Abstract
Notch signaling is active during the development of mosaic epithelial sheets and during their turnover and regeneration. After the loss of hair cells in the mosaic sheet of the vestibular sensory epithelium, new hair cells can be spontaneously generated by transdifferentiation of supporting cells. This regenerative process involves downregulation of the Hes5 gene and is known to be limited and incomplete, especially when the lesion is severe. Here, we test whether further downregulation of Hes5 gene accomplished by the use of siRNA after a severe lesion induced by an aminoglycoside in the mouse utricle can enhance the transdifferentiation of supporting cells and lead to the increased production of new hair cells. We demonstrate that Hes5 levels in the utricle decreased after the application of siRNA and that the number of hair cells in these utricles was significantly larger than following control treatment. The data suggest that siRNA technology may be useful for inducing repair and regeneration in the inner ear and that the Notch signaling pathway is a potentially useful target for specific gene expression inhibition.
Introduction
People lose vestibular function gradually with aging, or due to acute infection, trauma, vascular disease, or ototoxic drugs. In many cases, abrupt hair cell loss is the main pathology leading to acute peripheral vestibulopathy. Ability to regenerate hair cells may restore the balance function in such ears. There has been clinical evidence showing that vestibular symptoms after acute vestibulopathy are alleviated not only by central compensation but also by restoration of end organ function.1,2
A partial recovery of peripheral vestibular function may accompany spontaneous regeneration of hair cells, as described in mice.3 However, the spontaneous hair cell regeneration is not sufficient, in terms of quantity and quality. It is therefore necessary to augment and increase the extent of hair cell regeneration in the peripheral vestibular organs. Potential strategies for hair cell regeneration therapy include enhancing transdifferentiation of supporting cells to new hair cells, and/or implanting stem cells. The former strategy has been accomplished by overexpression of developmental genes using viral vectors.4
Better knowledge of the changes in gene expression that accompany the spontaneous hair cell regeneration may help us design means to enhance the process. This could conceivably be accomplished by further downregulating genes that inhibit the process. One possible way to inhibit specific genes is by siRNA technology, where specific mRNAs can be targeted for degradation, resulting in inhibition of the synthesis of the encoded protein.5 siRNA has been used to suppress specific gene expression, ever since therapeutic effects of Fas-specific siRNA with experimentally induced hepatitis was reported in 20036. Inner ear application of siRNA was successful in antagonizing the effect of a dominant negative mutation7 and in protecting against outer hair cells loss in animals treated with the antitumor drug cisplatin.8
Developmental studies have characterized the signaling molecules that guide cell fate determination and differentiation in the sensory epithelium.9 The differentiation into hair cells and supporting cells is regulated by Notch signaling and then by Atoh1, a basic helix–loop–helix transcription factor, which is a positive regulator of the hair cell phenotype. Deletion of Atoh1 blocks hair cell development in inner ear.10 Duration of Atoh1 expression has also been linked to the differentiation process,11 and unregulated continued expression of Atoh1 during hair cell maturation has been shown to cause hair cell damage and degeneration.12 These data suggest that forced, unregulated Atoh1 gene expression may have an adverse effect on regenerating hair cells. In designing the strategies for hair cell regeneration, it is therefore conceivable that blocking supporting cell-specific genes may be used instead of upregulation of Atoh1.
During normal development, progenitor cells that become supporting cells are prevented from expressing Atoh1 by Notch signaling. Notch ligands, Jagged 2 and Delta 1, activate Notch receptors of future supporting cells and make activated receptor domains enter the nucleus as transcriptional factors. This Notch activation increases the expression of two target genes, Hes1 and Hes5 in future supporting cells.9 Deletion of either Hes1 or Hes5 causes significantly increased number of hair cells in mouse inner ear.13 Cotransfection of Kölliker's organ cells with Atoh1 and Hes1 showed that Atoh1 transcription is a target of Hes1 in the ear.14 The developmental genes regulating the differentiation of hair cells and supporting cells in the vestibular epithelium are similar to these active in the cochlea, but some differences may exist in the specific role of each gene.15,16
Once tissues are mature, the developmental role of Notch signaling is diminished and the level of expression of Notch family genes is reduced. Nevertheless, when the sensory epithelium of the inner ear is exposed to a trauma that involves hair cell injury or loss, levels of specific Notch molecules change in both the cochlea and the vestibular system.16,17,18 In the mammalian vestibular epithelium where low-level spontaneous hair cell regeneration occurs, Hes5 mRNA appears to be downregulated, whereas Atoh1 mRNA levels increase during the process of regeneration.18 As such, Hes5 downregulation may be positively related with the inducing hair cell regeneration. On the basis of this observation, as well as on data from cultured utricles,19 and cultured organ of Corti20 showing that blocking Notch signaling enhances production of new hair cells, we speculated that Hes5 siRNA administration following an insult caused by the ototoxic aminoglycosides would be able to enhance the extent of regeneration in the utricle.
For this set of in vivo experiments, we induced a lesion in the utricles of mice by injecting streptomycin to the posterior semicircular canal (PSC) of the left ear. One week later, when a near-complete loss of hair cells occurred, we reopened the left PSC and injected Hes5 siRNA for the experimental group, or sterile normal saline for the control group. Animals were killed 2 weeks later (3 weeks after the lesion), utricles harvested and processed. Upon hair cell counting, Hes5 siRNA-treated utricles showed significant increase in the number of hair cells compared with controls, suggesting that Hes5 siRNA treatment increases the number of hair cells regenerated in the traumatized ears.
