear) bilateral SNHL without any other identified problem, mild bilateral SNHL and mild cerebral palsy, or moderate learning difficulties. Severe disability or multiple problems, for example, moderate or severe bilateral SNHL in association with other problem(s), moderate or severe cerebral palsy, or severe learning difficulties, were classified as severe impairment.
Statistical Analyses
Categorical variables were compared using χ2 test or Fisher exact test where appropriate. P values for comparison of IQ test scores were obtained with a 2-sample t test for overall IQ and performance IQ, and with the Kruskal-Wallis test for verbal IQ, as variances were significantly different (P = .01). Analyses were carried out in Stata version 11 (StataCorp, College Station, Texas).
RESULTS
Study Populations
Overall, 16 474 of 19 589 live newborns (84%) were tested for CMV in the Malmö study; 76 were positive, an incidence of 4.6 per 1000 births (95% confidence interval [CI], 3.6–5.8), and 62 controls were selected. In the London study, 19 354 of 21 213 live-born infants (91%) were tested, following screening of 21 917 women. Congenital CMV was diagnosed in 61 infants, an incidence of 3.2 per 1000 births (95% CI, 2.4–4.0). Fifty-nine screened women had confirmed primary infection in pregnancy; all delivered live-born infants, 25 with congenital CMV (included in the 61 above). An additional 39 congenitally infected infants were identified through newborn screening of approximately 15 000 infants whose mothers were not enrolled in pregnancy. In total, 152 London controls were selected. Combining the populations, about 50 000 infants were screened and 176 children with congenital CMV identified. A higher proportion of mothers of infants with congenital CMV in London were ≤20 years of age compared with Malmö (P < .001; Table 2). Seventy-one percent of mothers of infected infants in London were white, 22% black, and 7% Asian, whereas all Malmö mothers were white, reflecting the local populations.
Table 2.
Maternal Demographics and Infant Characteristics for Children With Congenital Cytomegalovirus and Controls in Malmö and London
| Malmö |
West London |
|||||||
|---|---|---|---|---|---|---|---|---|
| Children With Congenital CMV |
Controls |
Children With Congenital CMV |
Controls |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Total | 76 | 62 | 100 | 152 | ||||
| Maternal age at booking, y | ||||||||
| 15–19 | 2 | 2.6 | 3 | 4.8 | 22 | 24.2 | 35 | 23.0 |
| 20–24 | 31 | 40.8 | 15 | 24.2 | 23 | 25.3 | 40 | 26.3 |
| 25–29 | 23 | 30.3 | 22 | 35.5 | 24 | 26.4 | 40 | 26.3 |
| 30–34 | 16 | 21.1 | 19 | 30.6 | 14 | 15.4 | 27 | 17.8 |
| 35–39 | 4 | 5.3 | 3 | 4.8 | 8 | 8.8 | 10 | 6.6 |
| Parity | ||||||||
| Primiparous | 48 | 63.2 | 29 | 46.8 | 56 | 56.6 | 93 | 61.2 |
| Multiparous | 28 | 36.8 | 33 | 53.2 | 43 | 43.4 | 59 | 38.8 |
| Infant sexa | ||||||||
| Female | 39 | 51.3 | 29 | 58.0 | 47 | 47.0 | 72 | 47.4 |
| Male | 37 | 48.7 | 21 | 42.0 | 53 | 53.0 | 80 | 52.6 |
| Gestational agea | ||||||||
| Term | 72 | 94.7 | 49 | 98.0 | 85 | 93.4 | 141 | 92.8 |
| Preterm | 4 | 5.3 | 1 | 2.0 | 6 | 6.6 | 11 | 7.2 |
Abbreviation: CMV, cytomegalovirus.
a For control infants in the Malmö study, data were only available for the 50 who were followed up to at least age 4.
