Abstract
CD (cathepsin D) is a ubiquitous lysosomal hydrolase involved in a variety of pathophysiological functions, including protein turnover, activation of pro-hormones, cell death and embryo development. CD-mediated proteolysis plays a pivotal role in tissue and organ homoeostasis. Altered expression and compartmentalization of CD have been observed in diseased muscle fibres. Whether CD is actively involved in muscle development, homoeostasis and dystrophy remains to be demonstrated. Zebrafish (Danio rerio) is emerging as a valuable ‘in vivo’ vertebrate model for muscular degeneration and congenital myopathies. In this work, we report on the perturbance of the somitic musculature development in zebrafish larvae caused by MPO (morpholino)-mediated silencing of CD in oocytes at the time of fertilization. Restoring CD expression, using an MPO-non-matching mutated mRNA, partially rescued the normal phenotype, confirming the indispensable role of CD in the correct development and integrity of the somitic musculature. This is the first report showing a congenital myopathy caused by CD deficiency in a vertebrate experimental animal model.
Keywords: autophagy, cathepsin D, muscular dystrophy, proteolysis, zebrafish
Abbreviations: CD, cathepsin D; dpf, days post-fertilization; H&E, haematoxylin and eosin; MPO, morpholino; T-MPO, translation MPO
INTRODUCTION
CD (cathepsin D; EC 3.4.23.5) is an aspartic protease found in endosomes and lysosomes of all eukaryotic cells. The pathophysiological role of CD in organ development and homoeostasis can be inferred from the phenotype of knockdown and knockout animal models, as described in mouse [1], fruitfly [2] and zebrafish [3]. One main function of CD is to degrade long-lived proteins transferred into the lysosomes via endocytosis, phagocytosis and autophagy [4,5]. Besides, CD-mediated proteolysis is necessary for the bio-activation of specific substrates, such as hormones [6–8], growth factors [9] and lysosomal cathepsins [10,11]. This function is tissue-specific, and depends on the compartmentalization (endosome, lysosome, cytosol, extracellular space) of CD. In skeletal muscle, CD participates together with other cathepsins in the overall protein turnover. In skeletal muscle fibres from patients with muscular dystrophies, inflammatory myopathies, rhabdomyolysis and neurogenic atrophy, CD was found highly expressed, compared with normal skeletal muscle, and it was dispersed in the cytoplasm rather than concentrated in peri-nuclear vesicles as in normal skeletal fibres [12]. In hindlimb unloading muscle atrophy, apoptotic myofibres were characterized by dystrophin breakdown and cytosolic translocation of CD [13]. It is worth mentioning that dystrophin was shown to be degraded by CD at pH 5.5 [14]. CD immunoreactivity, both intra- and extra-lysosomes, were found particularly increased in small regenerating fibres of Duchenne-type muscular dystrophy [12]. This latter finding was interpreted as associated with an active role of CD in muscle regeneration. However, the direct involvement of CD in muscle development, homoeostasis and dystrophy remains to be demonstrated.
In the last decade, zebrafish (Danio rerio) has become a model of choice for the study of several human muscle disorders [15,16]. In our previous work, we found that zebrafish zygotes lacking CD develop to larvae that present with several phenotypic alterations, including failure of yolk absorption, reduced body length, microphthalmia and defective development of the digestive tract, the swim bladder and the retina-pigmented epithelium [3]. In this work, we focused our attention on the development of the somitic musculature in CD-knockdown zebrafish. We show for the first time that the absence of CD results in congenital myopathy in zebrafish. The present finding adds to the pathophysiological roles of CD, and opens new insights into the pathogenetic mechanisms of muscle degeneration.
MATERIALS AND METHODS
MPOs (morpholinos) and rescue-CD-mutated mRNA
The 25 bp antisense MPO targeting the ATG region [T-MPO (translation MPO)] of zebrafish CD RNA and the in vitro synthesized mutant zebrafish CD mRNA carrying eight mismatches (rescue-CD mRNA) towards the T-MPO have been previously described [3]. T-MPO (now on CD–MPO) was purchased from Gene Tools. The Gene Tools Standard Control 25-mer oligonucleotide MPO (CTRL) was used for sham injections.
Zebrafish husbandry and manipulation
Zebrafish were housed and maintained at the MBC (University of Torino, Italy) as previously described [17], and staged according to the published guidelines [18,19]. Following fertilization, the eggs at the one/two-cell stage were collected and micro-injected, and the embryos were raised at 28°C under standard laboratory conditions. Embryos were grown in the presence of 0.003% 1-phenyl-2-thiourea to prevent formation of melanin pigments. Micro-injection (5 ng/egg of CD–MPO plus or not 200 pg/egg of rescue-CD mRNA) was performed using a Nanoject II injection device (Drummond Scientific). MPO was diluted in Phenol Red micro-injection solution. All manipulations were performed according to the recommendation of the local ethical committee.
Immunoblotting
A pool of larvae was homogenized by ultrasonication in a buffer containing protease inhibitors and denatured in SDS-electrophoresis buffer. Where indicated, the larvae were mechanically de-yolked prior to homogenization. After electrophoresis, proteins were transferred on to a nitrocellulose sheet and CD was revealed with a rabbit polyclonal antibody against rat CD [20] that cross-reacts with zebrafish CD [3]. The filter was stripped and re-probed with a mouse monoclonal antibody against β-actin or tubulin (Sigma–Aldrich) for assessment of protein loading. HRP (horseradish peroxidase)-conjugated goat anti-rabbit or anti-mouse antibodies (Bio-Rad) were used as secondary antibodies as appropriate. Bands were imaged using the VersaDOC Imaging System (Bio-Rad) apparatus equipped with the software Quantity One (Bio-Rad). Protein concentration in homogenate was measured with the Bradford method.