Results
Vestibular hair cell loss: lesion model
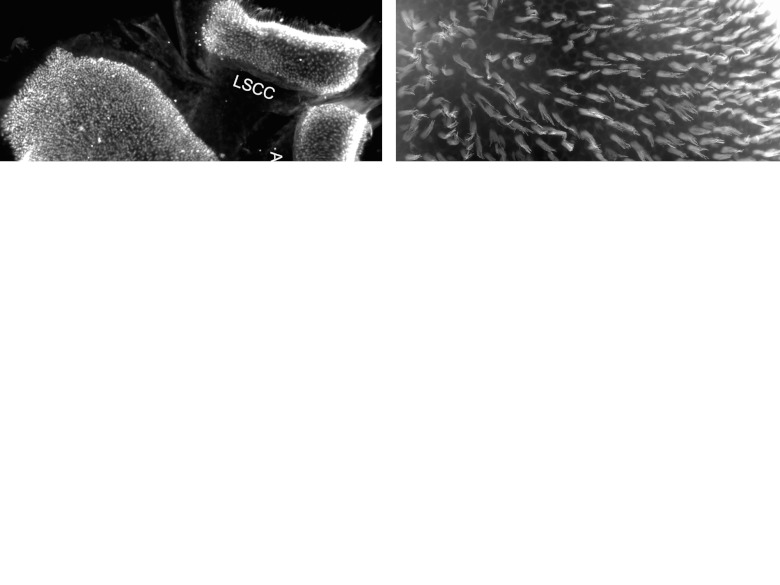
Mice showed acute vestibulopathy signs as soon as they woke up from surgical anesthesia. The major symptoms included longitudinal body axis rotation, left side head tilting and circling. These signs persisted for several days to several weeks, with longitudinal body axis rotation resolving first, and head tilt last, often evident until the end of the experiment. Tissues obtained for the histology included, in most cases, the utricle along with the two adjacent canal organs, but only the utricles were used for quantitative analysis. A low magnification epi-fluorescence image presents the general morphology of these vestibular organs and shows a uniform distribution of hair cells as depicted by the staining for Myosin VIIa (Figure 1a). Whole mounts stained with fluorescent phalloidin reveal the presence of stereocilia and the intercellular junctions, showing a normal distribution of hair cells in the sensory epithelium in normal controls (Figure 1b). In tissues obtained 1 week after the ototoxic insult, the density of Myosin VIIa-positive cells is drastically reduced (Figure 1c). Among the few cells that retain the specific hair cell staining, many appear dysmorphic and disorganized, possibly representing debris of dead cells. These finding were observed in both the utricle and the adjacent canal organs (Figure 1c). Higher magnification analysis of phalloidin-stained utricle tissue 1 week after the insult shows that the transitional epithelium bordering the sensory epithelium remained organized, whereas the sensory epithelium proper not only lost its hair cells, but also its typical epithelial organization (Figure 1d).
Figure 1.
Epi-fluorescence images of vestibular epithelia taken from normal ears (a,b) or an ear obtained 1 week after ototoxic trauma (c,d). (a) The sensory epithelium (SE) of the utricle (on left) and two semicircular canals (LSCC for lateral and ASCC for anterior) on the right, stained for Myosin VIIa, showing dense and even distribution of positive cells, with staining visible in stereocilia bundles. (b) Phalloidin staining in the utricle reveals long stereocilia bundles throughout the sensory epithelium. (c) After the lesion, the number of cells stained by Myosin VIIa appears greatly diminished and the few stained cells exhibit clumps or dysmorphic aggregations in both utricle and canal organs. (d) The contour of adherens junctions stained with phalloidin shows normal appearance in the transitional epithelium (TE) but the sensory epithelium regions lacks hair cell bundles and the apical junctional organization is perturbed. Scale bar: a and c are 50 µm; b and d are 20 µm.
Evaluation of siRNA delivery
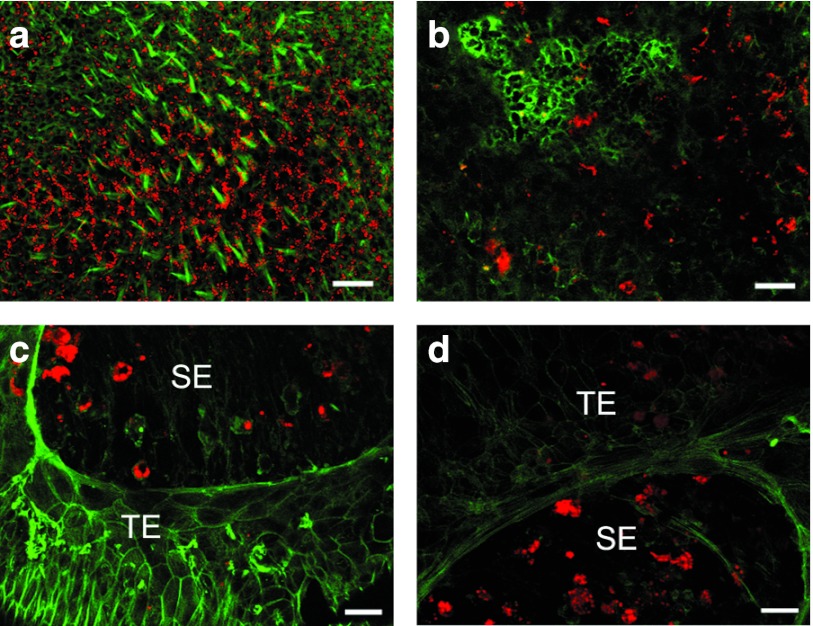
To determine whether siRNA can reach the sensory epithelium after the inoculation into the posterior canal, we injected a tracer siRNA with a red fluorescent marker (Cy3.5 labeled siRNA, 2 µl of 4 µg/µl solution) (Figure 2). Tissues were prepared as whole mounts 1 day after the inoculation and counter-stained with Alexa Fluor 488-conjugated phalloidin (green) to show tissue landmarks and cellular organization. In the normal sensory epithelium of the utricle (Figure 2a), widespread distribution of the red reporter siRNA is seen in the sensory epithelium. The red staining is not confined to a specific cell type. Costaining with phalloidin shows that the population of hair cells is preserved in qualitative appearance, without any evidence of hair cell loss. In other animals, a lesion was induced with streptomycin, and the ears received fluorescent siRNA 1 day (Figure 2b) or 3 days later (Figure 2c,d). In lesioned tissues obtained 1 day after the siRNA injection (8 days after the lesion was induced), a severe loss of hair cells is evident in the utricular sensory epithelium, and the siRNA is found in many cells in the epithelium (Figure 2b). Ears of animals that were killed 3 days after the siRNA injection had a similar appearance, exhibiting severe hair cell loss and prominent presence of the Cy3.5-siRNA in nonsensory cells that remained in the vestibular epithelium after the loss of hair cells (Figure 2c,d). The presence of the red siRNA fluorescent signal is not confined to the sensory epithelium. Rather, it was also seen in nonsensory areas adjacent to the sensory epithelium (Figure 2d).
Figure 2.
Confocal images of whole mounts of utricles costained with Alexa Fluor 488-conjugated phalloidin (green) after injection of fluorescent reference siRNA (Cy3.5 siRNA, 2 µl, 4 µg/µl). (a) Widespread red signal throughout the normal utricular sensory epithelium (SE) is observed 1 day after injection. The staining is present in all cell types. (b) One day after Cy3.5-siRNA injection (performed 1 week after streptomycin inoculation), fluorescent siRNA signal is present in moderate quantities. (c) Three days after reporter injection (performed 1 week after the insult) fluorescent siRNA signal is detectable throughout the tissue but in the sensory area it is more prominent, whereas the level of staining in the transitional epithelium is reduced at this stage. (d) Same sample as (c) showing Cy3.5-siRNA presence in nonsensory area of the transitional epithelium (TE). Scale bar: 20 µm.
