Abstract
Transplantation of bone marrow-derived mesenchymal stromal cells (MSCs) is an emerging treatment for heart failure based on their secretion-mediated “paracrine effects”. Feasibility of the scaffoldless cell sheet technique to enhance the outcome of cell transplantation has been reported using other cell types, though the mechanism underpinning the enhancement remains uncertain. We here investigated the role of this innovative technique to amplify the effects of MSC transplantation with a focus on the underlying factors. After coronary artery ligation in rats, syngeneic MSCs were grafted by either epicardial placement of MSC sheets generated using temperature-responsive dishes or intramyocardial (IM) injection. Markedly increased initial retention boosted the presence of donor MSCs persistently after MSC sheet placement although the donor survival was not improved. Most of the MSCs grafted by the cell sheet technique remained resided on the epicardial surface, but the epicardium quickly regressed and new vessels sprouted into the sheets, assuring the permeation of paracrine mediators from MSCs into the host myocardium. In fact, there was augmented upregulation of various paracrine effect-related genes and signaling pathways in the early phase after MSC sheet therapy. Correspondingly, more extensive paracrine effects and resultant cardiac function recovery were achieved by MSC sheet therapy. Further development of this approach towards clinical application is encouraged.
Introduction
Recent research has shown that transplantation of bone marrow-derived mesenchymal stromal cells (MSCs) is a promising new approach for the treatment of heart failure.1,2,3,4 Although differentiation of MSCs to cardiomyocytes does not occur to a significant extent in vivo,3,4 the ability of MSCs to secrete beneficial growth factors, cytokines, and chemokines is thought to be substantial enough to achieve therapeutic effects by restoring damaged cardiac tissues (known as the paracrine effect).1,2,5 There is also evidence that MSCs stimulate endogenous progenitor/stem cells towards myocardial regeneration in a paracrine manner.6,7 Based on these findings, many clinical trials of MSC transplantation are ongoing.8
Intramyocardial (IM), intracoronary, or intravenous injection of MSC suspensions is currently used for MSC delivery in the trials, however, these may be suboptimal in achieving the maximum effect from MSC-based therapy. Most importantly, these methods result in poor retention and impeded survival of donor cells, leading to poor donor cell engraftment and consequently to limited therapeutic effects.2,9,10,11 Usually, enzymatic digestion (i.e., trypsinization) is used for harvesting MSCs from culture dishes, but this may cause damage to the cells.12,13 In addition, IM injection causes mechanical damage and inflammation, which will also hinder donor cell survival.9,10,11 On the other hand, intracoronary injection of MSCs, having a relatively large cell size, has a risk of coronary embolism,14 particularly when injected into diseased and narrowed coronary arteries.
Okano and his colleagues have recently developed a novel bioengineering technology to generate scaffold-free “cell sheets ” using unique culture dishes, the surface of which is coated with a temperature-responsive polymer (poly-N-isopropylacrylamide).12,13 At 37 °C the dish surface is hydrophobic, and cells can adhere and grow. However, when the temperature is dropped to 25 °C, the polymer becomes hydrophilic and swollen, causing the cells to detach from the dish as a free cell sheet. In contrast to trypsinization, cell surface proteins, cell–cell junctions, and the underpinning extracellular matrix are well preserved in this method.12,13 As all polymer remains on the culture dishes throughout the process for cell sheet generation, the produced cell sheets are free of artificial scaffolds.
It has been reported that epicardial placement of cell sheets formed using various cell types improved cardiac function of the damaged heart.13,15,16,17,18 However, even though bone marrow-derived MSCs are one of the most promising donors for cell therapy, the efficiency of cell sheets formed of this cell type has not been reported. Although adipose tissue-derived MSCs have been previously examined,15,17 the fundamental biological features of these cell types, such as differentiation, viability, proliferation, and stress response, are considerably different.19 Furthermore, whereas the augmented therapeutic effects by the cell sheet technique have been suggested when compared with other cell delivery methods,16,20 the machinery underlying such superiority of this approach remains ill-identified. There were preliminary data showing that the cell sheet technique achieved greater donor cell presence,20 but these were not convincing enough to conclude whether this is because of increased initial retention, survival, or both of donor cells. In addition, regarding the paracrine effect, there is a concern that permeation of secreted factors from epicardially localized MSCs into the host myocardium might be blocked by the existing epicardium. Thus, we investigated the precise role of the cell sheet technique to augment the effects of MSC transplantation with particular focus on donor cell dynamics and paracrine effects.
Results
Augmented improvement of postinfarction cardiac function by MSC sheet therapy
At day 28 post-treatment in a rat coronary artery ligation model, echocardiography showed that left ventricular ejection fraction post-myocardial infarction (MI) was significantly improved in the IM injection of MSC suspensions (IM group) compared with the Control (sham-treatment) group, and more importantly this improvement was further enhanced by the epicardial placement of an MSC sheet (Sheet group) (Table 1). In addition, post-treatment left ventricular end-systolic dimensions in the Sheet group were smaller than those in the Control and IM groups. Consistently, catheterization showed improved cardiac function (increased maximum and minimum dP/dt, and contractility index) in the Sheet group compared with the other groups (Table 1). These results confirmed that the use of the cell sheet technique improved the therapeutic effects of transplantation of bone marrow-derived MSCs for the treatment of MI in comparison with IM injection.
Table 1. Cardiac function at day 28 after treatment.
