Abstract
Cranial defects can be caused by injury, infection, or tumor invasion. Large defects should be reconstructed to protect the brain and normalize the cerebral hemodynamics. The conventional method is to cover the defect with bone cement. Custom-made implants designed for the individual patient are now available. We report our experience with one such product in patients with large cranial defects (>7.6 cm in diameter). A CT scan with 2 mm slices and a three-dimensional reconstruction were obtained from the patient. This information was dispatched to the company and used as a template to form the implant. The cranial implant was received within four weeks. From 2005 to 2010, custom-made cranial implants were used in 13 patients with large cranial defects. In 10 of the 13 patients, secondary deep infection was the cause of the cranial defect. All the implants fitted well or very well to the defect. No infections were seen after implantation; however, one patient was reoperated on for an epidural hematoma. A custom-made cranial implant is considerably more expensive than an implant made of bone cement, but ensures that the defect is optimally covered. The use of custom-made implants is straightforward and timesaving, and they provide an excellent medical and cosmetic result.
Keywords: cranial implant, custom made, 3-D CT
Introduction
A cranial bone defect can occur after injury, infection, and tumor invasion or when autogenous bone is unsuited for replacement after a decompressive craniectomy due to brain infarction or hemorrhage. Bony defects smaller than 3 cm in diameter do not need reconstruction unless they are located in visible areas on the forehead or they cause discomfort when direct pressure is applied. Defects between 3 cm and 5 cm may need treatment primarily for cosmetic reasons because of visible and pulsating deepening. Defects larger than 5 cm represent an increased risk for brain injury from direct trauma and may disturb cerebral hemodynamics. Several studies have shown that replacement of large bone flaps normalizes intracranial pressure and the cerebrospinal fluid balance, and clinical improvement has been observed.1–3
Cranial defects have traditionally been repaired with metal plates or bone cement, sometimes enforced with stainless steel grids. The disadvantage of the conventional method is that perfect fit and curvature can be difficult to obtain in large reconstructions, especially when the defect involves the craniofacial junction. Prefabricated custom-made cranial implants in different composite materials have become available over the past few decades, as described by Karvounis et al in 1970.4 These are tailored to the specific defect of the individual patient.5–7
At the Department of Neurosurgery, Oslo University Hospital Rikshospitalet, we have begun using such an implant (Medpore biomaterial customized implant. Porex Surgical, Inc. 15 Dart Road, Newnan, GA 30265-1017 USA) to reconstruct large bony skull defects when the patient’s own bone is not available. Medpor is a porous biocompatible polyethylene material with a structure that allows rapid fibro-vascular growth and incorporation of the patient’s own tissue.8 The method and our experience with 13 surgical patients are presented here.
Methods
Thirteen patients with large cranial bone defects were fitted with custom-made cranial implants at the Department of Neurosurgery, Oslo University Hospital Rikshospitalet during the period 2005– 2010. All patients during this period needing a cranial substitute for their large bone defect were selected to participate in our study. Patients who received a cranioplasty with autologous cryopreserved bone were excluded. The cause of the cranial defect was bone infection in 10 patients. Six of the infections occurred after replacement of the patient’s own bone flap following decompressive hemicraniectomy, and four developed a secondary deep infection after craniotomy. Before undergoing cranioplasty, the patients were treated with antibiotics for at least 8 weeks. In one patient, the bone was spontaneously resorbed after primary craniotomy. One patient underwent decompressive craniectomy abroad, and was repatriated without the bone flap. In the last patient, replacement of the bone flap after hemicraniectomy was impossible due to persistent brain swelling. The operation was discontinued and the bone flap discarded due to the increased risk of infection from resterilization. None of the skull defects in our patients were due to trauma or tumor invasion.
Patients with large cranial defects were investigated using a computerized tomography (CT) scan with thin slices (<2 mm) and a three-dimensional reconstruction (Fig. 1). This information was forwarded to the company that produces the implant using measurements from the CT scan (Fig. 2).
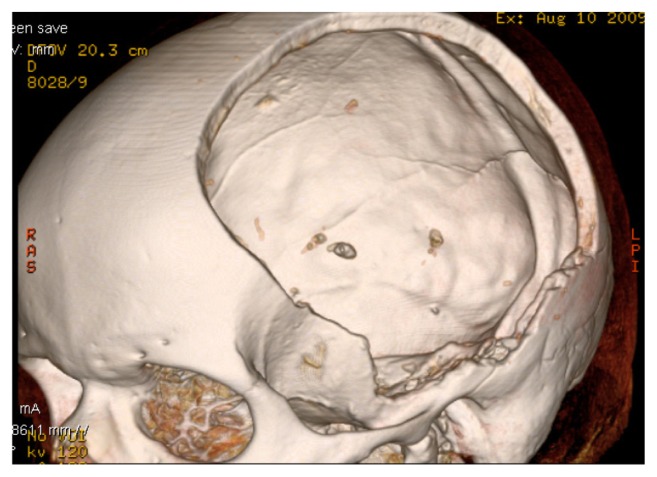
Figure 1.