Myosin VIIa and Atoh1 in the Vestibular sensory epithelium
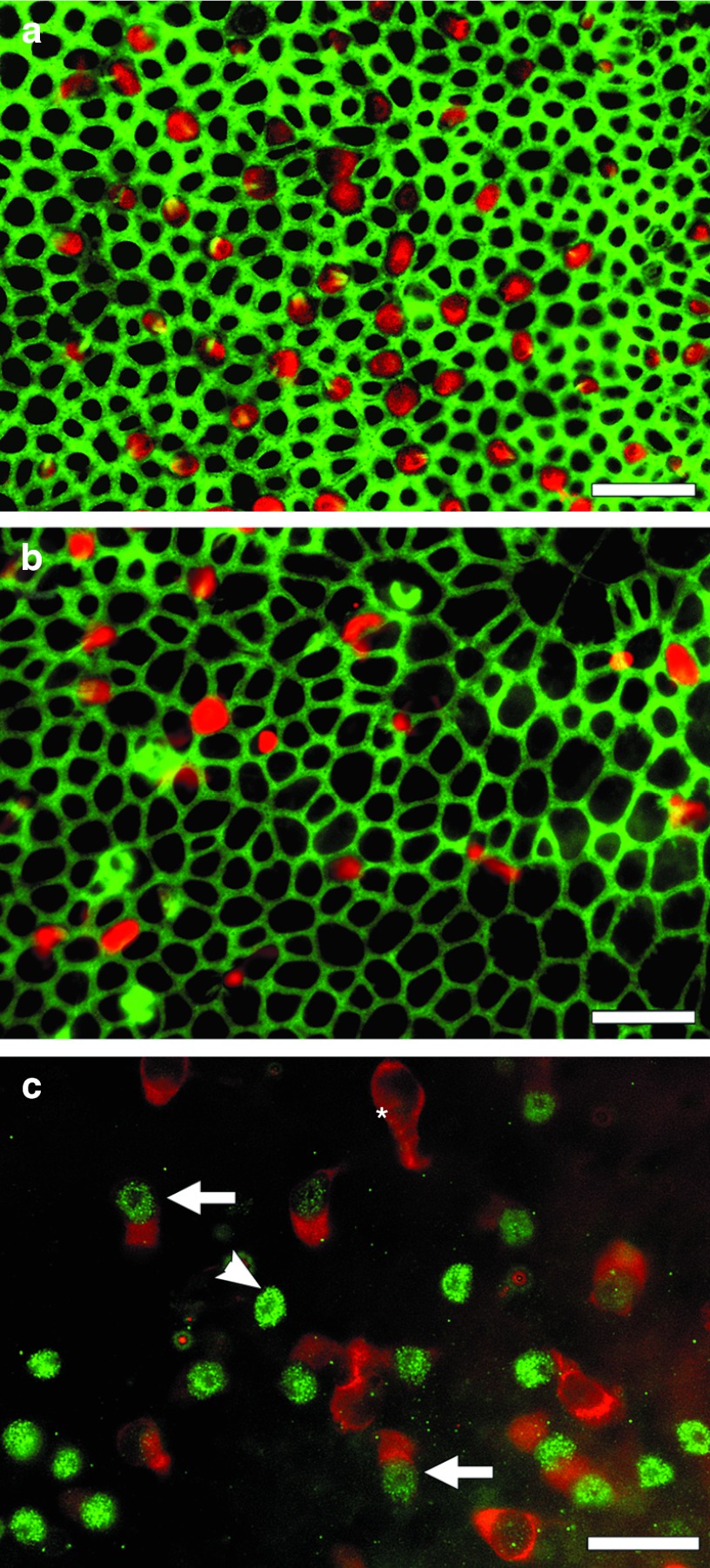
Whole mounts of utricles from Hes5 siRNA-treated mice taken 3 weeks after streptomycin administrations show numerous Myosin VIIa-positive cells, which had variable sizes and shapes (Figure 3a). In some cases, the cell body appears elongated and the length exceeds the typical dimensions of vestibular hair cells (data not shown). Myosin VIIa-positive cells are mostly found in the medial posterior region of the utricle and are least prevalent in the striolar region. These cells show short stereocilia resembling premature bundles, and exhibit irregular planar polarity, with bundles oriented randomly. The apical cell contour is variable with some cells appearing triangular and others oval, round, or polygonal (Figure 3a). Some of the Myosin VIIa-positive cells do not have a stereocilia bundle prominent enough to be detected with phalloidin staining. A whole mount image of vehicle control vestibular sensory epithelium taken at the same time-point shows significantly fewer Myosin VIIa-positive cells than Hes5 siRNA-treated mice (Figure 3b). Quantitation of the data appears in Figures 5 and 6. Myosin VIIa-positive cells found in control tissues (Figure 3b) often display irregular shape and appear as degenerating cells or debris, but their size appears similar to that of the red cells in the siRNA-treated utricle (Figure 3a).
Figure 3.
Whole mounts of utricles viewed in epi-fluorescence after ototoxic lesion followed by treatment with Hes5 siRNA (a,c) or control reagent (b). (a) Three weeks following the lesion (2 weeks after Hes5 siRNA injection), anti-Myosin VIIa antibody (red) and phalloidin (green) reveal numerous Myosin VIIa-positive cells in the tissue. These presumed regenerated hair cells appear in variable size and apical contour shape ranging from round to nearly triangular. The stereocilia bundles are viewed in green and appear to have irregular orientation and planar polarity. The density of cells is lower than in normal controls (Figure 1b). (b) Control-treatment sample at same time-point shows few poorly organized Myosin VIIA-positive cells. The density of cells appears lower in the control tissue and the width of the junction actin belt thinner than in the siRNA-treated utricle (a). The diameter of hair cells appears similar in a and b. (c) One week after Hes5 siRNA injection (2 weeks after the lesion) the sensory epithelium region of the utricle, stained for Atoh1 (green) and Myosin VIIa (red) includes Atoh1-positive/Myosin VIIa-negative cells (arrow head), Atoh1-negative/Myosin VIIa-positive cells (asterisk), and cells positive for both (arrow). Scale bar: 20 µm.
Whole mounts of utricles treated with Hes5 siRNA and obtained for analysis 2 weeks later show numerous Atoh1-positive cell nuclei with or without Myosin VIIa-positive cell body (Figure 3c). Atoh1-positive cells are found most frequently in posteromedial area with fewer cells in the striolar region (data not shown).
Three different types of cells are seen in the auditory epithelium area based on staining combinations of Atoh1 and Myosin VIIa. One type of cell is Atoh1-positive without obvious Myosin VIIa-positive cell body, suggesting that newly developing hair cells are derived from supporting cells undergoing transdifferentiation, based on the rationale that supporting cells do not express Myosin VIIa. The second type of cell is Atoh1-positive and Myosin VIIa-positive, most likely representing newly formed hair cells which are still immature, based on the rationale that mature hair cells do not express Atoh1. The third type of cell is Myosin VIIa-positive and Atoh1-negative. These cells are either original hair cells that survived the lesion or, more likely, newly generated hair cells that have completed the process of downregulating Atoh1 expression. This latter explanation is more likely due to the severe outcome of the insult, leaving behind a lesion with few or no hair cells.
