Abstract
A large randomized controlled trial, The National Lung Screening Study (NLST), has demonstrated that screening with low-dose spiral computed tomography saved lives from lung cancer when compared with screening with chest radiographs. This is the first test showing efficacy in screening for lung cancer as previous trials of chest radiographs and sputum cytology failed to result in fewer deaths with screening. This review will examine the problem of lung cancer, the issues presented by screening, and the results of computed tomography (CT) studies for lung cancer screening. Now that CT screening has been shown to be effective, implementation of screening becomes the next step.
Introduction
Cancer of the lung is the leading cause of cancer death in both women and men in the United States. Although it is hard to believe these days, lung cancer was a rare malignancy in the early 1900s and what happened thereafter correlates tightly with increased cigarette consumption. In the year 2013, there is estimated to be 228,190 new cases of carcinoma of the lung, and an estimated 159,480 deaths from lung cancer in the U.S [1]. Lung cancer accounts for approximately one third of all cancer-related death in men and one quarter of all cancer death in women. Lung cancer deaths for men and women are declining; however, more people still die of lung cancer each year than of breast, colon, prostate and pancreas cancer combined. As the five-year survival for lung cancer is currently 16%, the devastation due to this single cancer type alone is alarming [1].
A humbling feature of lung cancer is that the majority of patients have evidence of spread at the time of presentation. Approximately three quarters of patients with lung cancer will present with symptoms, and the majority of these have an advanced stage of tumor at the time of diagnosis. Early stage detection is infrequent and usually serendipitous. Surgery is often curative for early stage non-small cell carcinoma; 5-year survival following removal of Stage IA/B lung cancer is approximately 70% and for Stage IIA/B disease is in the range of 50% [2].
Given this scenario, early detection through screening clearly makes sense but, until recently, no test had been shown to reduce deaths from lung cancer. Studies from the late 70s and early 80s screened with chest radiographs and sputum cytology and failed to show reduction in mortality [3-6]. Recently released results from the Prostate Lung Colon and Ovary (PLCO) study have shown once and for all that chest radiograph screening does not result in lives saved from lung cancer [7]. CT has been in clinical use since the mid 70s but it wasn's until technical advances allowing for rapid scanning at low dose that CT screening became feasible (figure 1). Single arm (no comparison or control group) studies from the late 90s and early 2000s reported promising results with CT detection of more cancers, more early stage cancers and with a high resectability rate [8-15]. By their design, these studies were not able to prove mortality reduction. However, the encouraging results from these studies led to the pursuit of a large randomized control trial, the NLST, which compared screening with low-dose CT scanning with chest radiograph and has shown that CT screening results in fewer deaths from lung cancer [16].
Figure 1. Early stage detection.
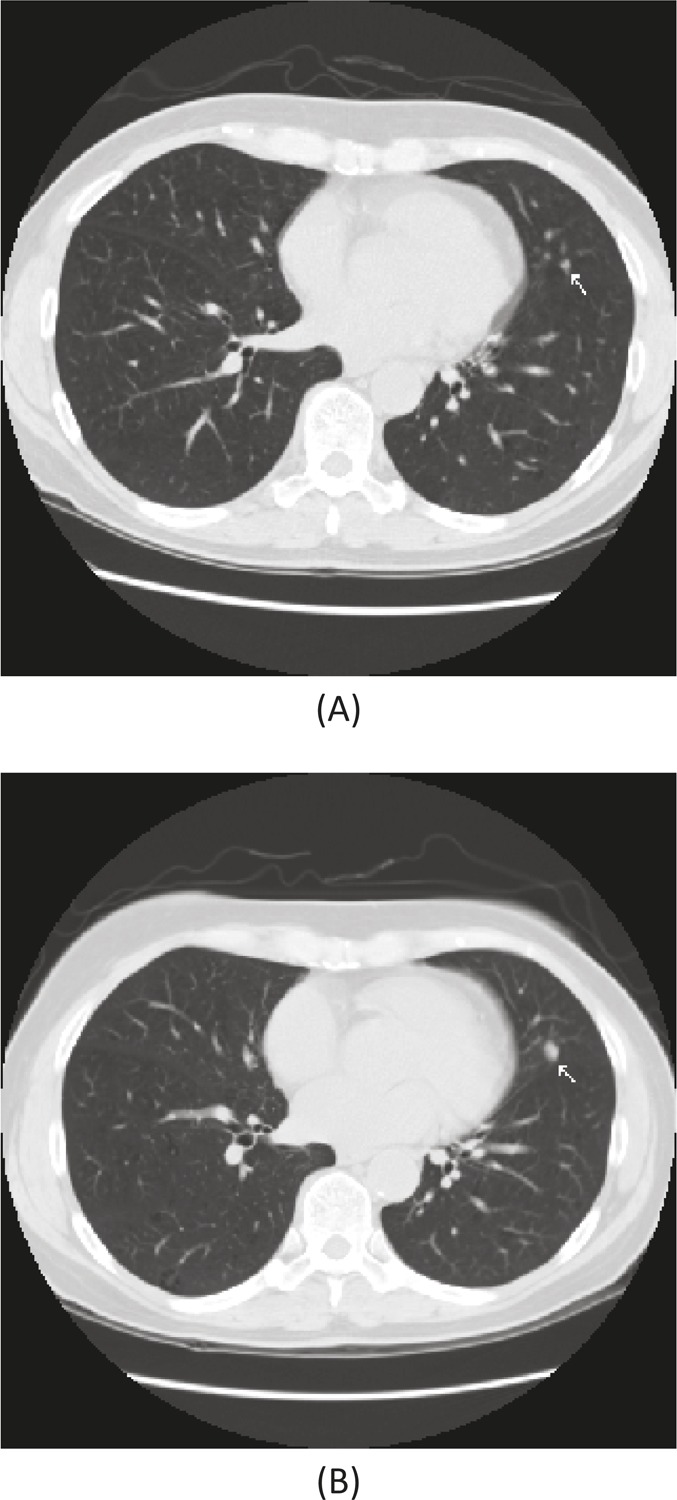
A 62 year old woman, former smoker with a 40-pack year history had a low dose screening CT showing a 3 mm nodule (A) in the left lung (lingula). At one-year follow-up, the nodule had grown (B) and at surgical resection was a 6 mm adenocarcinoma. She remains without evidence of disease seven years after removal.