Three-dimensional CT reconstruction of a large cranial defect in a 58-year-old man who underwent a decompressive craniectomy for an acute subdural hematoma and accompanying brain edema.
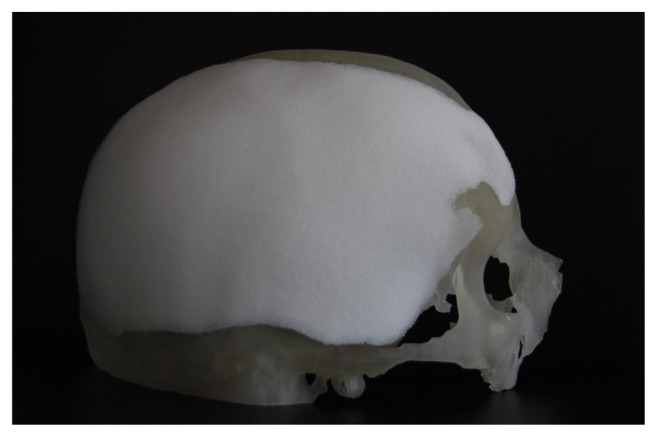
Figure 2.
CT-reconstructed cranium, with the implant covering the defect, to evaluate the shape and congruency of the implant.
All patients had a CT scan the day before planned surgery to evaluate the intracranial space before reconstruction of the skull defect. This preoperative scan was introduced as routine procedure after a cranioplasty in one of the first patients was unsuccessful due to persistent brain edema.
Cranioplasty was performed on average 5 months after the bone defect occurred (range 2.5–9 months). The scar from the primary surgery was opened and the bone margins were freed. Using a burr, six to eight small holes were made in the cranial implant for suturing the dura to the inside of the implant in order to avoid postoperative epidural hematoma. The cranial implant was fixed with three to four metal plates (CranioFix®, Braun Melsungen AG, Melsungen, Germany) or titanium miniplates (Lorentz, OrtoMedic AS, Lysaker, Norway) (Fig. 3). A subcutaneous drain was removed the day after surgery.
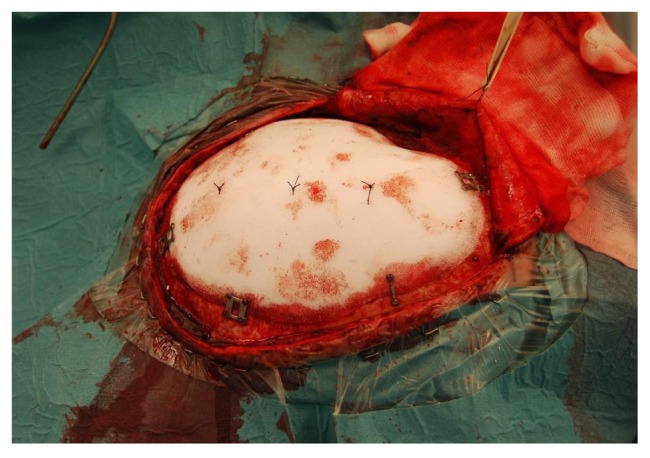
Figure 3.
The cranial implant in place and secured with miniplates (Lorenz) and three sutures attaching the dura to the implant to prevent an epidural blood clot.
The patients received prophylactic antibiotics preoperatively (cefalotin 2 g). A CT scan and follow-up after 3 months was performed for all patients (Fig. 4).
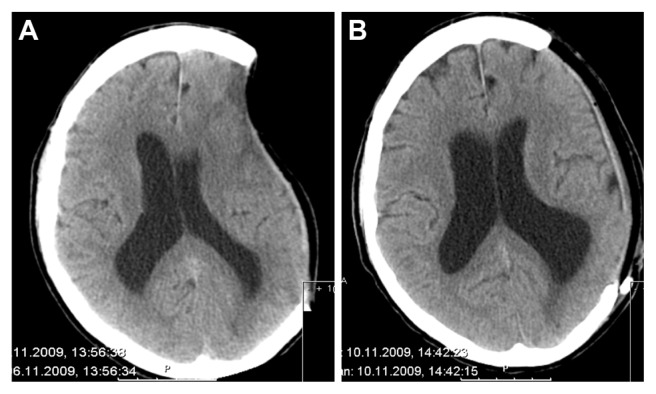
Figure 4.