Cross sections of utricular sensory epithelia
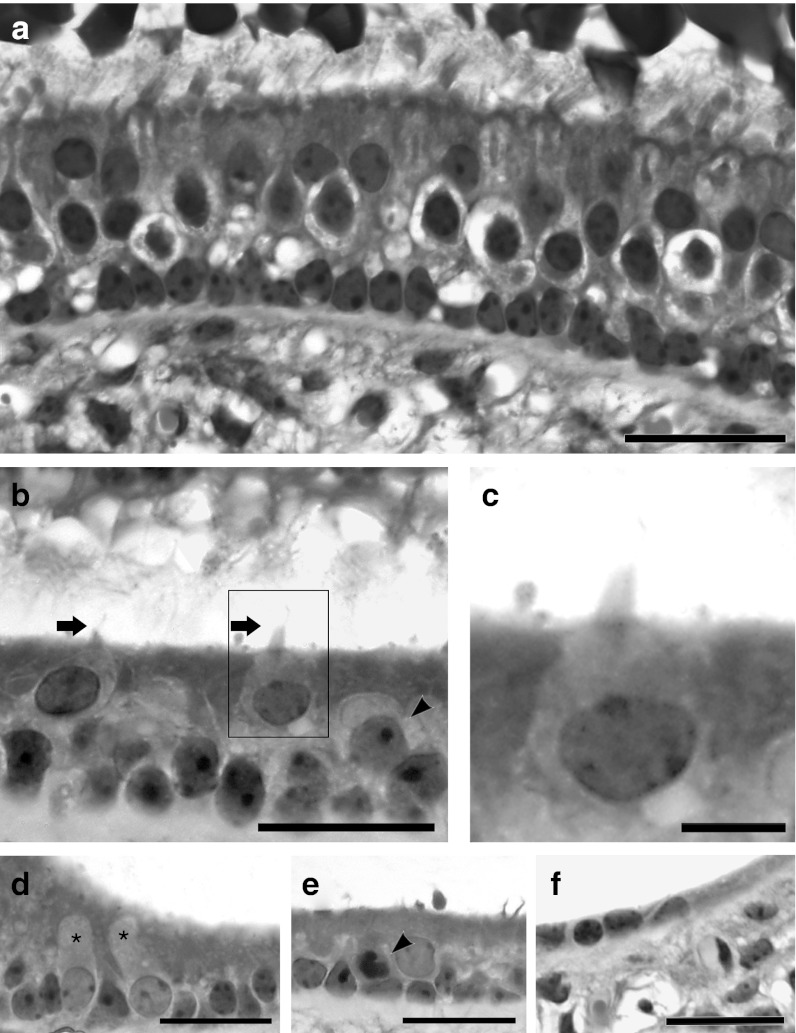
Plastic sections of normal utricles displayed the sensory epithelium including type I and II hair cells and nonsensory cells, whose nuclei reside near the basement membrane (Figure 4a). Utricles obtained from Hes5 siRNA-treated and control mice were obtained 3 weeks following the streptomycin injection (2 weeks after siHes5 or control vehicle injection), embedded in plastic, and sectioned. In the experimental treatment group, there are both hair cells and supporting cells (Figure 4b). The nuclei of hair cells appear to be in a higher (more luminal) position in the epithelium, as seen in Figure 4a and in published images of the normal utricle.21 In addition to the two distinctive rows of nuclei belonging to supporting cells (bottom) and hair cells (top), there are some nuclei in intermediate positions in siRNA-treated utricles (Figure 4c). These nuclei appear larger than nuclei in the supporting cell layer. Hair cells often display lighter-stained nuclei, larger cell body, and light cytoplasm (Figure 4b,c). Hair cells have short hair bundles (Figure 4b,c), which look premature compared with the long bundles of normal utricle hair cells (Figure 4a). In the marginal zone of utricles with regenerated hair cells, we observed some nuclei located in the supporting cell level and exhibiting features of hair cell nuclei based on size and density of chromatin (Figure 4d). This observation suggests that these cells could have been in the process of transdifferentiation from the supporting cell to the hair cell phenotype at the time tissues were obtained. There are a few cells exhibiting a darker nucleus possibly indicative of degenerative process (Figure 4e, arrow head). Representative cross sections of a control utricle observed at the same time-point (2 weeks after vehicle injection) are devoid of hair cells (Figure 4f).
Figure 4.
Light microscope images of cross-sectioned utricles from a normal (untreated) ear (a), or ears after ototoxic lesion followed by treatment with Hes5 siRNA (b–e) or vehicle control (f). (a) The normal utricular sensory epithelium contains hair cells and nonsensory cells. Type I hair cells are surrounded by a calyx. Stereocilia are tall and appear embedded in the otoconial membrane. Supporting cell nuclei are arranged in a orderly line just above the basement membrane. (b) In lesion and then siRNA-treated utricle, hair cells are close to the luminal surface of the epithelium and exhibit short stereocilia bundles (arrow). There were some nuclei in intermediate positions (arrow head). These cells have larger and lighter nuclei than cells in the supporting cell layer. Nuclei in the supporting cell layer are not as well organized near the basement membrane as in the normal utricle. (c) Zoom-in image of highlighted area in b shows one longest cilium. (d) Some nuclei located at the supporting cell level have distinguishing features based on size and density of chromatin, suggesting they are newly transdifferentiating cells (asterisks). (e) Cells containing a darker nucleus are possibly undergoing a degenerative process (arrow head). (f) Cross-section of a utricle observed at same time-point, 2 weeks after vehicle (control) injection. The tissue is devoid of hair cells. All scale bars: 20 µm.
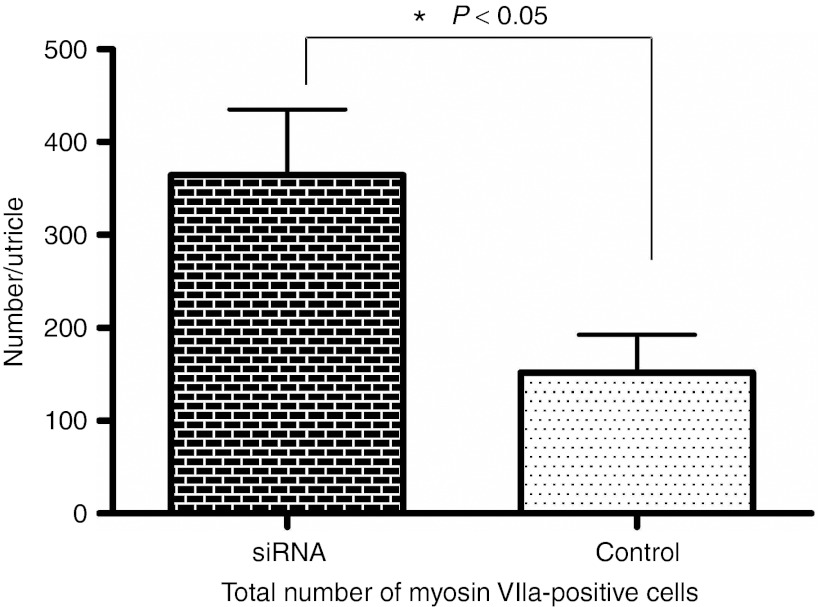
We counted hair cells in whole mounts of utricles of each group (Hes5 siRNA and vehicle control) 3 weeks after streptomycin administration (Figure 5). Average total hair cell number per utricle is 364.3 (±284.1, n = 16 utricles) in Hes5 siRNA-treated group. In contrast, the number of hair cells is 151.9 (±152.6, n = 14 utricles) in vehicle treated group. Hair cell number difference between each group is statistically significant (P < 0.05, Mann–Whitney U test).This result suggests that vestibular hair cell regeneration as observed 3 weeks after streptomycin damage is significantly enhanced by Hes5 siRNA administration. Nevertheless, despite the more than twofold increase in the number of hair cells in siRNA-treated utricles, the number of hair cells is far smaller compared with normal utricles which contain 3,246 ± 58 hair cells.21
Figure 5.