In short, finding lung cancer early through screening leads to lives saved.
Current screening studies
The NLST is by far the largest of the randomized controlled trials evaluating CT screening for lung cancer and included over 53,000 participants who were current or former heavy smokers (the equivalent of a pack per day for 30 years) ages 55 to 74 [16]. The study was designed to have 90% power to detect a 21% reduction in mortality [17]. Participants were randomized between low-dose spiral CT and chest x-ray at baseline, year 1, and year 2 and then followed for a median of over 6.5 years. A positive screen result in the CT arm of the NLST was defined as the finding of a non-calcified nodule of at least 4 mm; 27% had a positive baseline screen, and 96% of these were false positive – i.e. did not show lung cancer. There were 649 cancers that were detected using CT and 367 diagnosed during follow-up post screening in the CT arm. In the chest radiograph arm, 279 cancers were detected, and 525 diagnosed during follow-up post screening. Within the CT arm, 63% of lung cancers diagnosed from a positive screening test were Stage I; only 29.8% were Stages III or IV. Among cancers detected by chest radiograph, 47.6% were stage I, and 43.2% were stage III or IV. This reduction in advanced cancers in the CT arm demonstrated a shift in stage at diagnosis from advanced disease to early stage; this is what one would anticipate seeing if the screening modality was effective. At the end of the follow-up period, there were 354 lung cancer deaths among those in the CT arm versus 442 deaths among those in the chest radiograph arm, or in other words a 20.0% reduction with CT screening [16]. In the NLST, the number of high risk participants that were needed to screen with CT to save one life from lung cancer (“number need to screen”) was 320. This compares favorably to the number needed to screen with mammography for breast cancer of 781 and with fecal occult blood testing for colorectal cancer of 1,250 [18].
There are several other smaller randomized CT screening trials in Europe that are underway (table 1): the largest of these is the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial with 7,557 participants randomized to receive CT screening [19]; the ITALUNG study [20] has 3,206 participants randomized to low-dose CT versus no screening; the Depiscan study [21] randomized 621 participants between CT and chest radiograph, and the Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays (DANTE) trial [22] enrolled 2,472 participants who were randomized between CT versus no CT. No information has been published regarding mortality in these studies. The Danish Lung Cancer Screening Trial (DLCST), which randomized 4,104 participants to CT versus no screening, reported that after five rounds of screening there were 15 lung cancer deaths in the CT screen group versus 11 in the no-screen group [23]. In other words, despite CT finding more cancers and more early stage cancers, there were similar numbers of advanced cancers in both groups and there was no mortality reduction with CT screening. So far, the NLST study is the only randomized controlled trial that has shown mortality reduction. Why didn's the DLSCT study show mortality reduction? The answer is uncertain. Compared to the NLST, the participants were younger and had fewer pack years smoking (20 pack-years minimum compared to 30 pack-years for NLST) and so were at lower risk for lung cancer than the NLST participants. The DLSCT is also a much smaller study and would not have had the statistical power to show a similar sized reduction in mortality as the NLST.