Overall, 11% (19/176; 95% CI, 6.6–16.3) of infants were classified as symptomatic at birth, with a higher proportion in Malmö (18%) than in London (5%, P = .004; Table 3). Symptoms included petechiae alone (10, all in Malmö), tachypnea (3, all in London), hepatomegaly and/or splenomegaly (4, 3 also with petechiae), hypotonia (1), and hypertonia and microcephaly (1, severe clinical presentation). In addition, 6 infants (3%) classified as asymptomatic were small for gestational age. None received antiviral treatment. Three Malmö controls were reported to have petechiae.
Table 3.
Outcome According to Presence of Symptoms at Birth in Children With Congenital Cytomegalovirus
| Symptoms at Birth | No. | (%) | No. With Known Outcomesa | Outcome at Last Follow-up |
Any Adverse Outcome, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal |
Mild |
Moderate |
Severe |
|||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||||
| Combined | ||||||||||||
| Asymptomatic | 157 | (89.2) | 135 | 116 | (85.9) | 9 | (6.7) | 6 | (4.4) | 4 | (3.0) | 14.1 |
| Symptomatic | 19 | (10.8) | 19 | 11 | (57.9) | 2 | (10.5) | 1 | (5.3) | 5 | (26.3) | 42.1 |
| Total | 176 | (100.0) | 154 | 127 | (82.5) | 11 | (7.1) | 7 | (4.6) | 9 | (5.8) | 17.5 |
| Malmö | ||||||||||||
| Asymptomatic | 62 | (81.6) | 53 | 44 | (83.0) | 3 | (5.7) | 3 | (5.7) | 3 | (5.7) | 17.0 |
| Symptomatic | 14 | (18.4) | 14 | 10 | (71.4) | 1 | (7.1) | 0 | (0.0) | 3 | (21.4) | 28.6 |
| Total | 76 | (100.0) | 67 | 54 | (80.6) | 4 | (6.0) | 3 | (4.5) | 6 | (9.0) | 19.4 |
| West London | ||||||||||||
| Asymptomatic | 95 | (95.0) | 82 | 72 | (87.8) | 6 | (7.3) | 3 | (3.7) | 1 | (1.2) | 12.2 |
| Symptomatic | 5 | (5.0) | 5 | 1 | (20.0) | 1 | (20.0) | 1 | (20.0) | 2 | (40.0) | 80.0 |
| Total | 100 | (100.0) | 87 | 73 | (83.9) | 7 | (8.1) | 4 | (4.6) | 3 | (3.4) | 16.1 |
a Includes all children with follow-up information reported at ≥5 years of age, as well as 2 children with sequelae who were last seen at 2 and 4 years of age. All those lost to follow-up before 5 years were asymptomatic at birth.
Outcomes in Children With Congenital CMV
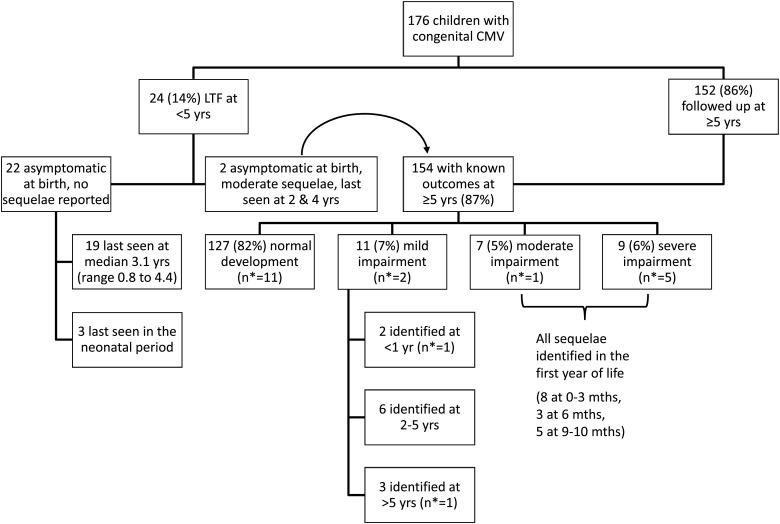
Follow-up information was available at age ≥5 years for 87% of children (Figure 1; Malmö, 88%; London, 87%; P = .82). Most children saw the study pediatrician at last visit, but for 4 children information was obtained from another healthcare professional or the parent. Half (77/152) were last seen at age 5 years, 5% (7/152) at age 6, and 45% (68/152) at 7 or older (all but 3 from Malmö). Two children with impairment were last seen at ages 2 and 4 years but are included in summary data as their problems would not have resolved by 5 years (bilateral SNHL and mental retardation, respectively). The remaining 22 children without follow-up information at 5 years were all asymptomatic at birth, with no health problems reported when last seen (Figure 1).