Improved initial retention, but not survival, of donor cells by MSC sheet therapy
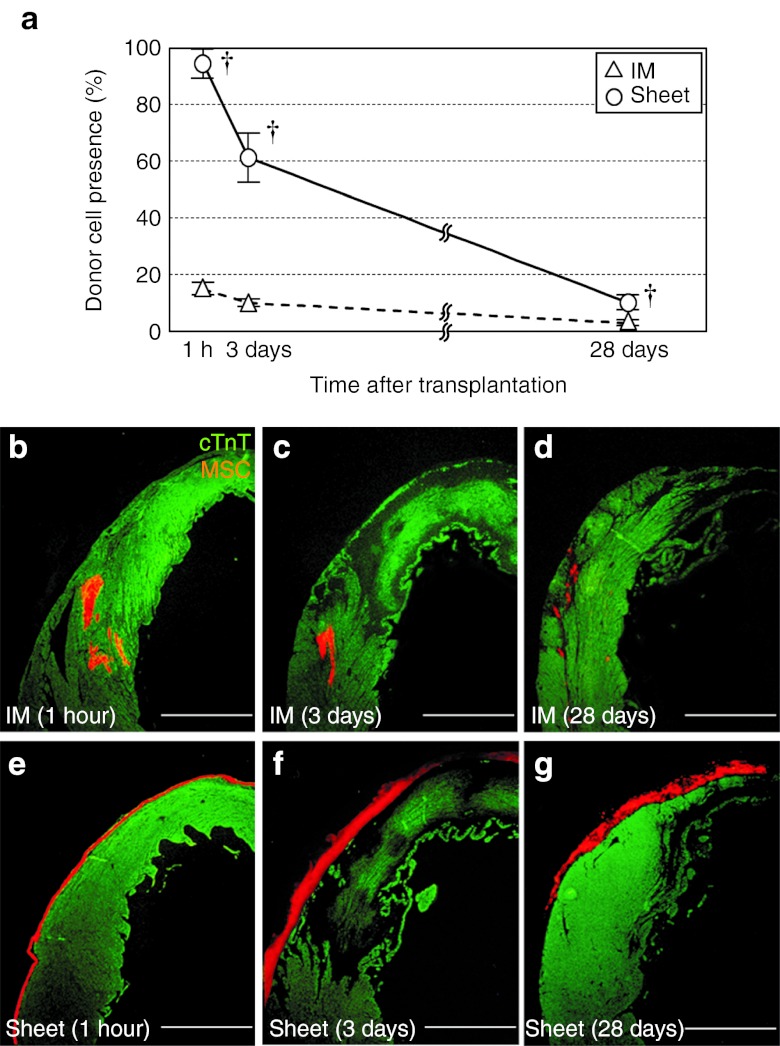
To elucidate the donor cell dynamics that should be related to the enhanced therapeutic outcome by the cell sheet technique, we carried out serial, quantitative assessments of the donor cell presence. Of note, the Sheet group showed 6.4-fold increased initial retention of donor MSCs at 1 hour of transplantation (Figure 1a). This led to the greater donor cell presence in the Sheet group both persistently (at least until day 28).
Figure 1.
Improved donor cell retention and distribution by MSC sheet therapy. (a) Quantitative assessments by using the detection of the male-specific sry gene in the female heart showed that the initial retention (at 1 hour) and successive presence of donor cells (% of the total donor cell number grafted) were improved in the Sheet group compared with the IM group. †P < 0.05 versus the IM group, mean ± SEM for n = 4–6 in each point. Histological evaluation showed that donor cell distribution was distinct between groups; (b–d) intramyocardial cell cluster formation in the IM group (for 1 hour, days 3 and 28, respectively) versus (e–g) retention on the epicardial surface in the Sheet group (for 1 hour, days 3 and 28, respectively). Orange signal for MSCs (DiI); green for cTnT. Bar = 1 mm. cTnT, cardiac troponin-T; IM, intramyocardial injection; MSC, mesenchymal stromal cell.
Histological observations uncovered distinct donor cell distributions between the groups. In the IM group, donor cells formed localized clusters within the myocardium at 1 hour and 3 days (Figure 1b,c), which persisted up to day 28 with a reduced size (Figure 1d). In contrast, most of the donor cells remained on the epicardial surface in the Sheet group throughout the time studied (Figure 1e–g). It was noted that the thickness of the MSC sheets was enlarged between 1 hour and 3 days after placement (Figure 1e,f). However, this did not necessarily indicate that the donor cell presence was increased during this period, as donor MSCs in the sheets were more compacted and dense at 1 hour (Supplementary Figure S1a–c). It was speculated that compacted cells during the cell sheet generation had relaxed and become looser after epicardial placement, making the sheets to appear thicker at day 3, even though the number of donor MSCs presence was actually decreased (Figure 1a). Picrosirius red and hematoxylin staining showed that there was accumulation of extracellular collagen within the sheets at day 3, with an increasing tendency by day 28 (Supplementary Figure S1d,e). However, there was no sign of constrictive heart failure (left ventricular end-diastolic pressure was actually reduced in the Sheet group, compared with the Control group; Table 1).
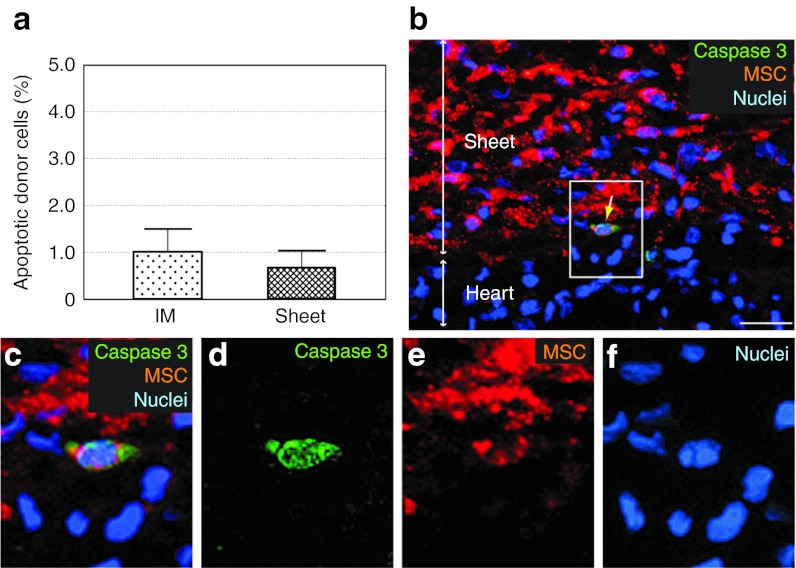
Unexpectedly, the survival rates of donor cells calculated from the serial changes in donor cell presence (Figure 1a) were not improved in the Sheet group (61.4/94.8 = 0.65 (Sheet) versus 10.1/14.9 = 0.68 (IM) between 1 hour and 3 days, P = 0.81; 10.2/61.4 = 0.17 (Sheet) versus 2.9/10.1 = 0.29 (IM) between 3 and 28 days; P = 0.18). Consistent with this, the frequency of donor cell apoptosis was similar between the groups at day 3 (Figure 2).