Histochemistry analysis
Transparent embryos were fixed overnight at 4°C in 10% NBF (neutral-buffered formalin; Sigma-Aldrich) and embedded in 1% (w/v) agarose for correct positioning and sectioning (longitudinal sectioning, rostral to caudal). The agarose blocks were dehydrated by ethanol gradient and after xylene diaphanization embedded in paraffin. Sections (4 μm) were cut with a manual microtome (Leica Microsystems), mounted on glass slides (Superfrost ultra plus microscope slides; Thermo Scientific), air-dried overnight at room temperature (25°C) and incubated at 70°C for 30 min before H&E (haematoxylin and eosin) staining. Observations were performed by two independent investigators with a Leica DMI 6000B fluorescence microscope (Leica Microsystems) equipped with the software Leica Application Suite version 1.8.0 (Leica Microsystems) or with a Leica MZ10F Modular stereo microscope interfaced to a CCD (charge-coupled device) camera and Leica Application Suite (version 1.8.0) software (Leica Microsystems). Representative images of at least three independent experiments are shown.
RESULTS
MPO disrupting the translation of CD mRNA affects the normal development of musculature in zebrafish larvae
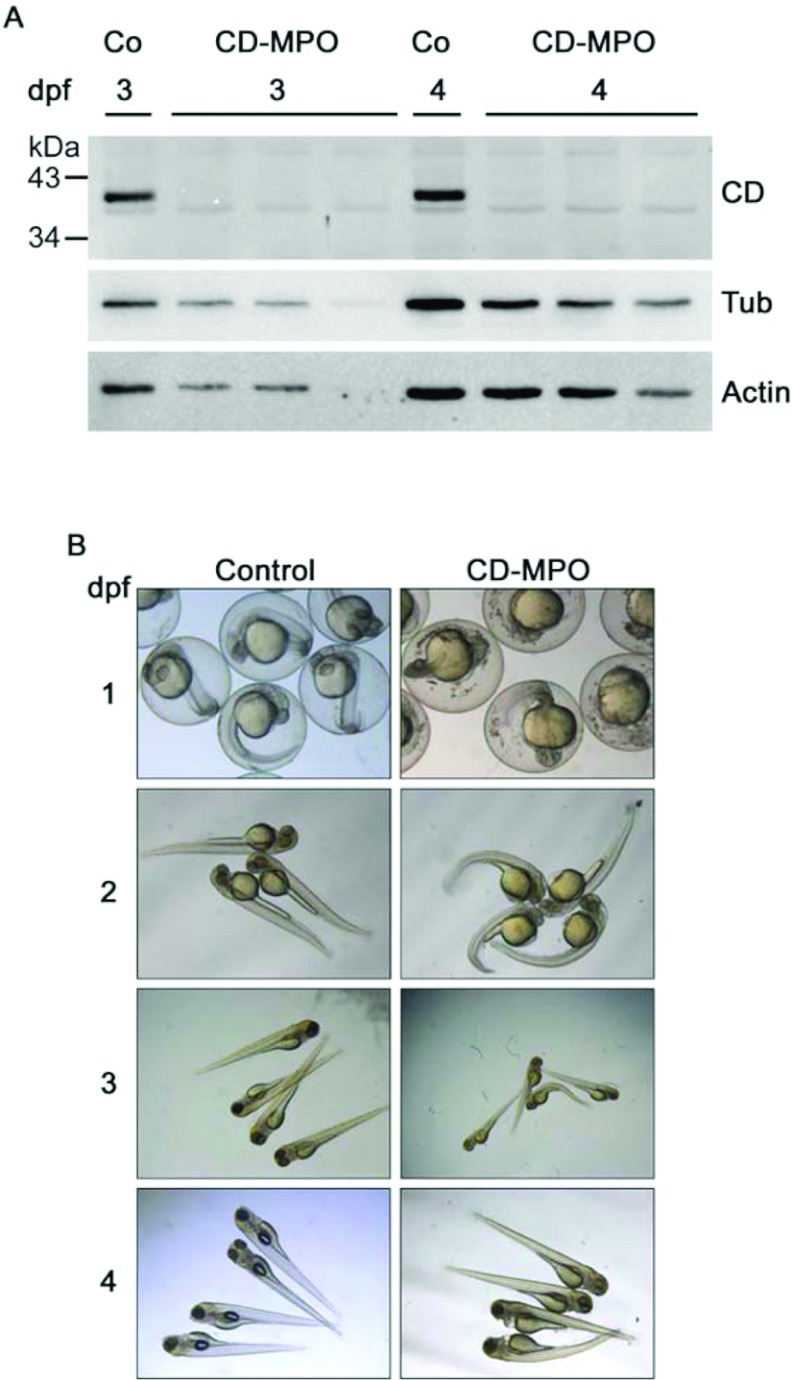
We have previously shown that maternal CD mRNA was present in unfertilized eggs, and that complete down-regulation of CD protein in zygotes could be achieved by disrupting the translation of both the mature maternal and the immature neo-synthesized CD RNAs with an MPO targeting the ATG starting codon [3]. In fact, CD expression was almost completely abolished in CD–MPO-injected embryo/larvae at 3 and 4 dpf (days post-fertilization) (Figure 1A). We focused on the gross alterations produced by CD–MPO. As compared with the paired counterpart injected with control MPO, a portion of zebrafish arising from CD–MPO-injected oocytes were bent, and showed impairment in their movements (Figure 1B). Many of zebrafish showing these alterations died by 4 dpf, and most of the survivors recovered an apparent ‘quasi-normal’ phenotype by 7 dpf (a time at which CD–MPO lost its activity). Typically, the mortality ratio in the CD–MPO-injected population were 13.5% at 1 dpf, 18.8% at 3 dpf and 26.7% at 4 dpf (average of three independent injections with, respectively, initial n=113; 54; 44). Thus, the proportion of bent zebrafish in the CD–MPO-injected population decreased with time from >90% at 2 dpf to 20% at 3 dpf. The bent phenotype recalled that reported in zebrafish models of different human myopathies, including Duchenne muscular dystrophy [21]. This observation prompted us to analyse the musculature in CD-knockdown morphants.