Figure 1.
Follow-up status, outcome, and timing of identification of sequelae in children with congenital cytomegalovirus. Abbreviations: CMV, cytomegalovirus; LTF, lost to follow-up; mths, months of age; n*, number with symptoms at birth; yr(s), year(s) of age.
Eighty-two percent of children with known outcome at 5 years had no developmental problems reported (Figure 1); 7% were classified as having mild, 5% moderate, and 6% severe impairment, with no difference by study (Table 3, P = .52). There were no deaths. Overall, 14 of 154 children (9%) had SNHL, which was bilateral moderate or severe in 8. One child with moderate sequelae, whose mother's infection was unclassified, was also diagnosed with fetal alcohol syndrome [7].
Forty-two percent of children classified as symptomatic at birth had sequelae, compared with 14% of asymptomatic children (P = .006; Table 3). The one child with neurological signs at birth was severely impaired (spastic quadriplegia, optic atrophy); visual problems were not reported in any other child.
All moderate and severe sequelae were reported in the first year of life (Figure 1). Mild impairment was first reported in 6 children between 2 and 5 years, and one at age 6. Three children were diagnosed with unilateral SNHL after 1 year of age (at 2, 3, and 4 years). Among 68 children with information on outcome at age ≥7 years, 2 with previously normal development had mild impairment reported (1 mild developmental and motor impairment [Stott test −4], the other a borderline abnormal Stott test [−3]) [9]. Forty children in Malmö were also seen at around age 10, with no major illnesses reported [10, 16].
Outcomes in Control Children
In the Malmö study, 50 of 62 controls (81%) were followed up to age 4 and 39 (63%) to age 7 years; at age 7, all were developing normally apart from 1 child with mild motor impairment (Stott test −3).
In the London study, 73% of control children (111/152) were followed up to median age 5.5 years (range, 4.7–7.2 years), and 97% of these (108) were developing normally. One child had unilateral SNHL, and 2 had mild motor impairment. All 41 control children lost to follow-up before age 5 were developing normally when last seen.
Cognitive Development
In the London study, mean development scores at age 2 among children with congenital CMV but no neurological symptoms at that time were similar to controls (101.1 ± 10.4, n = 35, vs 101.2 ± 10.4, n = 73), as reported elsewhere [14]. Among children with normal development or mild impairment at age 5, IQ was measured in 90% of cases (69/77) and 97% of controls (108/111). Overall and performance IQ scores were not significantly different between cases and controls (Table 4); congenitally infected children had a lower median verbal IQ score, but this did not reach statistical significance.
Table 4.