Total Myosin VIIa-positive cells counts in utricles of each group (Hes5 siRNA and vehicle control) at 3 weeks following the lesion. Average Myosin VIIa-positive cell numbers in Hes5 siRNA group are higher than vehicle group. Mann–Whitney U test. Error bars represent one standard error measurement (SEM). N = 16 for siRNA group, N = 14 for saline group.
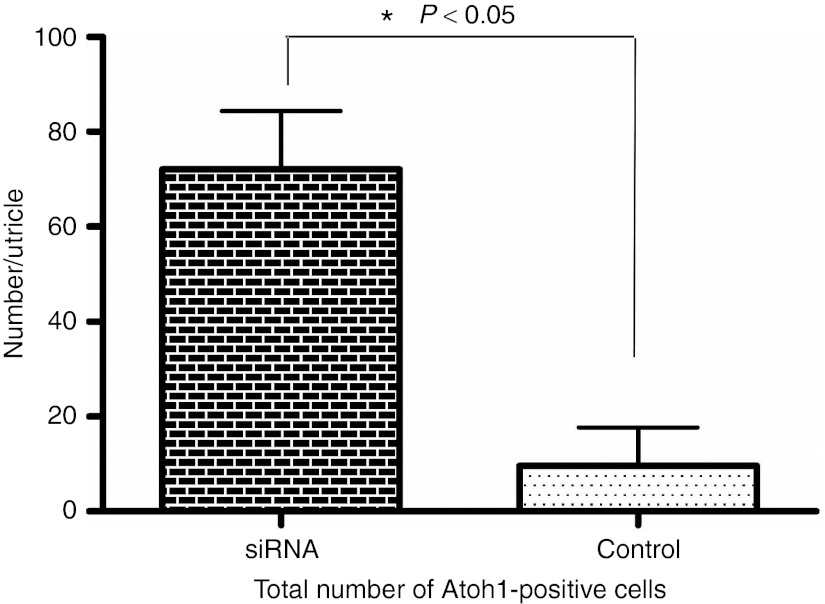
We also counted and compared Atoh1-positive cell number using the same method as for Myosin VIIa-positive cell counting. For counting Atoh1-positive nuclei, we picked a time-point 1 week earlier than that used for Myosin VIIa counting, because Atoh1 expression precedes that of Myosin VIIa expression during development and regeneration, and tends to decline as cells mature.13,18 Average total Atoh1-positive cell number in the utricle 2 weeks after streptomycin administration is 72.0 (±32.7, n = 7) in Hes5 siRNA treated group as compared with 9.6 (±18.2, n = 5) in vehicle treated group (Figure 6). Atoh1-positive cell number difference between each group was statistically significant (P < 0.05, Mann–Whitney U test). Atoh1-positive cell numbers at 2 weeks after the aminoglycoside insult are higher in Hes5 siRNA-treated mice.
Figure 6.
Total Atoh1-positive cells count in utricles of each group (Hes5 siRNA and vehicle control) at 2 weeks following the lesion. Average Atoh1-positive cell numbers in Hes5 siRNA group are higher than vehicle group. Mann–Whitney U test. Error bars represent one standard error measurement (SEM). N = 7 for siRNA group, N = 5 for saline group.
Taken together, the lower number of Atoh1-positive cells and Myosin VIIa-positive cells at 2 weeks and 3 weeks, respectively, in control tissues, as compared with the Hes5 siRNA-treated ears, suggest that Hes5 siRNA acts to induce hair cell regeneration in the vestibular epithelium.
Quantification of Hes5 and Atoh1 mRNA
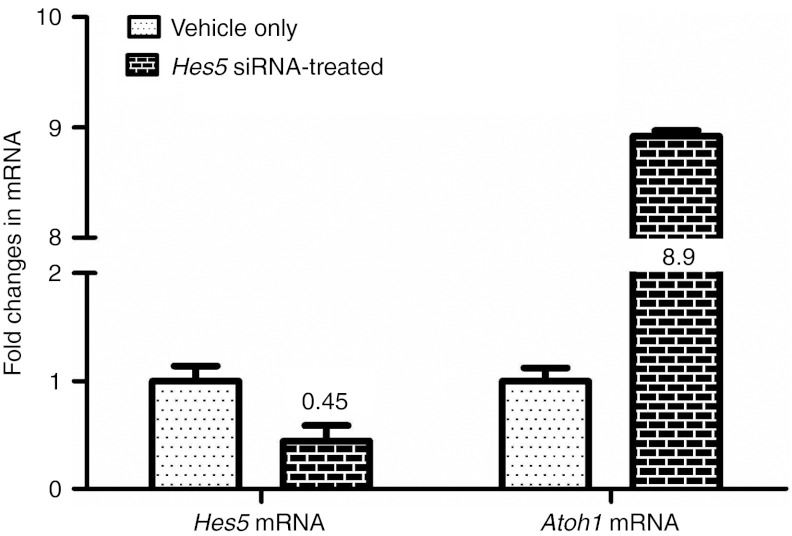
We performed quantitative real-time PCR (qRT-PCR) to see if Hes5 siRNA injection affected Hes5 mRNA expression with subsequent downstream Atoh1 mRNA upregulation at 2 weeks following the streptomycin injection (1 week after Hes5 siRNA or control vehicle injection). Relative mRNA amount fold changes of Hes5 siRNA-treated group as compared with vehicle group were calculated (Figure 7). Expression level of Hes5 mRNA in siHes5 injected ears is 45% of those detected in control ears (vehicle group). Expression of Atoh1 mRNA is 8.9-fold upregulated in siHes5-treated group. This result correlates with Atoh1-positive cell number difference with immunofluorescent staining.