Figure 1. CD-knockdown zebrafish morphants exhibit a bent phenotype.
(A) Fertilized eggs at the stage of one or two cells were injected with CD–MPO or control MPO (Co). A pool of (not de-yolked) larvae at 3 and 4 dpf was collected and analysed by Western blot for CD expression. The Western blot of three different pools of larvae from three different experiments is shown. In control-injected larvae, the 41 kDa mature CD was detected, whereas in CD–MPO-injected larvae CD was absent. Tubulin and actin were used as reference of protein loading in the lanes. (B) Control and CD–MPO morphants at 1–4 dpf were imaged under the stereo microscope. A representative selection from three different experiments is shown. The bent phenotype is clearly evident in CD–MPO morphants.
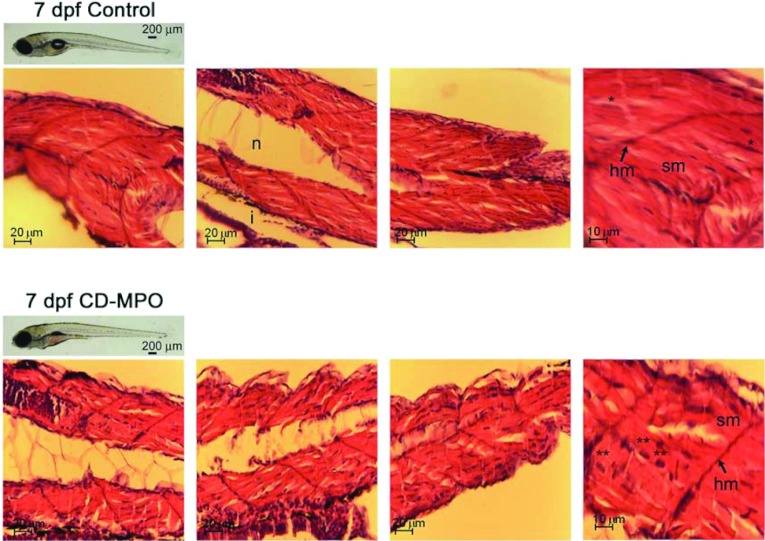
Zebrafish fertilized eggs were micro-injected with standard control MPO oligonucleotide (control injection, CTRL) or CD–MPO, and the musculature organization was analysed by histochemistry in 7 dpf larvae. At this age, the survival of zebrafish larvae was dramatically compromised. The survival rate of CD–MPO larvae at 7 dpf was 55±5% that of control-injected pairs (n=80; P<0.05; Student's t test), and it approximates to zero in the following days. To be noted, lethality in the CD-knockdown population showing an apparent ‘quasi-normal’ phenotype reached the maximum at a time in which CD–MPO lost its effectiveness. Interestingly, CD-knockout mice show an apparent ‘quasi-normal’ phenotype at birth, and then die in an anorexic state after 21 days [1]. These observations indicate that deficiency of CD causes alterations in vital organs that could be compensated for only shortly after birth. At 7 dpf, CD–MPO larvae had no inflated swim bladder and showed a reduced development of the oro-anal tract, compared with the normal counterpart (Figure 2). The motility of CD–MPO morphants was greatly reduced at this age, compared with matching controls. Longitudinal sections were stained with H&E and imaged under the microscope. The somitic musculature of CD–MPO larvae at 7 dpf presented with evident alterations in the organization (Figure 2). In fact, the histological analysis of the musculature in control zebrafish (Figure 2, upper panels) revealed an intense staining with eosin (indicative of the protein content), the presence of syncytial nuclei (indicative of correct myotube formation) and the presence of myosepta (indicative of correct organization of the fibres). By contrast, the somitic musculature of CD–MPO zebrafish (Figure 2, lower panels) showed a less compact architecture, as indicated by the presence of large portions not stained with eosin. This aspect is suggestive of a minor content of myo-fibres in the somitic musculature. In zebrafish, the formation of somitic muscles initiates from the segmented paraxial mesoderm soon after fertilization. By 1 dpf, the somites are separated by horizontal and vertical myosepta, sheets of laminar tendon that along with the notochord serve as attachment sites for somitic muscle fibres. Despite the horizontal myosepta were present, the muscle fibres of the CD-knockdown morphants showed an evident disorganization. In these muscles, in fact, myosepta appeared narrow and thin, as compared with those in muscles of controls. In addition, the number of syncytial nuclei was greatly reduced, and many nuclei showed an eccentric position, indicative of imperfect formation of myotubes. It is probably that these alterations concurred to the bent phenotype and the motility impairment observed in CD–MPO zebrafish. It is to be noted that the above alterations were found in CD–MPO morphants with an apparent ‘quasi-normal’ phenotype.
Figure 2. Histochemistry of the musculature in 7 dpf control and CD–MPO zebrafish.
Control and CD–MPO-injected zebrafish at 7 dpf showing an apparent ‘quasi-normal’ phenotype were subjected to longitudinal sectioning and H&E staining of the musculature. A selection of images from three independent experiments is shown. Symbols: hm, horizontal myoseptum; i, intestine; n, notochord; sm, somitic muscle.*syncytial nucleus; **eccentric nucleus.