Comparison of Intelligence Quotient Scores at Age 5 Years Between Children With Congenital Cytomegalovirus and Controls in the West London Study
| Children With Congenital CMV | Controls | P Valuea | |
|---|---|---|---|
| IQ Score | (n = 69) | (n = 108) | |
| Overall IQ | |||
| Median | 109.0 | 111.5 | |
| Mean | 111.7 | 113.0 | |
| Variance | 246.6 | 185.0 | .561 |
| Performance IQ | |||
| Median | 118.0 | 118.0 | |
| Mean | 119.3 | 118.1 | |
| Variance | 201.6 | 198.8 | .566 |
| Verbal IQ | |||
| Median | 97.0 | 105.5 | |
| Mean | 102.6 | 106.0 | |
| Variance | 315.6 | 186.2 | .056 |
Abbreviations: CMV, cytomegalovirus; IQ, intelligence quotient.
a Obtained with 2-sample t test for overall IQ and performance IQ, and with Kruskal-Wallis test for verbal IQ, as variances were significantly different (P = .01).
As reported elsewhere, there were no developmental or intellectual differences between cases and controls in Malmö at 21 months or 7 years, respectively [10]. Based on the Griffiths scale, mean scores (6.3 ± 2.3 among 32 cases, 6.1 ± 1.9 among 51 controls) and the proportion classified as abnormal (cases, 19%; controls, 16%) were similar. At age 7, there were no differences in mean scores on the Wechsler Intelligence Scale for Children (cases: 5.8 ± 2; controls: 6.4 ± 1.6), or in the proportion scoring below normal (cases, 3/25, controls, 2/41) [10].
Type of Maternal Infection
Type of infection (confirmed or presumed) was determined for 82% of Malmö and 65% of London women (Table 5). Eighty percent of London women were classified as having primary infection (confirmed or presumed), compared with 48% in Malmö (P < .001). Among the 16 children with moderate/severe outcomes, 2 had mothers with confirmed nonprimary infection, and 7 had mothers with presumed nonprimary infection (Table 5). SNHL was reported in 15% of children following nonprimary maternal infection (6/39, 5 moderate/severe bilateral; 1/17 for confirmed nonprimary infection) and 5% following primary infection (4/73, 2 moderate/severe bilateral; 0/53 for confirmed primary infection) (P = .09). Timing of primary infection (confirmed or presumed) for the 5 women whose infants had moderate/severe outcomes was first trimester (n = 3), first or second (n = 1), and second or third (n = 1).
Table 5.
Prevalence of Symptoms at Birth and Final Outcome (at 5–7 Years of Age) According to Type of Maternal Cytomegalovirus Infection
| Symptomatic at Birth |
Outcomea |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total | Normal/Mild |
Moderate/Severe |
||||||
| Type of Maternal Infection | No. | (%) | No. | (%) | No. | (%) | No. | (%) |
| Combined | ||||||||
| Confirmed primary | 59 | (33.5) | 4 | (6.8) | 52 | (98.1) | 1 | (1.9) |
| Presumed primary | 23 | (13.1) | 4 | (17.4) | 16 | (80.0) | 4 | (20.0) |
| Confirmed nonprimary | 21 | (11.9) | 2 | (9.5) | 15 | (88.2) | 2 | (11.8) |
| Presumed nonprimary | 24 | (13.6) | 4 | (16.7) | 15 | (68.2) | 7 | (31.8) |
| Not known | 49 | (27.8) | 5 | (10.2) | 40 | (95.2) | 2 | (4.8) |
| Total | 176 | (100) | 19 | (10.8) | 138 | (89.6) | 16 | (10.4) |
| Malmö | ||||||||
| Confirmed primary | 25 | (32.9) | 4 | (16.0) | 22 | (95.7) | 1 | (4.3) |
| Presumed primary | 5 | (6.6) | 3 | (60.0) | 4 | (80.0) | 1 | (20.0) |
| Confirmed nonprimary | 18 | (23.7) | 2 | (11.1) | 13 | (86.7) | 2 | (13.3) |
| Presumed nonprimary | 14 | (18.4) | 3 | (21.4) | 8 | (61.5) | 5 | (38.5) |
| Not known | 14 | (18.4) | 2 | (14.3) | 11 | (100) | 0 | (0.0) |
| Total | 76 | (100) | 14 | (18.4) | 58 | (86.6) | 9 | (13.4) |
| West London | ||||||||
| Confirmed primary | 34 | (34.0) | 0 | (0.0) | 30 | (100) | 0 | (0.0) |
| Presumed primary | 18 | (18.0) | 1 | (5.6) | 12 | (80.0) | 3 | (20.0) |
| Confirmed nonprimary | 3 | (3.0) | 0 | (0.0) | 2 | (100) | 0 | (0.0) |
| Presumed nonprimary | 10 | (10.0) | 1 | (10.0) | 7 | (77.8) | 2 | (22.2) |
| Not known | 35 | (35.0) | 3 | (8.6) | 29 | (93.5) | 2 | (6.5) |
| Total | 100 | (100) | 5 | (5.0) | 80 | (92.0) | 7 | (8.0) |
a Restricted to 154 children with outcomes reported.