Figure 7.
qRT-PCR data showing fold changes in mRNA expression between vehicle group and Hes5 siRNA-treated group at 2 weeks following streptomycin injection surgery. Expression of Hes5 mRNA was 0.45-fold downregulated in siRNA-treated group compared with vehicle group. Expression of Atoh1 mRNA was 8.9-fold upregulated in siRNA-treated group. Error bars represent one standard error measurement (SEM). N = 6 for each group.
Discussion
We show that inhibiting Hes5 with siRNA increases the number of hair cells that appear in the severely traumatized vestibular epithelium. The treatment also results in the decrease of Hes5 levels and increase in Atoh1 levels in the tissue, as detected by qRT-PCR. As spontaneous regeneration by itself is incomplete, augmentation of the regeneration is likely to contribute to better functional recovery after an insult to the epithelium. Accomplishing this task with one application of a nontoxic siRNA reagent provides an avenue for developing clinically relevant therapy for vestibular hair cell regeneration.
The method we used to eliminate vestibular hair cells was selected due to its ability to induce a complete or near-complete degeneration of hair cells in the utricle. Although this lesion does not mimic the majority of cases of vestibular pathology in human ears, it is advantageous because it allows us to determine the extent of regeneration, when the treatment is performed on ears devoid of hair cells. To minimize individual variability in terms of hair cell loss and obtain a complete or near-complete hair cell elimination, we used a concentration of 200 mg/ml streptomycin. The unilateral nature of the lesion reported here is another disadvantage, as it does not allow for reliable physiological testing of the vestibular system. This sensory system can compensate and improve function based on the remaining unlesioned ear and plasticity of the brain. Attempts to accomplish a drastic and severe lesion with systemic application of vestibulotoxic drugs failed to produce a lesion severe enough for experimental value. Similarly, our attempts to induce bilateral lesions via surgical injections into each ear also failed, due to the high mortality of the animals. Once a reliable method for inducing bilateral profound lesions is available, it will be important to extend these experiments to include balance tests.
We include the analysis of the utricle, which is amenable for observation due to its flat two-dimensional–like appearance. In contrast, the cristae of the semicircular canals have a more complicated three-dimensional structure, which makes hair cell counting in whole mount preparations more difficult. The saccule is largely ignored here, because this organ has shown the most variable (between animals) extent of lesion after the streptomycin insult we used. It is unclear whether the mild and variable saccular lesions are due to an internal feature associated with the saccule proper, or due to the distance of this organ from the site of aminoglycoside injection.
In this study, we used PSC approach to deliver Hes5 siRNA into the inner ear directly. This approach was chosen to obtain optimal control of the amount of siRNA delivered, so as to achieve consistent effects. However, this route for surgical approach does not seem to be ideal for clinical application. Rather, transtympanic or intratympanic injections are more likely clinical alternatives for delivering siRNA into the inner ear, and their feasibility has been demonstrated.8 With these approaches it is likely that the cochlea would receive a higher dose of the siRNA than the vestibular organs, such that consequences of the treatment on the cochlear epithelium should be carefully considered.
The basic helix–loop–helix genes Hes1 and Hes5 are target genes for mammalian Notch pathway and transcription factors that affect cell fate determination.22 During the development of mouse vestibular epithelium, both Hes1 and Hes5 play important roles in regulation of hair cell differentiation. Deletion of either Hes1 or Hes5 significantly increases the number of vestibular hair cells,13 with Hes1 deletion being the more prominent of the two. On the basis of these findings in developing tissues, it is necessary to determine if the same set of molecules can also induce regeneration of hair cells in mature tissues. Despite the indication that Hes1, rather than Hes5, is the dominant molecule determining hair cell phenotype during development of the vestibular epithelium, we have used here Hes5 siRNA, because according to a later study using Hes5-GFP transgenic mice and in situ hybridization, Hes5 expression was specifically upregulated in supporting cells of the mature vestibule after a lesion,16 suggesting that it can serve as a target for inhibition that may lead to hair cell regeneration. Nevertheless, it will be also important to test whether Hes1 siRNA alone, or the combination of Hes5 and Hes1 siRNAs, will further increase production of new hair cells in the traumatized vestibular epithelium.
Hes1 and Hes5 genes are negative regulators of hair cell differentiation during development, acting by antagonizing Atoh1. As such, an additional row of inner hair cells was detected in Hes1 mutant mice, and additional outer hair cells were found in Hes5 mutant mice.23 In accordance with these observations, spontaneous hair cell regeneration that occurs in the mouse utricle following an aminoglycoside lesion is accompanied by downregulation of Hes5 and upregulation of Atoh1.18 The upregulation of Hes5 was observed as early as 3 days after the insult and lasted for at least 4 weeks, whereas Atoh1 downregulation could not be observed 2 weeks after the insult. Data on spontaneous regeneration as studied by Wang, along with the outcome of the siRNA treatment studied here together, indicate that blocking Hes5 leads to enhanced level of Atoh1 expression and also increased duration of Atoh1 upregulation. It is possible that repeated doses of siRNA or its continuous delivery by slow release will result in even longer and more efficient block of Hes5, which in turn would further enhance the extent of new hair cell formation in the lesioned utricle.
Hair cells in mammalian vestibular sensory organs can regenerate after aminoglycoside induced damage without any intervention.3,24,25 After extremely severe lesions, regeneration may be absent altogether.26 When regeneration does occur, it is incomplete and may be insufficient for restoring function. Several options have shown feasibility for enhancing the extent of hair cell regeneration with better likelihood for functional recovery. These options include gene transfection using viral vectors,27,28 blocking Notch signaling with DAPT or use of siRNA. The main advantage of the siRNA technology is absence of adverse effects, as demonstrated here by showing hair cell preservation after infusion of siRNA into an intact (nonlesioned) ear. Another advantage is the possibility for diffusing the reagent into the tissue after application in the vicinity of the cochlear fluids, such as via transtympanic delivery, an approach not shown in this study. The latter approach needs to be confirmed and further advanced in order to enhance the clinical applicability of the method.
During spontaneous hair cell regeneration in the vestibular epithelium, the number of Atoh1-positive cells started to decrease as early as 1 week after insult.18 At the time-point of 2 weeks after the insult, Myosin VIIa-positive cells consisted of 60% of the Atoh1-positive cell population. As the lesion paradigm differs between this study and the Wang report, with the present lesion being more severe, comparison of time-points is not completely valid. Additional studies comparing spontaneous regeneration with that induced by siRNA blocking Hes gene(s) are necessary.
The lesion we induced was selected for its complete or near-complete elimination of hair cells, but it had a pronounced effect on the general well being of the mice and resulted in mortalities in some cases. Because of the general toxicity, we refrained from performing the additional procedure that could have further compromised the health of the animals. Future studies that will use a more moderate lesion may be able to address functional outcomes, for which a contralateral lesion is needed. Similarly, the issue of cell proliferation as part of the regenerative process could not be addressed with the current model due to the significant general toxicity associated with BrdU. We cannot, at present, determine if new cells were added to the epithelium.