Table 1. Randomized, controlled CT screening studies.
| Study | Screen modality: no. participants | Noncalcified nodules (baseline) | Total no. of cancers detected | Surgical stage I | Deaths from lung cancer | Mortality reduction |
|---|---|---|---|---|---|---|
| DANTE [22] | CT: 1276 | 27.5% | 58 (4.5%) | 71% | 11 | NR |
| No screen: 1196 | nr | 25 (2.1%) | 52% | 10 | ||
| Depiscan [21] | CT: 336 | 45.2% | 8 (2.4%) | 37.5% | nr | NR |
| CXR: 285 | 7.4% | 1 (0.4%) | 100% | nr | ||
| DLSCT [23] | CT: 2052 | 27.3% | 69 (3.4%) | 68% | 15 | None |
| No screen: 2052 | nr | 24 (1.2%) | 21 | 11 | ||
| ITALUNG [20] | CT: 1406 | 30%* | 21 (1.5%) | 47.6% | nr | NR |
| No screen: 1593 | nr | nr | nr | nr | ||
| NELSON [19] | CT: 7557 | 50.5% | 70 (0.9%) | 63.9% | nr | NR |
| No screen: nr | nr | nr | nr | nr | ||
| NLST [16] | CT: 26722 | 27.3** | 1060 | 50% | 356 | 20.0% |
| CXR: 26732 | 9.2 | 941 | 30.7% | 443 |
NR: not reported
* reported as positive if a nodule ≥ 5 mm was detected
** reported as positive if a nodule ≥ 4 mm was detected
Biases in screening
Use of a screening test introduces biases such as lead time, length time, and overdiagnosis, which are inherent in screening and lead to apparent improvement in survival even when there may not be any. The bias effect on survival is what necessitates a randomized controlled trial to demonstrate a change in mortality rather than just survival [24]. Lead-time bias describes the survival benefit of detecting a cancer earlier than it would have been in the absence of screening even if the natural history of disease is not changed. The measure of time between detection and death is increased, but true survival, time from disease onset to eventual death, is something we can's actually measure. We can measure time from diagnosis to death, and early detection improves apparent survival, even if mortality remains unchanged. The patients with cancer would appear to live longer simply because the disease was detected earlier, but this is not necessarily an indication of a change in the natural history. Lengthening apparent survival is desired, yet it is not sufficient proof of benefit from screening.
Applying a screening tool such as CT at specified intervals has a higher likelihood of detecting a cancer with a more indolent course than one with a more rapid course. Length-time bias is the apparent improvement in survival when that improvement is due to selective detection of cancers with a less aggressive course. Screening with CT is more likely to detect peripheral adenocarcinomas and these have a more favorable outcome than other types of lung cancer such as centrally located small cell and squamous lung cancers, which have a poorer prognosis.
Overdiagnosis can result from the identification of indolent lung cancers that would not have a fatal outcome if left undetected. Though a true cancer, an overdiagnosis cancer is one that the patient would have died with rather than died from – i.e. the patient would have died of a cause other than lung cancer had the lung cancer gone undetected. In such cases, measured survival improves but the natural history of these cancers does not need to be altered with detection and treatment.
Recognizing these biases in screening is important; measured survival can be expected to improve compared with no screening even if earlier detection and intervention did not alter the course of the disease. Mortality reduction, rather than survival improvement, is therefore the best measure of a screening tool's effectiveness for a lethal disease and this can be seen in the NLST results.
Problems with CT screening
Problems identified with CT screening include false positive scans, benign nodule resections, over diagnosis and the effect of radiation – not to mention the cost. For the most part, now that low dose CT has been shown to reduce mortality, the problems of CT screening are recognized as manageable, with the possible exception of cost.
If one considers any non-calcified nodule as a potential lung cancer, a positive result will be found in approximately 50% of nodules identified using CT screening with 98% of those being false positives. In the Mayo CT study, baseline scanning revealed one or more noncalcified nodules in 51% of the participants on the initial scan and, after 4 annual scans, 74% of the participants had one or more nodules [12]. In the Early Lung Cancer Action Project (ELCAP) study, 23% of the participants had a nodule at baseline [10]. The ELCAP study used a single detector scanner with 10 mm collimation (slice thickness) and the Mayo study used a 5 mm slice width. Data from these studies and others indicate that the frequency of nodule detection is a function of slice thickness on CT. Narrower slice thickness detects more nodules and, hopefully earlier detection of lung cancers, yet this also results in the identification of more non-cancerous nodules. The NLST required nodules to be 4 mm in diameter or greater to be considered ‘positive.’ At baseline the nodule detection rate was 27%, yet there was still a 96% false positive rate – in other words, only 4% of these were cancers [16].