Rescue of muscle fibre integrity by injection of mutated zebrafish CD mRNA in CD knockdown fertilized eggs
To confirm that CD was indeed indispensable for the correct development and integrity of the somitic musculature in zebrafish, we performed a rescue experiment by co-injecting the fertilized eggs with CD–MPO and an in vitro synthesized rescue-CD mRNA. The latter carries eight nucleotide mutations in the matching sequence targeted by CD–MPO. In this set of experiments, we looked for earlier effects of the lack of mature CD on muscle fibre organization. The musculature of zebrafish larvae was analysed at 4 dpf, a stage at which CD expression reaches the highest level [3]. We performed three separate experiments and collected altogether 20 larvae at 4 dpf for each type of injection (CTRL or CD–MPO or RESCUE). We first assessed the level of CD expression in the injected specimen. Zebrafish CD mRNA codifies for a 41 kDa monoglycosylated single-chain protein [22].
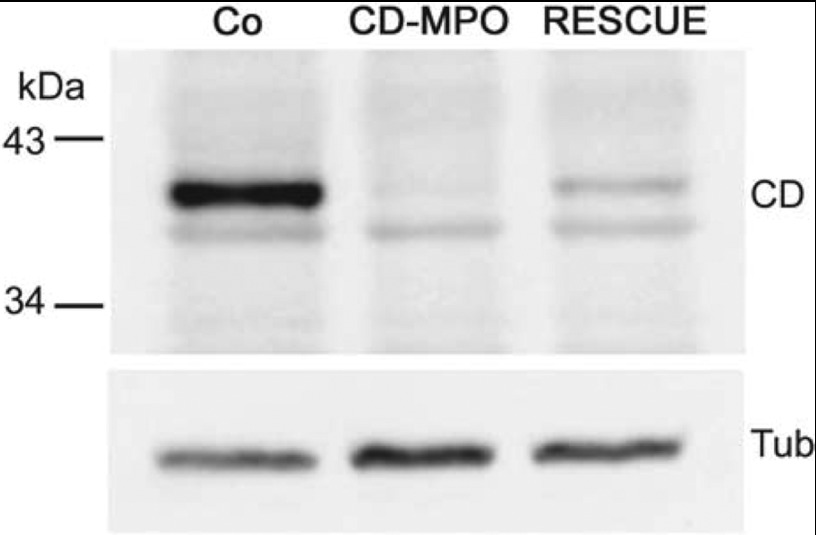
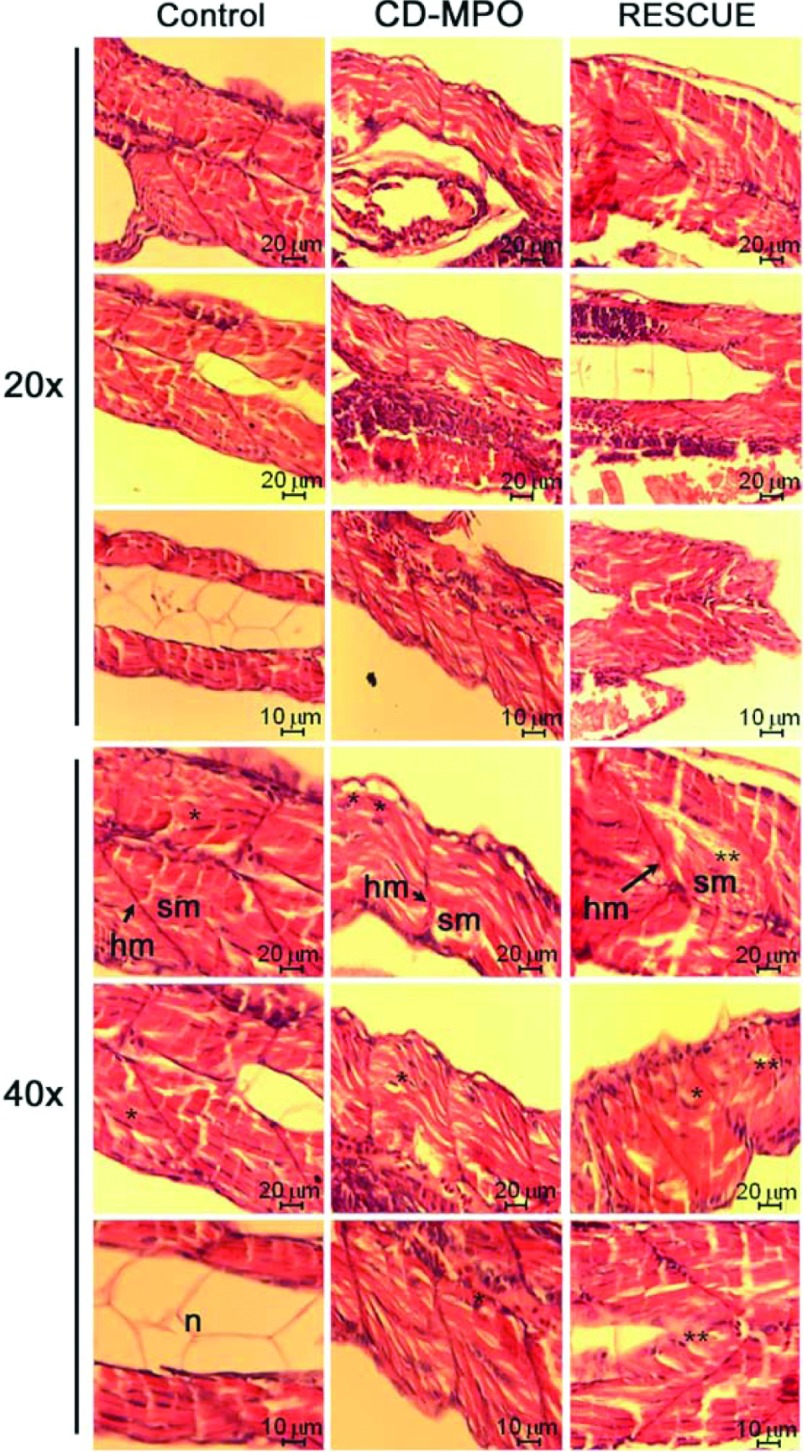
As shown by Western blot analysis (Figure 3), the CD–MPO greatly reduced the accumulation of CD and the injection of rescue-CD mRNA partially restored the level of mature CD in zebrafish larvae (see also [3]). The (most) cranial, trunk and caudal portions of the musculature of control and CD–MPO 4 dpf morphants were analysed by histochemistry (Figures 4 and 5). As above, the CD–MPO zebrafish with a bent phenotype were excluded from the analysis, to avoid biased considerations. In CD–MPO morphants the myofibres did not show a parallel and intact organization as in controls, rather they appeared rippled. Histochemistry revealed an uneven staining with eosin (suggestive of empty spaces between the myofibres), and a paucity of nuclei (indicative of scarce cells), a large number of which were in an eccentric position. Altogether, the syncytial formation of myotubes appeared dramatically compromised in CD-knockdown morphants. These alterations can be better appreciated in the images at high magnification (Figure 5). Strikingly, in CD-rescued morphants, the somitic musculature organization, in terms of myo-fibre horizontal and parallel disposition, cellularity and syncytial myotubes, resembled that of controls.