Figure 2.
Donor cell apoptosis after MSC sheet therapy. (a) Immunolabeling for cleaved caspase-3 detected a similar frequency of apoptotic donor cells in both treatment groups at day 3. (b) A representative image of apoptotic donor cells (yellow arrow) after MSC sheet therapy is shown. Enlarged images of white-gated area in b are presented in c–f with each marker separate and merged. Green signal for cleaved caspase-3; orange for MSCs (DiI); blue for nuclei (DAPI). Bar = 30 µm in b. DAPI, 4′,6-diamidino-2-phenylindole; IM, intramyocardial injection; MSC, mesenchymal stromal cell.
Differentiation of donor cells after MSC sheet therapy
Immunohistostaining for cardiac troponin-T (cTnT) after transplantation of DiI-labeled MSCs did not detect clear evidence of cardiomyogenic differentiation of MSCs; there were no cells positive for both cTnT and DiI in the IM or Sheet group. Isolectin B4 staining detected a number of neovascular formations within the MSC sheets (Figure 3a–d). Most of these consisted of host-derived (DiI−) endothelial cells, while the remaining contained donor-derived (DiI+) cells, suggesting endothelial differentiation (or fusion) of donor MSCs, consistent to the previous findings.21 However, in the host myocardium, there were no donor (DiI+) MSC-containing vessels found.
Figure 3.
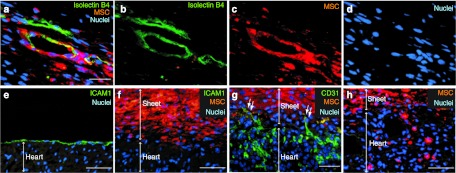
Donor cell behaviors and changes in the epicardium after MSC sheet therapy. (a–d) Isolectin B4 staining demonstrated that the vasculature in MSC sheets contained both host-derived (DiI−) and donor MSC-derived (DiI+) endothelial cells at day 28 after transplantation of DiI+ sheets. (e) ICAM1 staining identified the epicardium in the Control group, (f) though this was absent by day 3 after MSC sheet therapy. (g) CD31 staining detected host-derived (Dil−) endothelium sprouting into the MSC sheets at day 3. (h) The majority of donor MSCs retained on the surface of the heart (as in f), but a small number of MSCs had migrated into the host myocardium at day 3. Green signal for isolectin B4 in a,b, ICAM-1 in e,f or CD31 in g; orange for MSCs (DiI); blue for nuclei (DAPI). Bar = 30 µm in a–d, 50 µm in e–h. DAPI, 4′,6-diamidino-2-phenylindole; ICAM, intercellular adhesion molecule; MSC, mesenchymal stromal cell.
Differentiation of donor MSCs to mesenchymal lineages, which was confirmed in our MSCs in vitro (Supplementary Figure S2), could be a concern if it occurs in the heart. However, our histological study showed that donor MSCs did not differentiate to adipocytes or osteocytes in the heart in vivo (Supplementary Figure S3), assuring the safety of MSC sheet therapy.
Findings to support the permeation of paracrine factors from MSC sheets into the heart
Given that the majority of donor cells were retained at the epicardial surface after MSC sheet therapy (Figure 1e–g), one concern might be that the presence of the epicardium may inhibit the permeation of paracrine mediators from MSC sheets to the host myocardial tissues. Our study here provided several findings to ease it. First, after MSC sheet placement, the epicardium promptly regressed and disappeared. ICAM1 staining demonstrated the presence of the epicardial layer in the Control group (Figure 3e), while these ICAM1+ epicardial cells were absent at both day 3 (Figure 3f) and day 28 (Supplementary Figure S4a) in the Sheet group. Second, isolectin B4 staining showed that many vasculatures formed within the MSC sheets were composed of host-derived (DiI−) cells (Figure 3a–d), indicating migration of vascular cells from the host myocardium into the sheets. Third, CD31 staining detected host-derived (DiI−) endothelial cells migrating into the MSC sheets by day 3, forming sprouting vessels from the host myocardium into the MSC sheet (Figure 3g). Fourth, there were donor MSCs, though relatively few in number, occasionally migrating into the host myocardium (Figure 3h and Supplementary Figure S4b–e). These findings collectively indicate that the epicardium disappeared shortly after MSC sheet placement, enabling early exchange of cells between the myocardium and MSC sheets, which could contribute to establishing vascular networks to feed the cell sheets. Such early establishment of communication between MSC sheets and myocardium assures permeation of molecules secreted from donor MSCs into the myocardium to facilitate the paracrine effect.