DISCUSSION
In these 2 large population-based studies with comprehensive follow-up to at least age 5, all moderate and severe sequelae of congenital CMV infection were apparent in the first year of life, and about half resulted from confirmed or probable nonprimary maternal infection. Mild problems, many of minimal consequence, were identified in 7% of children with congenital CMV, and 2% of uninfected controls, and were mostly identified after 1 year of age. At 5–7 years, there was little evidence of cognitive deficit among children with otherwise normal development. Visual impairment was reported in one child and there were no recorded deaths.
Birth prevalence of congenital CMV in Malmö and West London (4.6 and 3.2 per 1000 births, respectively) was similar to rates reported in other population-based studies in resource-rich settings [4, 17–19]. The higher rate in Malmö could relate to higher maternal seroprevalence, 72% [20] compared with 54% in London [13]. In line with other studies [21–23], we found no evidence of an excess of preterm births in congenitally infected infants, and the overall rate of 6% was consistent with population rates in the United Kingdom and Sweden [24, 25].
In these 2 studies, only 1 child had obvious neurological manifestations at birth, and most other neonatal symptoms of congenital CMV were mild. The proportion of newborns with symptoms reported (11%) is consistent with a recent review, in which 12.7% of 810 newborns across 15 studies were symptomatic [1]. However, there was variation in prevalence and type of symptoms reported between the Malmö and London studies, and across those included in the review, highlighting the need for standardized protocols and definitions [2]. Differences could have been due to differential ascertainment of mild symptoms such as petechiae or low-grade hepatosplenomegaly, which might not have been routinely recorded. In the United Kingdom, as in Sweden, children diagnosed with congenital CMV through routine clinical care usually have multiple symptoms and/or neurological signs at birth [26]. In the absence of our studies, other than the child with severe clinical presentation at birth, most children would probably have remained undiagnosed.
Among children developing normally at age 1, there was little evidence of subsequent deterioration. Late-onset hearing loss has been reported in other studies at ages ranging from 8 months to 6 years [4, 5, 27]. In our studies, mild or moderate unilateral hearing loss was reported in 3 children after age 1. No differences in cognitive performance were identified at age 5 between normal/mildly impaired children with congenital CMV and controls in the London study, or at age 7 in the Malmö study [10, 16]. Similarly, in a comparable population-based study of 64 congenitally infected children and matched controls in Canada (birth prevalence: 0.4%), IQ scores were reportedly within the normal range for cases and controls at 3–5 years of age [28].
This analysis highlights the contribution of nonprimary maternal infection to the burden of congenital CMV disease in childhood, even in countries where maternal seroprevalence is relatively low [29]. In our studies, type of maternal infection was unclassified or presumed for a substantial proportion of children, limiting the interpretation of our results. A better understanding of the risk of sequelae according to type of maternal infection is needed, ideally from studies with good ascertainment of seroconversion, reactivation and reinfection, and updated virological tools (eg, IgG avidity testing). However, it is clear that concerted prevention strategies should target both primary and nonprimary infection in pregnancy.