A cerebral CT shows the deepening according to the bone defect (A) and the result after the cranioplasty has been performed (B).
Results
There were eight male and five female patients. The mean age was 57 years (range 29–75 years). The largest average diameter of the bone defect was 12.3 cm (range 7,6–15,3 cm) (Table 1).
Table 1.
Background data of each patient showing primary disease affecting left or right hemisphere, largest craniectomy diameter, the reason why cranioplasty with a prefabricated implant was performed, and complications to the cranioplasty procedure.
| Case | Age | Sex | Primary disease | Location | Size (mm) | Reason for cranioplasty | Complication |
|---|---|---|---|---|---|---|---|
| 1 | 65 | F | SAH | R | 138 | Infection | None |
| 2 | 29 | M | SAH | R | 149 | Infection | None |
| 3 | 45 | M | MCA inf. | L | 132 | Infection | None |
| 4 | 55 | F | SAH | L | 115 | Infection | None |
| 5 | 54 | F | SAH | L | 153 | Bone resorption | None |
| 6 | 58 | M | Meningeoma | R | 103 | Infection | Epidural hematoma |
| 7 | 76 | M | Meningeoma | L | 104 | Infection | None |
| 8 | 39 | M | MCA inf. | L | 130 | Infection | None |
| 9 | 50 | M | Meningeoma | R | 129 | Infection | None |
| 10 | 38 | M | MCA inf. | R | 136 | Own cranium unsuitable | None |
| 11 | 58 | M | ASDH | L | 137 | Own cranium missing | None |
| 12 | 34 | F | Meningeoma | L | 114 | Infection | None |
| 13 | 75 | F | Meningeoma | L | 76 | Infection | None |
Notes: Own cranium unsuitable = failure to replace the patient’s bone graft. Own cranium missing = primary operation performed abroad and the removed cranium after hemicraniectomy was not sent with the patient back to Norway.
Abbreviations: ASDH, acute subdural hematoma; f, female; l, left; M, male; MCA inf., middle cerebral artery infarction; SAH, subarachnoid hemorrhage; r, right.
Most of the patients presented with a considerable deepening corresponding to the cranial defect (Fig. 4A). In two of the 13 patients, however, the preoperative CT scan showed persistent brain swelling or hydrocephalus, and the patients were treated with lumbar drainage of cerebrospinal fluid before a cranioplasty was performed. The cranial implant fitted perfectly in 10 out of 13 patients. The remaining three implants needed small adjustments to achieve optimal fitting to the bone margins. The duration of surgery was on average 121 minutes (range 63–200 minutes).
One patient developed an epidural hematoma that was reoperated on after a few hours, and the implant was replaced. No infections were recorded after cranioplasty.
Discussion
Reconstructing large cranial bone defects has previously been challenging both technically and cosmetically. Particularly when the bone defect has involved the craniofacial junction, the cosmetic result of such a reconstruction has not always been satisfactory. The traditional method has been to cover larger defects with steel mesh secured to the bone margins using steel wires. This framework acts as a support for bone cement which is then formed to the defect, as described by Galicich and Hovind.9 For the last two decades we have used bone cement (polymethyl-methacrylate) mounted with metal plates after hardening.
Over the course of the last decade, custom-made cranial implants tailored to the individual patient have become available. The implants are made of different plastic materials, one of which is transparent. Studies have shown that customized fabricated patient-matched implants make it simpler to reconstruct the curvature of the skull.7,10–12 We ordered the implants for all our patients from the same manufacturer (Porex Surgical, Inc.).
The main cause of cranial defects in our patient population was secondary deep infection. In six patients, the infection occurred after their own bone flap was replaced following decompressive craniectomy. The bone flaps were preserved in a freezer at −80 °C. The risk of infection after craniotomy is approximately 4%,12–15 and keeping a bone flap sterilized in a freezer constitutes no major increased risk of infection after replacement (3.9%–5.1%).16–19 Although some authors have treated bacteriologically contaminated free bone flaps successfully without discarding the flaps,20,21 the usual procedure when infection occurs is to remove the original bone flap and replace it with allograft at a delayed cranioplasty.18,19,22–25
The results from the use of custom-made cranial implants have so far been encouraging. The delivery of the ordered cranial implants has been reliable, with time-to-delivery an average of 4 weeks (no more than 6 weeks). All the implants have fitted well or perfectly to the cranial defects of the patients. The procedure saved time and made it simpler to reconstruct large cranial defects. All kinds of implants and foreign objects represent an increased risk of infection. It has not been shown, however, that prefabricated cranial implants represent a higher risk of infection than replacement of autogenous bone flaps.25,26 No infection was seen in our 13 patients. It is unlikely that prefabricated implants represent an enhanced risk of infection compared to a cranioplasty using cement that requires a prolonged manipulation of foreign material in the operating field. Moreover, using a prefabricated implant means that the toxic gas produced during the mixing and hardening of cement in the operating theatre is avoided.