Improved paracrine effects: recovery of post-MI failing cardiac tissues by MSC sheet therapy
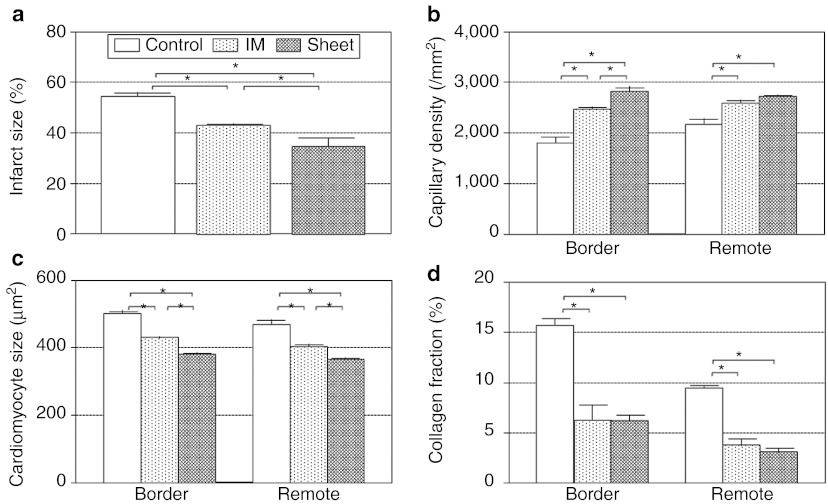
We then looked at histological evidence for the paracrine effects. At day 28, our picrosirius red staining showed that the infarct size in the Sheet group was the smallest among the three groups at day 28 (Figure 4a and Supplementary Figure S5a–c). This was associated with increase in the capillary density and reduction of the cardiomyocyte hypertrophy in both border and remote areas in the Sheet group compared with other groups (Figure 4b,c and Supplementary Figure S5d–i). Extracellular collagen deposition in the Control group was markedly and widely attenuated in the Sheet and IM groups, with a slight tendency of further attenuation in the Sheet group (Figure 4d and Supplementary Figure S5j–l). These data suggest that both cell delivery methods achieved a similar pattern of paracrine effects to recover the failing myocardium post-MI, but the degree of the effects was more substantial after MSC sheet therapy compared with IM injection.
Figure 4.
Histological recovery of post-MI failing cardiac tissues by MSC sheet therapy. (a) Histological studies detected reduced infarct size (Supplementary Figure S5a–c), (b) increased capillary density (Supplementary Figure S5d–f), (c) reduced cardiomyocyte hypertrophy (Supplementary Figure S5g–i), and (d) attenuated collagen deposition (Supplementary Figure S5j–l) at day 28 in the Sheet group compared with the Control group (and to the IM group in most points).*P < 0.05, mean ± SEM for n = 4–5 in each point. IM, intramyocardial injection; MI, myocardial infarction; MSC, mesenchymal stromal cell.
Improved paracrine effects: increased endogenous regeneration activity by MSC sheet therapy
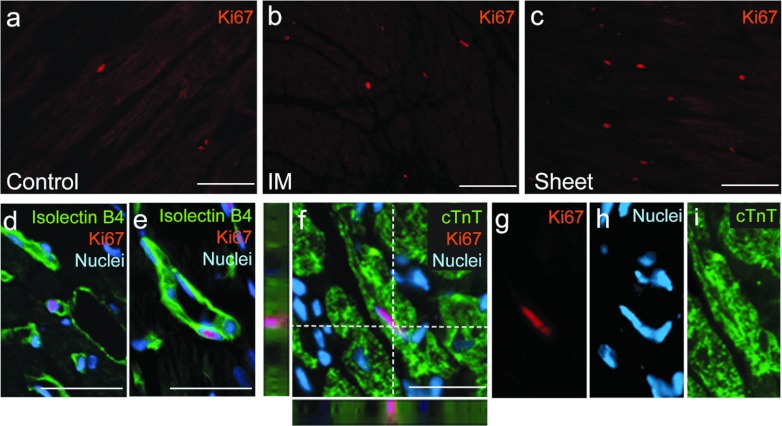
Another target of the MSC-mediated paracrine effects is an activation of endogenous progenitor cells to regenerate cardiomyocytes and vasculature,6,7 but this ability remains controversial.22,23 Our in vivo studies added new information. The number of Ki67+ proliferating cells markedly increased in both border and remote areas in the Sheet group at day 28, compared with the Control and IM groups (Table 2, Figure 5a–c, Supplementary Figure S6a–d). Ki67+ cells were negative for CD45. In addition, the number of Sca-1+/DiI− cells, which are reported to be cardiac progenitor cells,24 as well as the number of Ki67+/Sca-1+/DiI− cells, were increased in the Sheet group.
Table 2. Endogenous regeneration by MSC sheet therapy.
Figure 5.
Improved endogenous regenerative activity by MSC sheet therapy. The number of Ki67+ cells was increased in the Sheet group compared with the IM and Control groups. Representative images of Ki67+ cells in the border areas of the (a) Control, (b) IM, and (c) Sheet groups at day 28 are shown (Table 2). Ki67+/isolectin B4+ cells were found in (d) capillaries as well as in (e) larger vessels after MSC sheet therapy. There were an increased number of Ki67+/cTnT+ proliferating cardiomyocytes in the Sheet group. (f–i) A representative image of Ki67+/cTnT+ cells after MSC sheet therapy is presented for each marker and merged. Green signal for isolectin B4 in d,e, cTnT in f,i; orange for Ki67; blue for nuclei (DAPI). Bar =100 µm in a–c and 30 µm in d–f. cTnT, cardiac troponin-T; DAPI, 4′,6-diamidino-2-phenylindole; IM, intramyocardial injection; MSC, mesenchymal stromal cell.
It was found that most of Ki67+ proliferating cells were also positive for isolectin B4 and/or CD34 (Table 2, Figure 5d,e, Supplementary Figure S6e–h), suggesting an acceleration of endogenous vascular regeneration. Ki67+/isolectin B4+ cells were found in capillaries (Figure 5d) as well as in larger vessels (Figure 5e). This enhanced vasculogenesis might contribute to the enhanced capillary density observed after MSC sheet therapy (Figure 4b). In addition, there were a small, but significantly increased, number of Ki67+/cTnT+ cells in the Sheet group, representing proliferating cardiomyocytes, compared with the Control group (Table 2 and Figure 5f–i). These cells were not donor (DiI+)-derived and thus could be stemmed from differentiation of endogenous progenitor cells or from re-entry of host cardiomyocytes to the cell cycle. However, the number of these Ki67+/cTnT+ cells was not large enough to expect that they had contributed significantly to the improvement of cardiac function after MSC sheet therapy.