Figure 3. Immunoblotting of CD.
Fertilized eggs were injected with control (Co) or CD–MPO or both CD–MPO and rescue-CD mRNA. At 4 dpf the corresponding larvae were de-yolked, pooled and homogenized for Western blot analysis of CD expression. At 4 dpf the expression of CD reached its maximum level. CD–MPO abrogated the synthesis of CD. Rescue-CD mRNA allowed the synthesis of a certain amount of CD. The position of molecular mass standards is indicated. The Western blot shown is representative of three independent experiments.
Figure 4. Histochemistry of the musculature in 4 dpf control, CD–MPO and rescue-CD zebrafish.
4 dpf zebrafish larvae arising from zygotes injected with control or CD–MPO or CD–MPO plus rescue-CD mRNA were subjected to longi-tudinal sectioning and H&E staining of the musculature. Only zebrafish with an apparent ‘quasi-normal’ phenotype were analysed. A selection of images from three independent experiments is shown. The longitudinal section of wild-type larvae at the same age (http//zfatlas.psu.edu/) is reported for reference. Symbols: n, notochord; sb, swim bladder.
Figure 5. Particulars of the musculature of 4 dpf control, CD–MPO and rescue-CD zebrafish.
The most cranial, trunk and caudal portions of the skeletal musculature of the zebrafish described in Figure 4 were imaged at a high magnification as indicated. Symbols: hm, horizontal myoseptum; n, notochord; sm, somitic muscle.*syncytial nucleus; **eccentric nucleus.
DISCUSSION
In zebrafish, somitic muscles constitute a large portion of the body and are easily accessible for analysis. In addition, zebrafish begin to move very soon after fertilization, which allows the early detection of muscle dysfunction. These features make the zebrafish an amenable vertebrate model for studies on muscle development and atrophy [15,16], and for the identification of molecular targets to be used for early diagnosis and therapy of myopathies [23,24]. Muscle tissue can be subjected to a variety of inherited, congenital and acquired diseases. Previous studies have linked CD to acquired muscle diseases. Abnormally high levels of expression and cytoplasmic localization of CD were reported in damaged skeletal muscles [25], and in muscle fibres from patients with muscular dystrophies, inflammatory myopathies, rhabdomyolysis and atrophy [12,13]. Cytoplasmic translocation of CD, following a primitive oxidative stress-mediated permeabilization of the lysosomal membrane, can in fact lead to the activation of cytosolic pro-apoptotic factors [26].
The present study provides the first evidence of an active and indispensable role of CD in the correct development and function of the skeletal musculature in zebrafish, demonstrating the involvement of CD in congenital myopathies. This was proved by MPO-mediated knockdown of both maternally supplied and newly synthesized CD mRNAs in fertilized eggs at the stage of one or two cells. A rescue experiment, in which an MPO-non-matching mRNA for zebrafish CD was co-injected with CD–MPO, definitively confirmed the role of CD in the development of the zebrafish musculature. At 2 dpf, a large proportion of CD–MPO morphants exhibited a bent phenotype, a characteristic of mutant zebrafish reproducing human myopathies [21]. Muscle fibres form through a differentiation process characterized by the arrest in cell proliferation and synthesis of myosin (transition from myoblasts to myocytes) followed by syncytial fusion of myocytes that leads to polynucleated myotubes. As compared with control pairs, the musculature of CD–MPO zebrafish appeared to have a less content of myotubes and much thinner myo-septa. The somitic lesions in CD-knockdown morphants could be documented in 4 dpf larvae with an apparent ‘quasi-normal’ phenotype. It is conceivable that more dramatic alterations in the musculature were present in morphants with the bent phenotype. At 7 dpf, a time at which the MPO nearly lost its effectiveness, the CD–MPO morphants still presented with abnormalities in the somitic musculature, indicating that the congenital deficiency of CD permanently compromised the development and integrity of the muscle tissue. Consistent with our findings, it was shown that the early somitic lesions in the zebrafish model of Duchenne muscular dystrophy lasted for long time [21].