The only complication encountered was a postoperative epidural hematoma in one patient. The patient was reoperated on and the hematoma evacuated within a few hours. During a craniotomy the dura is sutured to the bone margins of the craniotomy in addition to the underside of the bone flap to prevent an epidural blood accumulation. In the patient who developed an epidural hematoma the dura was secured to the bone flap in three places, but the sutures were torn.
The use of implants and the resulting costs in neurosurgery have increased almost exponentially in recent years. This increase is mostly due to expensive stimulators for movement disorders and epilepsy, drug pumps for spasticity and pain, and fixating devices for the spine. The cost of a conventional cranioplasty made of cement is approximately €300, whereas the cost of a prefabricated cranial implant is around €5000, depending on the size of the implant. There is no need for additional special equipment.
By using prefabricated cranial implants, the operating time in our study seemed to be reduced in accordance with the findings of others.25,26 The savings from the reduced load on the operating staff compensates for some of the extra implant costs.26,27 The trend in neurosurgery and other surgical specialties is towards the increasing use of advanced technical equipment and implants to replace, stimulate, or fixate the normal tissue to improve function. When introducing new methods, however, we have to show moderation and prevent the rejection of well-proven methods that work satisfactorily in most situations. The challenge in the near future will be to make our voices heard and ensure that the increasing need for various implants is mirrored in budget planning.
Conclusion
Computer-aided design of prefabricated cranial implants is a time-saving method to reconstruct large cranial defects. The method ensures that the defect is optimally covered and provides a cosmetically acceptable result. The increased implant cost can, therefore, be justified in selected cases.
Footnotes
Author Contributions
Conceived and designed the experiments: JS, JBJ. Analysed the data: JS. Wrote the first draft of the manuscript: JBJ. Contributed to the writing of the manuscript: JS, JBJ. Agree with manuscript results and conclusions: JS, JBJ. Jointly developed the structure and arguments for the paper: JS, JBJ. Made critical revisions and approved final version: JS, JBJ. All authors reviewed and approved of the final manuscript.
Competing Interests
Authors disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication authors have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Short statement explaining how our paper is relevant to Journal of central nervous system diseases: Our article describes a safe method of reconstructing large cranial defects when the patients’ own skull bone can no longer be used. This is important knowledge in clinical neurosurgical and neurological practice. This study was approved by the Data Protection Official for Research at Oslo University Hospital Rikshospitalet.
Funding
Authors disclose no funding sources.
References
- 1.Kuo JR, Wang CC, Chio CC, Cheng TJ. Neurological improvement after cranioplasty—analysis by transcranial doppler ultrasonography. J Clin Neurosci. 2004;11(5):486–9. doi: 10.1016/j.jocn.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93(1):53–61. doi: 10.3171/jns.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]
- 3.Won YD, Yoo DS, Kim KT, et al. Cranioplasty effect on the cerebral hemodynamics and cardiac function. Acta Neurochir Suppl. 2008;102:15–20. doi: 10.1007/978-3-211-85578-2_3. [DOI] [PubMed] [Google Scholar]
- 4.Karvounis PC, Chiu J, Sabin H. The use of prefabricated polyethylene plate for cranioplasty. J Trauma. 1970;10(3):249–54. doi: 10.1097/00005373-197003000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann B, Sepehrnia A. Taylored implants for alloplastic cranioplasty— clinical and surgical considerations. Acta Neurochir Suppl. 2005;93:127–9. doi: 10.1007/3-211-27577-0_21. [DOI] [PubMed] [Google Scholar]