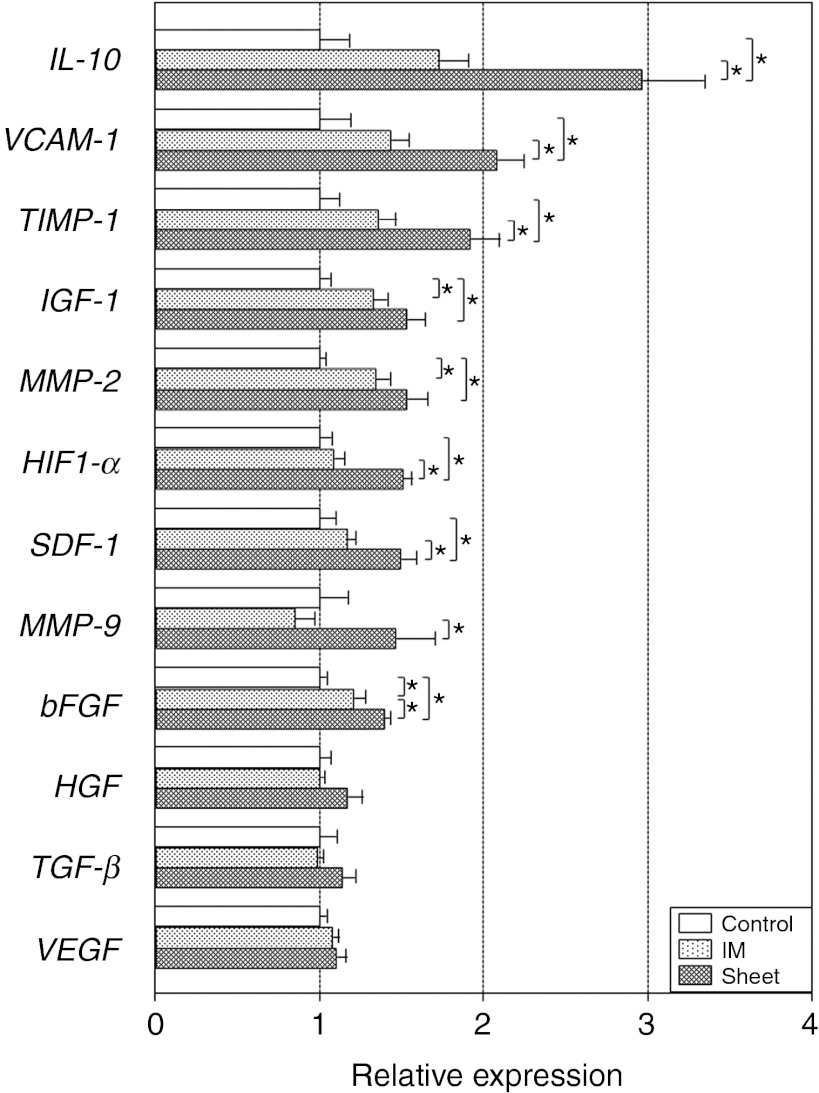
Amplified upregulation of paracrine mediators and signaling pathways by MSC sheet therapy
To gain a further insight into the augmented paracrine effects by MSC sheet therapy, we performed quantitative reverse transcription-PCR screening for genes presumed to be relevant to the MSC-mediated paracrine effects.2,3,21,24,25,26,27,28,29 As a result, we observed that myocardial expression of IL-10, VCAM-1, TIMP-1, IGF-1, MMP-2, HIF1-α, SDF-1, MMP-9, and bFGF was upregulated in the Sheet group at day 3 compared with the Control group (Figure 6). These data corresponded well with the above-discussed histological changes in neovascular formation, fibrosis, and endogenous myocardial regeneration (Figures 4 and 5). Of note, upregulation of these genes was also observed in the IM group, but to a lesser extent compared with the Sheet group. Major signaling pathways relevant to the observed histological changes22,23 have also shown the same trend; JNK and p38 MAPK were both activated at day 3 in the Sheet and IM groups, with more substantial activation in the Sheet group, as compared with the Control group (Supplementary Figure S7). Of note, this amplified upregulation of paracrine-related genes and signaling pathways after MSC sheet therapy matched with both the above-mentioned increase in donor cell presence and augmented paracrine benefits. Upregulation of most of these genes was, however, reduced to the baseline (values in the Control group) by day 28 after MSC sheet therapy (Supplementary Figure S8), corresponding with the largely reduced donor cell presence by this time (Figure 1a).
Figure 6.
Amplified upregulation of paracrine effect-relevant genes by MSC sheet therapy. Quantitative RT-PCR screening detected upregulation of a group of genes likely to be relevant to the MSC-derived paracrine effects in the Sheet group, and in the IM group to a lesser extent, at day 3. All expression levels were normalized to that in the Control group, which was assigned a value of 1.0. *P < 0.05, mean ± SEM for n = 5–6 in each group. IM, intramyocardial injection; MSC, mesenchymal stromal cell; RT-PCR, reverse transcription-PCR.