In conclusion, we have shown that Hes5 siRNA administration via the PSC is an effective way to downregulate Hes5 mRNA expression during regenerative period after hair cell damage in the vestibular system, and that downregulation of Hes5 gene induced robust Atoh1 upregulation. Along with the increase in the number of Atoh1-positive cells, the number of Myosin VIIa-positive cells also increased in the mouse utricle after streptomycin damage. Once a less invasive method for introducing the siRNA to the target tissue is available, along with a reliable method for inducing a bilateral lesion, it will be possible to expand the analysis and include functional measures of balance perception. Future experiments using transtympanic delivery of reagents for generating the lesion and of the therapeutic siRNA would enhance assessment of clinical efficacy for this therapeutic approach.
Materials and Methods
Animals. We used female CD1 mice purchased from Charles River Laboratories International (Wilmington, MA). CD1 mice were selected because they lack a vestibular hair cell pathology and display no visible balance deficits, and because the spontaneous vestibular hair cell regeneration has been documented in these mice vis-a-vis changes in expression of Notch signaling genes.18 Animals were housed at the animal care facility at the University of Michigan. All animal experiments were approved by the University of Michigan Institutional Committee on the Use and Care of Animals and were performed using acceptable veterinary standards. Mice were 3–4 weeks old at the onset of the experimental procedures (streptomycin injection surgery).
siRNA. All siRNA molecules used in this study were chemically stabilized by alternating 2′-O-methylation as described previously29 and were synthesized at BioSpring (Frankfurt, Germany). Hes5 siRNA, targeting Hes5 mRNA, was used for activity experiments in vitro and efficacy experiments in vivo. Target knockdown activity and nuclease stability of Hes5 siRNA, and its integrity, are demonstrated in Supplementary Figures S1 and S2, respectively. Hes5 siRNA has the following sense strand sequence 5′-GGGTTCTA TGATATTTGTA-3′. Fluorescent siRNA was as previously reported.30
Surgery for creating a vestibular lesion. A partial lesion in the vestibular epithelium involves more interanimal variability than a complete lesion. We therefore used an ototoxic regimen for hair cell elimination leading to a complete or near-complete hair cell loss in the utricle. Mice were anesthetized with an intraperitoneal injection of xylazine (7 mg/kg) and ketamine HCl (120 mg/kg). Ketoprofen (10 mg/kg) was applied subcutaneously before surgery. The surgical approach and procedures were similar to those described previously.18 The method used here differed from that described in the Wang paper in that the elliptical shape hole was drilled on the PSC canal using a 30G needle. A total of 1.5 µl streptomycin solution (200 mg/ml; X-gen Pharmaceuticals, Northport, NY) was injected through the opening in the PSC with a microcannula at the rate of 0.5 µl/minute. After the injection, the hole was plugged with a piece of muscle and the skin was sutured with 6-0 Prolene suture (Ethicon, Somerville, NJ).
Second surgery for Hes5 siRNA or control (vehicle) injection. One week after streptomycin injection, a second surgery for siRNA injection was performed. The surgical technique itself was identical to the first surgery other than the injected materials, although presence of fibrotic and granulation tissues around the suture site were frequently observed. In such cases, the granulation tissue was gently removed to reveal the previous site of canalostomy. As the first surgery caused PSC obstruction in some cases, it was necessary to verify that the canal was patent, by removing the seal and checking for a fluid flow. When needed, we used a 6-0 Prolene (Ethicon, Somerville, NJ) suture to gently probe the perforation and induce recanalization. Once opening, the canal was accomplished, Hes5 siRNA (or control solution) was injected into the canal. We injected 8 µg of Hes5 siRNA in 2 µl volume, prepared by adding sterile normal saline to the lyophilized siRNA. Control ears received normal saline (2 µl/ear) alone.
Histology. Tissues were fixed and dissected as described.18 Saccules were examined and the lesions observed in these organs appeared to vary between mild and moderate. As such, saccular data are not included here. We focused on utricles as these organs exhibited severe lesions with complete or nearly complete loss of hair cells. To assess the number of hair cells present in utricles, we killed mice 3 weeks following streptomycin injection and stained the utricles with antibodies specific for Myosin VIIa. Vestibular organs from other mice were obtained 2 weeks after the streptomycin treatment (1 week after the siRNA treatment) and stained with antibody to Atoh1 and Myosin VIIa. Some of the specimens were also stained with fluorescence-conjugated phalloidin to view actin which serves as a useful marker for stereocilia and apical cell junction belt, thereby depicting cytoarchitecture in the tissue.
For immunocytochemistry, tissues were dissected out and treated with 0.3% Triton X-100 in phosphate-buffered saline for 15 minutes, and then blocked using 5% normal donkey serum (Jackson Immunoresearch, West Grove, PA) in phosphate-buffered saline for 30 minutes at room temperature. Tissues were then incubated overnight at 4 °C in a primary antibody. The following primary antibodies were used: rabbit anti-Myosin VIIa (diluted 1:200; Proteus BioSciences, Ramona, CA), mouse anti-Myosin VIIa (diluted 1:200, Hybridoma Bank, The University of Iowa, IA, USA), and rabbit anti-Atoh1 (diluted 1:200, a kind gift from Dr. Jane Johnson, University of Texas Southwestern Medical Centre) (Helms and Johnson, 1998).
After rinsing in phosphate-buffered saline, samples were incubated in fluorescence-labeled secondary antibodies (Alexa Fluor 488 or 594; Invitrogen/Molecular Probes, Carlsbad, CA) at a dilution of 1:200 for 30 minutes at room temperature. For actin labeling, tissues were incubated with Alexa Fluor 488 or 594-conjugated phalloidin (diluted 1:200; Invitrogen/Molecular Probes) for 30 minutes. After staining, specimens were mounted on glass slides with Fluoro-Gel mounting media (Electron Microscopy Sciences, Hatfield, PA). For epi-fluorescence analysis and photography, we used a Leica DMRB epi-fluorescence microscope (Leica, Eaton, PA) and obtained images with a SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI) with SPOT-RT Software c3.3.
For confocal microscopy analysis, we used a Zeiss LSM 510-META Laser Scanning Confocal Microscope (Carl Zeiss, Oberkochen, Germany) with ×40 objective lens. Confocal images were cropped, labeled, and spaced with Zeiss LSM Image Browser software (Carl Zeiss) and Adobe Photoshop software (Adobe Systems, San Jose, CA).
For plastic sectioning, we used normal mice or animals killed 3 weeks following the streptomycin injection. The tissues were decalcified in 3% EDTA for 7 days, embedded in JB-4 (Electron Microscopy Sciences, Hatfield, PA) and sectioned with glass knives at 3 µm. Plastic sections were obtained from normal mice, two mice that received siRNA and two that received control vehicle.