CD-knockdown morphants also showed no swim bladder inflation. Noteworthily, both the myopathy and the abnormal development of the swim bladder, are common features to dystrophin-knockdown zebrafish morphants [27]. Interestingly, dystrophin-knockdown, dystroglycan-knockdown and CD-knockdown zebrafish morphants all show a delayed development, besides the somitic lesions ([3,27,28] and the present study). Remarkably, in our study the (small) amount of CD driven by the rescuing mRNA in the initial stages was sufficient to largely prevent the developmental anomalies imparted by CD–MPO, underscoring the importance of CD-mediated proteolysis in tissue development and homoeostasis. It is to be noted, however, that in CD-rescued morphants a portion of nuclei maintained an eccentric position, indicative of an imperfect recovery in the formation of myotubes. This could be related to the small amount of mature CD and/or to the fact that the somitic lesions occurred before a sufficient amount of mature CD could be synthesized.
What could be the link between CD-mediated lysosomal proteolysis and muscle fibre integrity? In other words, why does the lack of lysosomal CD negatively affect muscle tissue development and integrity? At present, we have no definitive answer to these questions. We can offer a hypothesis based on the known role of CD-mediated proteolysis in muscle tissue macromolecular turnover. Protein degradation in skeletal muscle is controlled by the two major proteolytic systems: the ubiquitin–proteasome system and the autophagy–lysosome system. The abnormal hyperactivation of these two systems contributes to muscle loss and weakness. Although excessive autophagy causes muscle wasting and atrophy [29,30], inhibition of autophagy–lysosomal degradation is associated with myopathies such as Danon disease and collagen VI muscular dystrophy [31,32]. Basal autophagy plays a beneficial role in controlling muscle mass. In fact, the ablation of Atg7, an essential autophagy gene, compromises autophagy in muscle, and this results in the accumulation of abnormal mitochondria and disorganization of sarcomeres [33]. In muscle, the persistence of dysfunctional organelle may cause the hyperactivation of catabolic processes that eventually lead to muscle atrophy. Therefore an efficient basal autophagy flux seems fundamental to prevent sarcopenia, as it guarantees the continuous turnover of organelles and membranes. Consistent with a beneficial role of cathepsin-mediated proteolysis in myogenesis, the expression of cathepsin B is increased in myoblasts committed to differentiation [34], and that of CD is increased in small regenerating fibres of Duchenne muscular dystrophy [12]. However, the activity of lysosomal CD decreases during differentiation and maturation of muscles, from mononuclear cells to myotubes [35]. On these grounds, we hypothesize that in the early stage of development lysosomal CD accomplishes an indispensable proteolytic function associated with autophagic clearance of protein aggregates and dysfunctional mitochondria generated during the muscle differentiation.
Besides, CD-mediated proteolysis could play a crucial role in the assembly of myotomes and in their interaction with the extracellular matrix. The myoseptum in the musculature of CD-knockdown morphants appeared much thinner than in the musculature of control zebrafish. The myoseptum plays a critical role in myotome organization and in the transmission of the contractile force. In zebrafish, the myoseptum develops along with the lateral-to-medial migration of slow-twitch fibres, its thickness increases with time (it reaches a 500 nm thickness by 3 dpf) and it becomes infiltrated by fibroblast-like cells at 6 dpf [36]. Therefore extracellular matrix components of myosepta, including collagen, laminin and fibronectin, are initially produced by muscle cells. Fibronectin, which is a ligand for integrin receptor, plays a critical role in somite differentiation. In fact, fibronectin is down-regulated during slow-twitch fibres migration, and is degraded in the proximity of multinucleated fast muscle cells but not in the proximity of mononucleate slow muscle cells [37]. Intriguingly, fibronectin is a substrate of CD [38]. Therefore the primitive absence of CD could compromise the post-translational down-regulation of fibronectin and, consequently, the correct organization of myotomes. Remarkably, the expression of both CD and fibronectin progressively decline during muscle differentiation and maturation.
Congenital alterations in the expression, compartmentalization and functional activity of CD were shown to result in embryo developmental defects and in neurodegenerative diseases [1,3,39–43]. In this work, we provide the first evidence that the congenital absence of CD is detrimental for the functional organization of the somitic musculature in zebrafish, indicating that CD plays a critical and indispensable role in the development and homoeostasis of muscle fibres. Given the high similarity between human and zebrafish muscles [44–46], the present data suggest that defective CD-mediated lysosomal proteolysis may contribute to several myopathies also in humans.
AUTHOR CONTRIBUTION
Carlo Follo, Matteo Ozzano and Claudia Isidoro conceived and designed the experiments. Carlo Follo, Matteo Ozzano and Claudia Montalenti performed the experiments. Carlo Follo, Matteo Ozzano and Claudia Isidoro analysed the data. Massimo Mattia Santoro and Ciro Isidoro contributed reagents/materials/analysis tools. Carlo Follo and Ciro Isidoro wrote the paper.
FUNDING
C.I. is supported by Consorzio InterUniversitario per le Biotecnologie (Trieste), Comoli & Ferrari SpA (Novara) and Associazione per la Ricerca Medica Ippocrate-Rhazi (Novara).