Discussion
This translational study has, for the first time, validated that the epicardial placement of bone marrow-derived MSC-sheets is feasible, safe, and more effective in treating heart failure, compared with IM MSC injection. As a primary factor responsible for the superiority of the cell sheet technique, we identified the improved initial retention of donor MSCs. Soon after epicardial placement, MSC sheets were found to firmly adhere to the heart probably due to the preserved extracellular matrix underlying the cell sheets, achieving 6.4-fold higher initial retention of MSCs, compared with IM injection of MSC suspensions. Even though the successive donor cell survival rate was not increased, this improved initial retention was substantial enough to achieve the significant increase of donor cell presence for at least 28 days. Importantly, this effect was robust enough to amplify the paracrine effects to recover the post-MI failing myocardium and to augment functional recovery of the hearts. Observed paracrine effects included the increase in neovascular formation, decrease in fibrosis, attenuation of cardiomyocyte hypertrophy, and improvement of endogenous myocardial regeneration. Underpinning these changes, there was amplified myocardial upregulation of a group of relevant molecules and related signaling pathways after MSC sheet therapy compared with IM injection.
In addition, we observed that the epicardium disappeared shortly after MSC sheet placement with establishment of early communication between the sheets and host myocardium, suggesting that the pre-existing epicardium did not hinder the permeation of molecules secreted from donor MSCs into the myocardium to facilitate the paracrine effect. As regards differentiation of donor MSCs, we observed donor cell-derived endothelial cells within the sheets (but not in the host myocardium), while there was no finding suggesting myogenic differentiation.
Recent research has shed light on the ability of MSCs to stimulate endogenous progenitor cells towards cardiomyocyte regeneration in a paracrine manner.6,7 However, this effect after IM MSC injection remains debated.22,23 Here, our study provided new evidence to understand this event. MSC sheet therapy enhanced cardiomyogenic regeneration via an endogenous route, compared with the sham control, in association with upregulation of IGF-1 and SDF-1, both of which are known to increase recruitment and/or activation of endogenous stem/progenitor cells.27,28,29 In contrast, the regenerative activity was much less extensive after IM MSC injection, corresponding to lower upregulation of SDF-1. These data collectively suggest that MSCs do have a capability to stimulate endogenous cardiomyogenic regeneration, and that the extent of MSC presence after IM injection may not be sufficient to activate this pathway. While on the other hand, the cell sheet technique achieved the markedly increased presence of MSCs, which would enable the production of a necessary magnitude of paracrine stimuli for regeneration. Having said this, cardiomyocyte regeneration even after MSC sheet therapy was not extensive enough to influence the global cardiac function. Further refinement is needed to realize therapeutic myocardial regeneration based on MSC-mediated paracrine effects.
Our quantitative data of donor cell presence demonstrated that the survival rate of grafted MSCs after the cell sheet technique was so low that ~90% of the initially retained MSCs were dead or disappeared by day 28. Corresponding to this, the myocardial upregulation of paracrine effect-related genes after MSC sheet therapy diminished by day 28. However, despite these, cardiac function and structure were improved for at least 28 days. This let us speculate that the contribution of the paracrine factors would take place mainly during the early phase after MSC sheet therapy, and this is sufficient to initiate long-lasting changes in the cardiac tissue components (i.e., neovascular formation, reduced fibrosis, attenuation of cardiomyocyte hypertrophy, and endogenous myocardial regeneration) and resultant recovery of global cardiac function. Then, once established, the improved cardiac function could last for a longer time.
It was unexpected that donor cell survival was not improved by the cell sheet technique compared with IM injection. There was apoptosis in donor cells in the sheet to the same ratio to IM injection. We speculate that cell death in the sheet could be caused by relative hypoxia and/or insufficient nutrition. There was increased neovascular formation within the host myocardium and vessel formation within the sheets. However, the donor cells are likely to die before the neovascular formation is functionally complete enough for supply to the sheet. In addition, there were sprouting vessels from the myocardium to the sheets (Figure 3g); however, the extent of these connecting vessels did not appear to be sufficient. These would collectively result in inadequate oxygen/nutrition delivery to donor cells and inhibiting long-term survival of donor cells. In order to improve donor cell survival after cell sheet technique, we believe it is important to further improve vascular formation in the sheets and vascular connections between the host myocardium and sheets for enhancing perfusion of the sheets. This might be achieved by co-treatment with angiogenesis factors such as HGF30 or cotransplantation with endothelial cells or endothelial progenitor cells.31,32
A limitation of the present study may be that IM cell injection was carried out into two sites in a heart. Although this is one of the most frequently used, standard protocols for IM cell injection in rat,11,33,34 we cannot deny a possibility that an increase in the number of IM injections might enhance the therapeutic efficacy.
In conclusion, the cell sheet technique enhanced initial retention and following presence of bone marrow-derived MSCs, amplified subsequent paracrine effects to recover/regenerate the damaged heart, and improved therapeutic outcomes, compared with IM injection. This timely information will provide important clinical implication to refine MSC-based therapy that has recently entered clinical trials. As the cell sheet placement requires open chest procedures, it would be a reasonable idea to carry out MSC sheet therapy in conjunction with routine cardiac surgery, like coronary artery bypass grafting, left ventricular assist device implantation, and so on. Further preclinical investigations on the safety and efficiency of MSC sheet therapy are warrantied for enabling early clinical application of this promising approach.
Materials and Methods
All studies were performed with the approval of the institutional ethics committee and the Home Office, UK. The investigation conforms to the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication, 1996). All in vivo and in vitro assessments were carried out in a blinded manner. Please see the Supplementary Materials and Methods for additional details.
Generation of MSC suspensions and MSC sheets. MSCs were isolated from the bone marrow from the tibias and femurs of male Lewis rats (100–150 g; Charles River Laboratories, Margate, UK) as described previously35 and characterized (Supplementary Figure S2). To generate an MSC sheet, 4 × 106 MSCs were seeded at passages 3–5 on a 35 mm temperature-responsive culture dish (UpCell; CellSeed, Tokyo, Japan). Following incubation for 12 hours at 37 °C under 5% CO2, the culture temperature was lowered to 20–22 °C enabling the MSC sheet to detach from the dish.15 The collected MSC sheets were 12–15 mm in diameter. For injection, 4 × 106 MSCs were collected using trypsinization and suspended in 200 µl phosphate-buffered saline. Cell number counting by digesting a sheet with trypsin demonstrated that a freshly generated MSC sheet contained 4.1 ± 0.2 × 106 cells (n = 5), assuring that there was no difference in the MSC number between cell sheet and IM injection groups. For graft tracking studies, MSCs were labeled with CM-DiI (DiI; Molecular Probes, Paisley, UK) according to the manufacturer's protocol.