Cell counts and data analysis. We captured multiple epi-fluorescence microscope images (using the ×40 objective lens) that covered the entire surface area of the whole mounted utricle, and then constructed a composite picture which encompassed the whole area of each utricle. We then counted Myosin VIIa-positive cells in composite pictures using tpsDig software (Version 2.12; F. James Rohlf, Ecology & Evolution, SUNY at Stony Brook, NY).
RNA isolation, reverse transcription and quantitative real-time PCR. RNA pools were prepared from tissues collected 2 weeks after the lesion surgery, a time-point which was 1 week after the second surgery (injection of either Hes5 siRNA or vehicle). For each group, vestibular tissues from six mice were dissected out in RNA later (Ambion, Austin, TX). Total RNA was isolated using TRIzol (Invitrogen) and treated with RNase-free DNase I (Invitrogen). RNA quality and quantity were assessed with Agilent Bioanalyzer 2100 (Agilent Technologies, New Castle, DE). cDNA was synthesized by SuperScript III first-strand synthesis supermix for qRT-PCR (Invitrogen).
qRT-PCR was performed in StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) with TaqMan Gene Expression Assays (Applied Biosystems) using StepOne Software v2.0 (Applied Biosystems). We examined transcripts for Hes5 and Atoh1. As the invariant control, we used mouse glyceraldehyde-3-phosphate dehydrogenase as a reference. The following assays were used: Hes5 (Assay ID: Mm00439311_g1), Atoh1 (Assay ID:Mm00476035_s1), and glyceraldehyde-3-phosphate dehydrogenase (Assay ID: Mm99999915_g1). For each gene, triplicate cDNA samples derived from each RNA pool were assayed twice (total six cDNA samples were used and averaged). To estimate changes in mRNA expression levels after the lesion, the 2−ΔΔCT method was used.18
SUPPLEMENTARY MATERIAL Figure S1. Target knockdown activity and nuclease stability of Hes5 siRNA. Figure S2. Analysis of Hes5 siRNA integrity on native 15% polyacrylamide gel following incubation at 37 °C in rat cerebrospinal fluid for the indicated periods of time: 1, 3, 8, or 24 hours. Supplementary Data.
Acknowledgments
We appreciate technical assistance and helpful comments provided by Hagit Ashush, Sharon Avkin, Lisa Beyer, Hagar Kalinski, Brandon Kappy, Igor Mett, Asaf Ori, and Donald Swiderski. This work was supported by Quark Pharmaceuticals Inc, The Williams Professorship, and NIH/NIDCD grants R01-DC001634, R01-DC007634, and P30-DC05188. Authors S.A., E.A., and E.F. are present or past employees of Quark Pharmaceuticals Inc. The work was done at Kresge Hearing Research Institute, The University of Michigan Medical School, 1150 W. Med. Cntr. Dr., Ann Arbor, Michigan 48109-5648, USA. The other authors declared no conflict of interest.
Supplementary Material
References
- Lee H, Yi HA, Chung IS., and, Lee SR. Long-term outcome of canal paresis of a vascular cause. J Neurol Neurosurg Psychiatr. 2011;82:105–109. doi: 10.1136/jnnp.2009.180497. [DOI] [PubMed] [Google Scholar]
- Ochi K., and, Ohashi T. Recovery of vestibular-evoked myogenic potential: relationship to other neural disorders in two patients with acute sensorineural hearing loss. ORL J Otorhinolaryngol Relat Spec. 2002;64:346–351. doi: 10.1159/000066083. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Izumikawa M, Beyer LA, Atkin GM., and, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K., and, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Akhtar S., and, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J.et al. (2003RNA interference targeting Fas protects mice from fulminant hepatitis Nat Med 9347–351. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Nishizaki K., and, Smith RJ. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet. 2005;14:1641–1650. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP., and, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011;2:e180. doi: 10.1038/cddis.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA.et al. (1999Math1: an essential gene for the generation of inner ear hair cells Science 2841837–1841. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B., and, Fritzsch B. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS ONE. 2012;7:e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O.et al. (2012Age-dependent in vivo conversion of mouse cochlear pillar and Deiters' cells to immature hair cells by Atoh1 ectopic expression J Neurosci 326600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R.et al. (2001Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear J Neurosci 214712–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K.et al. (2005Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit Dev Dyn 234633–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailam R, Lanford PJ, Dolinsky CM, Norton CR, Gridley T., and, Kelley MW. Expression of proneural and neurogenic genes in the embryonic mammalian vestibular system. J Neurocytol. 1999;28:809–819. doi: 10.1023/a:1007009803095. [DOI] [PubMed] [Google Scholar]
- Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O., and, Reh TA. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10:321–340. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts SA, Shoemaker CR., and, Raphael Y. Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hear Res. 2009;249:15–22. doi: 10.1016/j.heares.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, Chatterjee I, Batts SA, Wong HT, Gong TW, Gong SS.et al. (2010Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle Hear Res 26761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC., and, Stone JS. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31:15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N.et al. (2011Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats PLoS ONE 6e23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Zeh C., and, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005;93:251–266. doi: 10.1152/jn.00746.2003. [DOI] [PubMed] [Google Scholar]
- Kageyama R., and, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Zine A., and, de Ribaupierre F. Notch/Notch ligands and Math1 expression patterns in the organ of Corti of wild-type and Hes1 and Hes5 mutant mice. Hear Res. 2002;170:22–31. doi: 10.1016/s0378-5955(02)00449-5. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT., and, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Tanyeri H, Lopez I., and, Honrubia V. Histological evidence for hair cell regeneration after ototoxic cell destruction with local application of gentamicin in the chinchilla crista ampullaris. Hear Res. 1995;89:194–202. doi: 10.1016/0378-5955(95)00137-7. [DOI] [PubMed] [Google Scholar]
- Meiteles LZ., and, Raphael Y. Scar formation in the vestibular sensory epithelium after aminoglycoside toxicity. Hear Res. 1994;79:26–38. doi: 10.1016/0378-5955(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Baker K, Brough DE., and, Staecker H. Repair of the vestibular system via adenovector delivery of Atoh1: a potential treatment for balance disorders. Adv Otorhinolaryngol. 2009;66:52–63. doi: 10.1159/000218207. [DOI] [PubMed] [Google Scholar]
- Schlecker C, Praetorius M, Brough DE, Presler RG, Jr, Hsu C, Plinkert PK.et al. (2011Selective atonal gene delivery improves balance function in a mouse model of vestibular disease Gene Ther 18884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czauderna F, Fechtner M, Dames S, Aygün H, Klippel A, Pronk GJ.et al. (2003Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells Nucleic Acids Res 312705–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E.et al. (2009siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury J Am Soc Nephrol 201754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N.et al. (2011Ocular neuroprotection by siRNA targeting caspase-2 Cell Death Dis 2e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.