References
- 1.Saftig P., Hetman M., Schmahl W., Weber K., Heine L., Mossmann H., Koster A., Hess B., Evers M., von Figura K., et al. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myllykangas L., Tyynela J., Page-McCaw A., Rubin G. M., Haltia M. J., Feany M. B. Cathepsin D-deficient Drosophila recapitulate the key features of neuronal ceroid lipofuscinoses. Neurobiol. Dis. 2005;19:194–199. doi: 10.1016/j.nbd.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Follo C., Ozzano M., Mugoni V., Castino R., Santoro M., Isidoro C. Knock-down of cathepsin D affects the retinal pigment epithelium, impairs swim-bladder ontogenesis and causes premature death in zebrafish. PLoS ONE. 2011;6:e21908. doi: 10.1371/journal.pone.0021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castino R., Demoz M., Isidoro C. Destination ‘lysosome’: a target organelle for tumour cell killing? J. Mol. Recognit. 2003;16:337–348. doi: 10.1002/jmr.643. [DOI] [PubMed] [Google Scholar]
- 5.Nicotra G., Castino R., Follo C., Peracchio C., Valente G., Isidoro C. The dilemma: does tissue expression of cathepsin D reflect tumor malignancy? The question: does the assay truly mirror cathepsin D mis-function in the tumor? Cancer Biomarkers. 2010;7:47–64. doi: 10.3233/CBM-2010-0143. [DOI] [PubMed] [Google Scholar]
- 6.Diment S., Martin K. J., Stahl P. D. Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J. Biol. Chem. 1989;264:13403–13406. [PubMed] [Google Scholar]
- 7.Erdmann S., Ricken A., Merkwitz C., Struman I., Castino R., Hummitzsch K., Gaunitz F., Isidoro C., Martial J., Spanel-Borowski K. The expression of prolactin and its cathepsin D-mediated cleavage in the bovine corpus luteum vary with the estrous cycle. Am. J. Physiol. Endocrinol. Metab. 2007;293:e1365–e1377. doi: 10.1152/ajpendo.00280.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lkhider M., Castino R., Bouguyon E., Isidoro C., Ollivier-Bousquet M. Cathepsin D released by lactating rat mammary epithelial cells is involved in prolactin cleavage under physiological conditions. J. Cell Sci. 2004;117:5155–5164. doi: 10.1242/jcs.01396. [DOI] [PubMed] [Google Scholar]
- 9.Takei Y., Higashira H., Yamamoto T., Hayashi K. Mitogenic activity toward human breast cancer cell line MCF-7 of two bFGFs purified from sera of breast cancer patients: co-operative role of cathepsin D. Breast Cancer Res. Treat. 1997;43:53–63. doi: 10.1023/a:1005749925296. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura Y., Kawabata T., Kato K. Identification of latent procathepsins B and L in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Arch. Biochem. Biophys. 1988;261:64–71. doi: 10.1016/0003-9861(88)90104-x. [DOI] [PubMed] [Google Scholar]
- 11.van der Stappen J. W., Williams A. C., Maciewicz R. A., Paraskeva C. Activation of cathepsin B, secreted by a colorectal cancer cell line requires low pH and is mediated by cathepsin D. Int. J. Cancer. 1996;67:547–554. doi: 10.1002/(SICI)1097-0215(19960807)67:4<547::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker J. N., Bertorini T. E., Mendell J. R. Immunocytochemical studies of cathepsin D in human skeletal muscle. Ann. Neurol. 1983;13:133–142. doi: 10.1002/ana.410130205. [DOI] [PubMed] [Google Scholar]
- 13.Nagano K., Suzaki E., Nagano Y., Kataoka K., Ozawa K. The activation of apoptosis factor in hind limb unloading-induced muscle atrophy under normal and low-temperature environmental conditions. Acta Histochem. 2008;110:505–518. doi: 10.1016/j.acthis.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Maltin C. A., Jones P., Mantle D. Effect of protease inhibitors and clenbuterol on the in vitro degradation of dystrophin by endogenous proteases in human skeletal muscle. Biosci. Rep. 1993;13:159–167. doi: 10.1007/BF01149960. [DOI] [PubMed] [Google Scholar]
- 15.Bassett D. I., Currie P. D. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum. Mol. Genet. 2003;12:265–270. doi: 10.1093/hmg/ddg279. [DOI] [PubMed] [Google Scholar]
- 16.Guyon J. R., Steffen L. S., Howell M. H., Pusack T. J., Lawrence C., Kunkel L. M. Modeling human muscle disease in zebrafish. Biochim. Biophys. Acta. 2007;1772:205–215. doi: 10.1016/j.bbadis.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Santoro M. M., Samuel T., Mitchell T., Reed J. C., Stainier D. Y. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat. Genet. 2007;39:1397–1402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 19.Parichy D. M., Elizondo M. R., Mills M. G., Gordon T. N., Engeszer R. E. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev. Dyn. 2009;238:2975–3015. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Démoz M., Castino R., Dragonetti A., Raiteri E., Baccino F. M., Isidoro C. Transformation by oncogenic ras-p21 alters the processing and subcellular localization of the lysosomal protease cathepsin D. J. Cell. Biochem. 1999;73:370–378. [PubMed] [Google Scholar]
- 21.Guyon J. R., Mosley A. N., Jun S. J., Montanaro F., Steffen L. S., Zhou Y., Nigro V., Zon L. I., Kunkel L. M. Delta-sarcoglycan is required for early zebrafish muscle organization. Exp. Cell Res. 2005;304:105–115. doi: 10.1016/j.yexcr.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Follo C., Ozzano M., Montalenti C., Ekkapongpisit M., Isidoro C. Similarities and differences in the biogenesis, processing and lysosomal targeting between zebrafish and human pro-cathepsin D: functional implications. Int. J. Biochem. Cell Biol. 2012;45:273–282. doi: 10.1016/j.biocel.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara G., Karpf J. A., Myers J. A., Alexander M. S., Guyon J. R., Kunkel L. M. Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5331–5336. doi: 10.1073/pnas.1102116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telfer W. R., Nelson D. D., Waugh T., Brooks S. V., Dowling J. J. Neb: a zebrafish model of nemaline myopathy due to nebulin mutation. Dis. Models Mech. 2012;5:389–396. doi: 10.1242/dmm.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decker R. S., Crie J. S., Poole A. R., Dingle J. T., Wildenthal K. Resistance to ischemic damage in hearts of starved rabbits: correlation with lysosomal alterations and delayed release of cathepsin D. Lab. Invest. 1980;43:197–207. [PubMed] [Google Scholar]