Animal models. Female Lewis rats (150–200 g; Charles River Laboratories) underwent left coronary artery ligation to generate MI as described previously.11 The animals were randomly assigned to undergo transplantation of 4 × 106 MSCs from syngeneic male rats by either Sheet group or IM group. For the Sheet group, an MSC sheet was placed to cover the infarct and surrounding border areas. After epicardial placement, MSC sheets were found to stably adhere to the heart within 20–30 minutes. For the IM group, MSCs suspended in 200 µl phosphate-buffered saline were intramyocardially injected with a 31G needle into two sites (100 µl each), targeting the border and infracted areas. Sham-treated (MI only) rats served as the Control group.
Cardiac performance measurement. Cardiac function and dimensions, and hemodynamic parameters were measured by using echocardiography (Vevo-770; VisualSonics, Amsterdam, The Netherlands) and catheterization (SPR-320 and PVAN3.2; Millar Instruments, Houston, TX) under general anesthesia using isoflurane inhalation in a blinded manner by well-experienced staff as previously described.11,36,37 All data were collected from at least three different measurements and averaged.
Quantitative assessment of donor cell presence. DNA was extracted from the whole left ventricular walls and the presence of male cells in the female hearts was quantitatively assessed to define donor cell presence/survival using real-time PCR (Prism 7900HT; Applied Biosystems, Paisley, UK) for the Y-chromosome-specific sry gene as previously described.36
Histological analysis. At chosen time points, the hearts were excised, fixed with 4% paraformaldehyde, and frozen. Cryosections were cut and incubated with polyclonal anti-cTnT antibody (1:200 dilution; HyTest, Turku, Finland), polyclonal anti-cleaved caspase-3 antibody (1:250; Cell Signaling Technology, Danvers, MA), biotin-conjugated Griffonia simplicifolia lectin I-isolectin B4 (1:100; Vector Laboratories, Peterborough, UK), monoclonal anti-CD45 antibody (1:50; BD, Oxford, UK), monoclonal anti-CD11b antibody (1:50; Chemicon, Watford, UK), monoclonal anti-granulocyte antigen antibody (1:20; AbD Serotec, Kidlington, UK), monoclonal anti-OX62 antibody (1:25; AbD Serotec), polyclonal anti-CD3 antibody (1:100; Abcam, Cambridge, UK), polyclonal anti-CD34 antibody (1:700; R&D, Abingdon, UK), monoclonal anti-CD31 antibody (1:50; AbD Serotec), monoclonal anti-ICAM1 antibody (1:50; Abcam), monoclonal anti-Ki67 antibody (1:50; Dako, Cambridgeshire, UK), or polyclonal anti-Sca-1 antibody (1:25; Abnova, Heidelberg, Germany) followed by visualization using fluorophore-conjugated secondary antibodies (Molecular Probes). Sections were analyzed by fluorescence microscopy (BZ8000; Keyence, Milton Keynes, UK) with or without nuclear counterstaining using 4′,6-diamidino-2-phenylindole (DAPI). Ten different fields from each of the border and remote areas per heart were randomly selected and assessed. Another set of sections were stained with 0.1% picrosirius red for assessing infarct size (% of scar length to total left ventricular circumference) and for detecting collagen deposition using NIH image-analysis software.11,36 To evaluate the cardiomyocyte size, the cross-sectional area of appropriately detected cardiomyocytes (transversely cut; having central nuclei and surrounded by circle-shaped capillaries)37 was measured for 50 cardiomyocytes per area. In addition, for detecting adipogenic and osteogenic differentiation, staining with Oil red O and Alizarin red was performed as above.38,39,40
Analysis of myocardial gene expression and signaling pathway activation. Total RNA was extracted from the whole left ventricular walls and assessed for myocardial gene expression by quantitative reverse transcription-PCR (Prism 7900HT) as previously described.36 TaqMan primers and probes were purchased from Applied Biosystems. Expression was normalized using Ubiquitin C. In addition, protein was extracted and assessed by western blotting. Primary antibodies were obtained from Cell Signaling Technology; anti-phosphorylated ERK1/2 (#4377), phosphorylated JNK (#9251), phosphorylated p38 (#9216), phosphorylated Akt (#4051), and phosphorylated PI3K (#4228). The labeled membrane was stripped, and then re-probed with anti-ERK1/2 (#9102), JNK (#9252), p38 (#9212), Akt (#9272), PI3K (#4292) antibodies. Blots were imaged and quantitative analysis was performed using Alpha view software (Alpha Innotech, Santa Clara, CA).36
Statistical analysis. All values are expressed as mean ± SEM. Statistical comparison of the data was performed using the Student's unpaired t-test for the analysis of donor cell apoptosis. All other data were statistically analyzed with one-way analysis of variance followed by Fisher's post-hoc analysis to compare groups. A value of P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Supplementary histological findings to Figure 1 (Less densely packed donor cells in the MSC sheets after epicardial placement). Figure S2. In vitro characterization of rat bone marrow-derived MSCs. Figure S3. Adipogenic and osteogenic differentiation of MSCs in vivo. Figure S4. Supplementary histological findings to Figure 3 (Donor cell behaviors and changes in the epicardium after MSC sheet therapy). Figure S5. Supplementary histological findings to Figure 4 (Histological recovery of post-MI failing cardiac tissues by MSC sheet therapy). Figure S6. Supplementary histological findings to Figure 5 and Table 2 (Improved endogenous regeneration activity by MSC sheet therapy). Figure S7. Elevated activation of paracrine effect-related signal pathways by MSC sheet therapy. Figure S8. Supplementary data to Figure 6 (Myocardial gene expression at day 28 after MSC sheet therapy). Figure S9. Materials and Methods
Acknowledgments
This research forms of the research themes contributing to the translational research portfolio of Barts Cardiovascular Biomedical Research Unit which is supported and funded by the UK National Institute of Health Research (New and Emerging Applications of Technology Progarmme (NEAT L018) and the Barts and The London Charity (ETHG1B8R)). The authors declared no conflict of interest.