Although these 2 studies were carried out >20 years ago, they remain unique in that both cases and controls were identified through large-scale population-based screening with no external referral. The presence of national health systems in both countries meant that all pregnant women residing within the hospital catchment areas were eligible for inclusion. Follow-up was carried out within the healthcare setting at frequent and regular intervals, and similar standard protocols were used, with detailed examinations including physical, developmental, and cognitive assessments. Identification of symptoms at birth and adverse outcomes in some controls highlights the importance of selecting appropriate comparison groups. Nevertheless, although follow-up was longer than in many other studies, the full spectrum of cognitive difficulties may not be apparent at 5–7 years, and continued formal evaluation at later ages is desirable.
In conclusion, although these studies shed light on the natural history of congenital CMV in untreated populations, a better understanding of the relationship between presentation at birth and subsequent outcome is required to inform consideration of the potential risks and benefits of treatment. Future large-scale studies should ensure that cases and controls are followed up long-term, and that appropriate maternal blood samples are collected to adequately characterize type of maternal infection.
Notes
Acknowledgments. We are grateful to the late Dr Sten Harris for his earlier contributions as an audiologist to the Malmö study, and to Drs Philip Preece and Stuart Logan, the West London study pediatricians. We also thank the midwives who collected the samples and the mothers and children who participated in the 2 studies.
Financial support. This work was supported by the Wellchild Trust through a Research Training Fellowship (to C. L. T.). The Malmö Study was supported by the Swedish Medical Research Council, National Board of Occupational Safety and Health; the Sävstaholm Association, Stockholm; and the Osterlund Foundation, Malmö. The West London Study of Cytomegalovirus in Pregnancy was supported by the UK Medical Research Council; the Wellcome Trust; and Action Research, the National Fund for Research into Crippling Diseases. This work was undertaken at the Centre for Paediatric Epidemiology and Biostatistics, which benefits from funding support from the UK Medical Research Council (MRC) in its capacity as the MRC Centre of Epidemiology for Child Health (grant number G0400546). The UCL Institute of Child Health, University College London, receives a proportion of funding from the Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355–63. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–76. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Fowler KB, McCollister FP, Dahle AJ, et al. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–30. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 4.Numazaki K, Fujikawa T. Chronological changes of incidence and prognosis of children with asymptomatic congenital cytomegalovirus infection in Sapporo, Japan. BMC Infect Dis. 2004;4:22. doi: 10.1186/1471-2334-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153:84–8. doi: 10.1016/j.jpeds.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 6.Ahlfors K, Harris S, Ivarsson S, Svanberg L. Secondary maternal cytomegalovirus infection causing symptomatic congenital infection. N Engl J Med. 1981;305:284. doi: 10.1056/NEJM198107303050514. [DOI] [PubMed] [Google Scholar]
- 7.Peckham CS, Chin KS, Coleman JC, et al. Cytomegalovirus infection in pregnancy: preliminary findings from a prospective study. Lancet. 1983;1:1352–5. doi: 10.1016/s0140-6736(83)92138-4. [DOI] [PubMed] [Google Scholar]
- 8.Ahlfors K, Ivarsson SA, Harris S, et al. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand J Infect Dis. 1984;16:129–37. doi: 10.3109/00365548409087131. [DOI] [PubMed] [Google Scholar]
- 9.Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand J Infect Dis. 1999;31:443–57. doi: 10.1080/00365549950163969. [DOI] [PubMed] [Google Scholar]