- 26.Castino R., Bellio N., Nicotra G., Follo C., Trincheri N. F., Isidoro C. Cathepsin D-Bax death pathway in oxidative stressed neuroblastoma cells. Free Radical Biol. Med. 2007;42:1305–1316. doi: 10.1016/j.freeradbiomed.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Bassett D. I., Bryson-Richardson R. J., Daggett D. F., Gautier P., Keenan D. G., Currie P. D. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development. 2003;130:5851–5860. doi: 10.1242/dev.00799. [DOI] [PubMed] [Google Scholar]
- 28.Parsons M. J., Campos I., Hirst E. M., Stemple D. L. Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development. 2002;129:3505–3512. doi: 10.1242/dev.129.14.3505. [DOI] [PubMed] [Google Scholar]
- 29.Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., Burden S. J., Di Lisi R., Sandri C., Zhao J., et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Carmignac V., Svensson M., Körner Z., Elowsson L., Matsumura C., Gawlik K. I., Allamand V., Durbeej M. Autophagy is increased in laminin α2 chain-deficient muscle and its inhibition improves muscle morphology in a mouse model of MDC1A. Hum. Mol. Genet. 2011;20:4891–4902. doi: 10.1093/hmg/ddr427. [DOI] [PubMed] [Google Scholar]
- 31.Nishino I., Fu J., Tanji K., Yamada T., Shimojo S., Koori T., Mora M., Riggs J. E., Oh S. J., Koga Y., et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 32.Grumati P., Coletto L., Sabatelli P., Cescon M., Angelin A., Bertaggia E., Blaauw B., Urciuolo A., Tiepolo T., Merlini L., et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010;16:1313–1320. doi: 10.1038/nm.2247. [DOI] [PubMed] [Google Scholar]
- 33.Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Béchet D. M., Ferrara M. J., Mordier S. B., Roux M. P., Deval C. D., Obled A. Expression of lysosomal cathepsin B during calf myoblast-myotube differentiation. Characterization of a cDNA encoding bovine cathepsin B. J. Biol. Chem. 1991;266:14104–14112. [PubMed] [Google Scholar]
- 35.Oron U. Proteolytic enzyme activity in rat hind limb muscles in fetus and during post-natal development. Int. J. Dev. Biol. 1990;34:457–460. [PubMed] [Google Scholar]
- 36.Charvet B., Malbouyres M., Pagnon-Minot A., Ruggiero F., Le Guellec D. Development of the zebrafish myoseptum with emphasis on the myotendinous junction. Cell Tissue Res. 2011;346:439–449. doi: 10.1007/s00441-011-1266-7. [DOI] [PubMed] [Google Scholar]
- 37.Snow C. J., Henry C. A. Dynamic formation of microenvironments at the myotendinous junction correlates with muscle fiber morphogenesis in zebrafish. Gene Exp. Patterns. 2009;9:37–42. doi: 10.1016/j.gep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ugarova T. P, Ljubimov A. V, Deng L., Plow E. F. Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin. Biochemistry. 1996;35:10913–10921. doi: 10.1021/bi960717s. [DOI] [PubMed] [Google Scholar]
- 39.Yoshizaki N., Soga M., Ito Y., Mao K. M., Sultana F., Yonezawa S. Two-step consumption of yolk granules during the development of quail embryos. Dev. Growth Differ. 2004;46:229–238. doi: 10.1111/j.1440-169X.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 40.Zuzarte-Luis V., Montero J. A., Torre-Perez N., Garcia-Porrero J. A., Hurle J. M. Cathepsin D gene expression outlines the areas of physiological cell death during embryonic development. Dev. Dyn. 2007;236:880–885. doi: 10.1002/dvdy.21076. [DOI] [PubMed] [Google Scholar]
- 41.Castino R., Thepparit C., Bellio N., Murphy D., Isidoro C. Akt induces apoptosis in neuroblastoma cells expressing a C98X vasopressin mutant following autophagy suppression. J. Neuroendocrinol. 2008;20:1165–1175. doi: 10.1111/j.1365-2826.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- 42.Tyynela J., Sohar I., Sleat D. E., Gin R. M., Donnelly R. J., Baumann M., Haltia M., Lobel P. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 2000;19:2786–2792. doi: 10.1093/emboj/19.12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koike M., Shibata M., Ohsawa Y., Nakanishi H., Koga T., Kametaka S., Waguri S., Momoi T., Kominami E., Peters C., et al. Involvement of two different cell death pathways in retinal atrophy of cathepsin D-deficient mice. Mol. Cell. Neurosci. 2003;22:146–161. doi: 10.1016/s1044-7431(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 44.Chambers S. P., Dodd A., Overall R., Sirey T., Lam L. T., Morris G. E., Love D. R. Dystrophin in adult zebrafish muscle. Biochem. Biophys. Res. Commun. 2001;286:478–483. doi: 10.1006/bbrc.2001.5424. [DOI] [PubMed] [Google Scholar]
- 45.Chambers S. P., Anderson L. V., Maguire G. M., Dodd A., Love D. R. Sarcoglycans of the zebrafish: orthology and localization to the sarcolemma and myosepta of muscle. Biochem. Biophys. Res. Commun. 2003;303:488–495. doi: 10.1016/s0006-291x(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 46.Guyon J. R., Mosley A. N., Zhou Y., O’Brien K. F., Sheng X., Chiang K., Davidson A. J., Volinski J. M., Zon L. I., Kunkel L. M. The dystrophin associated protein complex in zebrafish. Hum. Mol. Genet. 2003;12:601–615. [PubMed] [Google Scholar]