Supplementary Material
References
- Menasche P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol. 2011;50:258–265. doi: 10.1016/j.yjmcc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Passier R, van Laake LW., and, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kurtz A., and, Stamm C. Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther. 2011;22:3–17. doi: 10.1089/hum.2010.211. [DOI] [PubMed] [Google Scholar]
- Boyle AJ, McNiece IK., and, Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol. 2010;660:65–84. doi: 10.1007/978-1-60761-705-1_5. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H.et al. (2005Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells Nat Med 11367–368. [DOI] [PubMed] [Google Scholar]
- Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS.et al. (2010Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation Circ Res 107913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y.et al. (2008Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells Biochem Biophys Res Commun 37411–16. [DOI] [PubMed] [Google Scholar]
- < http://clinicaltrials.gov/ >. 4 September 2012.
- Suzuki K, Murtuza B, Beauchamp JR, Brand NJ, Barton PJ, Varela-Carver A.et al. (2004Role of interleukin-1beta in acute inflammation and graft death after cell transplantation to the heart Circulation 110suppl. 1II219–224. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Beauchamp JR, Smolenski RT, Varela-Carver A, Fukushima S.et al. (2004Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart FASEB J 181153–1155. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J.et al. (2007Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model Circulation 1152254–2261. [DOI] [PubMed] [Google Scholar]
- Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F.et al. (2005Cell sheet engineering: recreating tissues without biodegradable scaffolds Biomaterials 266415–6422. [DOI] [PubMed] [Google Scholar]
- Elloumi-Hannachi I, Yamato M., and, Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Brand NJ, Smolenski RT, Jayakumar J, Murtuza B., and, Yacoub MH. Development of a novel method for cell transplantation through the coronary artery. Circulation. 2000;102 suppl. 3:III359–III364. doi: 10.1161/01.cir.102.suppl_3.iii-359. [DOI] [PubMed] [Google Scholar]
- Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H.et al. (2006Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction Nat Med 12459–465. [DOI] [PubMed] [Google Scholar]
- Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S.et al. (2005Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets J Thorac Cardiovasc Surg 1301333–1341. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M.et al. (2009Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice J Clin Invest 1192204–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L.et al. (2010Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells Circulation 122suppl. 11S118–S123. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U.et al. (2005Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood Exp Hematol 331402–1416. [DOI] [PubMed] [Google Scholar]
- Sekiya N, Matsumiya G, Miyagawa S, Saito A, Shimizu T, Okano T.et al. (2009Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart J Thorac Cardiovasc Surg 138985–993. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M.et al. (2004Mesenchymal stem cells can be differentiated into endothelial cells in vitro Stem Cells 22377–384. [DOI] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Gannon J., and, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Zhai P, Maejima Y., and, Sadoshima J. Myocardial injection with GSK-3ß-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108:478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y.et al. (2003Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction Proc Natl Acad Sci USA 10012313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM.et al. (2008Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction Circ Res 103203–211. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH.et al. (2004Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart FASEB J 181138–1140. [DOI] [PubMed] [Google Scholar]
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M.et al. (2003Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy Lancet 362697–703. [DOI] [PubMed] [Google Scholar]
- Haider HKh, Jiang S, Idris NM., and, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E.et al. (2007Enhancing repair of the mammalian heart Circ Res 1001732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siltanen A, Kitabayashi K, Lakkisto P, Mäkelä J, Pätilä T, Ono M.et al. (2011hHGF overexpression in myoblast sheets enhances their angiogenic potential in rat chronic heart failure PLoS ONE 6e19161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M.et al. (2008Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts Circulation 118suppl. 14S145–S152. [DOI] [PubMed] [Google Scholar]
- Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M.et al. (2010Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering Biomaterials 313903–3909. [DOI] [PubMed] [Google Scholar]
- Mazo M, Cemborain A, Gavira JJ, Abizanda G, Araña M, Casado M.et al. (2012Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity Cell Transplant 211023–1037. [DOI] [PubMed] [Google Scholar]
- Kim SH, Moon HH, Kim HA, Hwang KC, Lee M., and, Choi D. Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol Ther. 2011;19:741–750. doi: 10.1038/mt.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H.et al. (2008Topical transplantation of mesenchymal stem cells accelerates gastric ulcer healing in rats Am J Physiol Gastrointest Liver Physiol 294G778–G786. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Fukushima S, Varela-Carver A, Lee J, Coppen SR, Takahashi K.et al. (2009Donor cell-type specific paracrine effects of cell transplantation for post-infarction heart failure J Mol Cell Cardiol 47288–295. [DOI] [PubMed] [Google Scholar]
- Litwin SE, Raya TE, Anderson PG, Litwin CM, Bressler R., and, Goldman S. Induction of myocardial hypertrophy after coronary ligation in rats decreases ventricular dilatation and improves systolic function. Circulation. 1991;84:1819–1827. doi: 10.1161/01.cir.84.4.1819. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI., and, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P.et al. (2006Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia Stem Cells 242202–2208. [DOI] [PubMed] [Google Scholar]
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM.et al. (2007Potential risks of bone marrow cell transplantation into infarcted hearts Blood 1101362–1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.