- 6.Lee SC, Wu CT, Lee ST, Chen PJ. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci. 2009;16(1):56–63. doi: 10.1016/j.jocn.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26(6):E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 8.Couldwell WT, Chen TC, Weiss MH, Fukushima T, Dougherty W. Cranioplasty with the Medpor porous polyethylene flexblock implant. Technical note. J Neurosurg. 1994;81(3):483–6. doi: 10.3171/jns.1994.81.3.0483. [DOI] [PubMed] [Google Scholar]
- 9.Galicich JH, Hovind KH. Stainless steel mesh-acrylic cranioplasty. Technical note. J Neurosurg. 1967;27(4):376–8. doi: 10.3171/jns.1967.27.4.0376. [DOI] [PubMed] [Google Scholar]
- 10.Dean D, Min KJ, Bond A. Computer aided design of large-format prefabricated cranial plates. J Craniofac Surg. 2003;14(6):819–32. doi: 10.1097/00001665-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Stoodley MA, Abbott JR, Simpson DA. Titanium cranioplasty using 3-D computer modelling of skull defects. J Clin Neurosci. 1996;3(2):149–55. doi: 10.1016/s0967-5868(96)90009-0. [DOI] [PubMed] [Google Scholar]
- 12.Joffe J, Harris M, Kahugu F, Nicoll S, Linney A, Richards R. A prospective study of computer-aided design and manufacture of titanium plate for cranioplasty and its clinical outcome. Br J Neurosurg. 1999;13(6):576–80. doi: 10.1080/02688699943088. [DOI] [PubMed] [Google Scholar]
- 13.Korinek AM. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidémiologie Hygiène et Prévention. Neurosurgery. 1997;41(5):1073–9. doi: 10.1097/00006123-199711000-00010. discussion 1079–81. [DOI] [PubMed] [Google Scholar]
- 14.Lietard C, Thébaud V, Besson G, Lejeune B. Risk factors for neurosurgical site infections: an 18-month prospective survey. J Neurosurg. 2008;109(4):729–34. doi: 10.3171/JNS/2008/109/10/0729. [DOI] [PubMed] [Google Scholar]
- 15.Korinek AM, Golmard JL, Elcheick A, et al. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. 2005;19(2):155–62. doi: 10.1080/02688690500145639. [DOI] [PubMed] [Google Scholar]
- 16.Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26(6):E9. doi: 10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 17.Grossman N, Shemesh-Jan HS, Merkin V, Gideon M, Cohen A. Deepfreeze preservation of cranial bones for future cranioplasty: nine years of experience in Soroka University Medical Center. Cell Tissue Bank. 2007;8(3):243–6. doi: 10.1007/s10561-006-9032-x. [DOI] [PubMed] [Google Scholar]
- 18.Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52(3):591–6. doi: 10.1227/01.neu.0000047891.86938.46. discussion 595–6. [DOI] [PubMed] [Google Scholar]
- 19.Inamasu J, Kuramae T, Nakatsukasa M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J Trauma. 2010;68(1):183–7. doi: 10.1097/TA.0b013e3181c45384. [DOI] [PubMed] [Google Scholar]
- 20.Widdel L, Winston KR. Pus and free bone flaps. J Neurosurg Pediatr. 2009;4(4):378–82. doi: 10.3171/2009.5.PEDS0963. [DOI] [PubMed] [Google Scholar]
- 21.Auguste KI, McDermott MW. Salvage of infected craniotomy bone flaps with the wash-in, wash-out indwelling antibiotic irrigating system. Technical note and case series of 12 patients. J Neurosurg. 2006;105(4):640–4. doi: 10.3171/jns.2006.105.4.640. [DOI] [PubMed] [Google Scholar]
- 22.Bhaskar IP, Zaw NN, Zheng M, Lee GY. Bone flap storage following craniectomy: a survey of practices in major Australian neurosurgical centres. ANZ J Surg. 2011;81(3):137–41. doi: 10.1111/j.1445-2197.2010.05584.x. [DOI] [PubMed] [Google Scholar]
- 23.Blomstedt GC. Infections in neurosurgery: a retrospective study of 1143 patients and 1517 operations. Acta Neurochir (Wien) 1985;78(3–4):81–90. doi: 10.1007/BF01808684. [DOI] [PubMed] [Google Scholar]
- 24.Dashti SR, Baharvahdat H, Spetzler RF, et al. Operative intracranial infection following craniotomy. Neurosurg Focus. 2008;24(6):E10. doi: 10.3171/FOC/2008/24/6/E10. [DOI] [PubMed] [Google Scholar]
- 25.Goh RC, Chang CN, Lin CL, Lo LJ. Customised fabricated implants after previous failed cranioplasty. J Plast Reconstr Aesthet Surg. 2010;63(9):1479–84. doi: 10.1016/j.bjps.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Balossier A, Durand A, Achim VV, Noudel R, Hurel S, Emery E. Reconstruction of the cranial vault using CAD/CAM-fabricated glass bioceramic implants. Neurochirurgie. 2011;57(1):21–7. doi: 10.1016/j.neuchi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Eppley BL, Kilgo M, Coleman JJ., 3rd Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002;109(3):864–71. doi: 10.1097/00006534-200203000-00005. [DOI] [PubMed] [Google Scholar]