- 10.Ivarsson SA, Lernmark B, Svanberg L. Ten-year clinical, developmental, and intellectual follow-up of children with congenital cytomegalovirus infection without neurologic symptoms at one year of age. Pediatrics. 1997;99:800–3. doi: 10.1542/peds.99.6.800. [DOI] [PubMed] [Google Scholar]
- 11.Harris S, Ahlfors K, Ivarsson S, Lernmark B, Svanberg L. Congenital cytomegalovirus infection and sensorineural hearing loss. Ear Hear. 1984;5:352–5. doi: 10.1097/00003446-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths R. The abilities of babies, a study in mental measurement. London, UK: University of London; 1954. [Google Scholar]
- 13.Tookey PA, Ades AE, Peckham CS. Cytomegalovirus prevalence in pregnant women: the influence of parity. Arch Dis Child. 1992;67(7 Spec No):779–83. doi: 10.1136/adc.67.7_spec_no.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearl KN, Preece PM, Ades A, Peckham CS. Neurodevelopmental assessment after congenital cytomegalovirus infection. Arch Dis Child. 1986;61:323–6. doi: 10.1136/adc.61.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1967. [Google Scholar]
- 16.Ivarsson SA, Jonsson K, Jonsson B. Birth characteristics and growth pattern in children with congenital cytomegalovirus infection. J Pediatr Endocrinol Metab. 2003;16:1233–8. doi: 10.1515/jpem.2003.16.9.1233. [DOI] [PubMed] [Google Scholar]
- 17.Larke RP, Wheatley E, Saigal S, Chernesky MA. Congenital cytomegalovirus infection in an urban Canadian community. J Infect Dis. 1980;142:647–53. doi: 10.1093/infdis/142.5.647. [DOI] [PubMed] [Google Scholar]
- 18.Andersen HK, Brostrom K, Hansen KB, et al. A prospective study on the incidence and significance of congenital cytomegalovirus infection. Acta Paediatr Scand. 1979;68:329–36. doi: 10.1111/j.1651-2227.1979.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 19.Barbi M, Binda S, Caroppo S, et al. Multicity Italian study of congenital cytomegalovirus infection. Pediatr Infect Dis J. 2006;25:156–9. doi: 10.1097/01.inf.0000199261.98769.29. [DOI] [PubMed] [Google Scholar]
- 20.Ahlfors K, Ivarsson SA, Johnsson T, Svanberg L. Primary and secondary maternal cytomegalovirus infections and their relation to congenital infection. Analysis of maternal sera. Acta Paediatr Scand. 1982;71:109–13. doi: 10.1111/j.1651-2227.1982.tb09380.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto AY, Mussi-Pinhata MM, Cristina P, et al. Congenital cytomegalovirus infection in preterm and full-term newborn infants from a population with a high seroprevalence rate. Pediatr Infect Dis J. 2001;20:188–92. doi: 10.1097/00006454-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Koyano S, Inoue N, Oka A, et al. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: feasibility and outcomes from a multicentre study. BMJ Open. 2011;1:e000118. doi: 10.1136/bmjopen-2011-000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis. 2009;49:522–8. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser K, Stanfield KM, Leon DA. Birthweight and gestational age by ethnic group, England and Wales 2005: introducing new data on births. Health Stat Q. 2008 (Autumn):22–55. [PubMed] [Google Scholar]
- 25.Morken NH, Kallen K, Hagberg H, Jacobsson B. Preterm birth in Sweden 1973–2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstet Gynecol Scand. 2005;84:558–65. doi: 10.1111/j.0001-6349.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 26.Townsend CL, Peckham CS, Tookey PA. Surveillance of congenital cytomegalovirus in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed. 2011;96:F398–403. doi: 10.1136/adc.2010.199901. [DOI] [PubMed] [Google Scholar]
- 27.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135:60–4. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 28.Saigal S, Lunyk O, Larke RP, Chernesky MA. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. Am J Dis Child. 1982;136:896–901. doi: 10.1001/archpedi.1982.03970460026006. [DOI] [PubMed] [Google Scholar]
- 29.Peckham C, Tookey P, Logan S, Giaquinto C. Screening options for prevention of congenital cytomegalovirus infection. J Med Screen. 2001;8:119–24. doi: 10.1136/jms.8.3.119. [DOI] [PubMed] [Google Scholar]