Although the finding of an abnormality (eventually found to be non-cancerous) may cause unnecessary concern for a period of time, a greater concern with false positives is the use of biopsy and surgery used to identify them. In the single arm CT screening studies, 18% to 27% of the surgical procedures were performed for benign histology [13-15,25]. Nodule evaluation to identify malignancy is imperfect; however, the aim is to keep resections for a benign process to a minimum. The literature suggests the screening centers have a much lower rate of benign nodule resections than usual practice; this is a concern if screening is to be done for the general population at centers with less experience.
Overdiagnosis is recognized as an issue within breast cancer and particularly prostate cancer screening, and is a problem within lung cancer screening as well. Most eventually lethal lung cancers have doubling times of 50 to 150 days, yet CT screening studies also identify a subset of tumors with long tumor-doubling times of 400 days or more. These slow-growing cancers tend to appear as non-solid – either pure glass opacities or part solid nodules on CT (figure 2). One CT screening study from Japan reported tumor doubling times ranging from 662 to 1,486 days with a mean of 880 days among malignancies presenting as pure glass opacities [26]. In the Mayo CT screening study, 13 of 48 (27%) detected cancers exhibited doubling times that were over 400 days, suggesting these may have been overdiagnosis cancers [27]. However, overall, enough lives are saved through CT screening by detecting fast-growing cancers for the benefit to outweigh the risk of overdiagnosis.
Figure 2. Probable overdiagnosis.
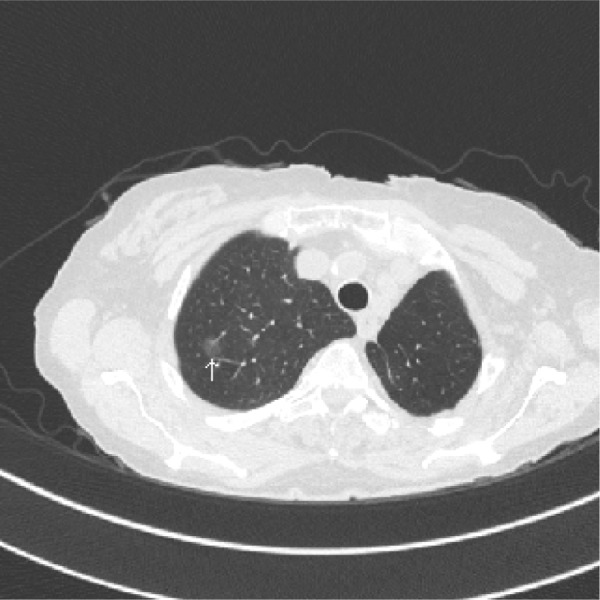
An 80 year old woman, former smoker, with a history of a left upper lobectomy for a stage I adenocarcinoma, has an 11 mm (arrow) ground glass opacity in the right upper lobe. The nodule has not changed in over 3-years and is currently being followed with annual CT. If this is a cancer it is likely to be an adenocarcinoma in situ and may represent and overdiagnosis cancer.
CT imaging involves radiation and, with it, the chance of actually inducing lung cancer, but estimates of the risk of low-dose CT are low even if it were performed annually over decades. The effective dose of radiation absorption is expressed in milliseverts (mSv). The average effective dose for a standard CT of the chest is about 7 mSv; a low-dose scan is about 1.5 mSv and this is about half of the natural ambient radiation exposure of approximately 3 mSv per year [28,29]. Authors of the NLST estimated that the radiation risk from CT screening 55-year old smokers results in 1 to 3 lung cancer deaths per 10,000 people screened and 0.3 new breast cancers per 10,000 females [16]. This potential harm from screening highlights the importance of having proven mortality reduction through a randomized controlled trial.
Recent estimates of the cost of CT screening in high-risk individuals have varied from as low as $19,000 to about $126,000 per life-year saved, reflecting differing modeling and assumptions [30,31]. However, neither of these estimates used data from the NLST, which is forthcoming and will have a significant impact on the acceptability of screening from a cost perspective.
Conclusions and future directions
Now that CT screening has been shown to save lives, implementation of screening appears appropriate, yet this must be done safely and effectively. The NLST selected a high-risk group to enhance the likelihood of developing lung cancer during the short period of the study to prove or disprove benefit from screening. In this sense, the participants were chosen to maximize the effect seen in the study rather than to define, once and for all, who would benefit from screening. In the transition of screening from the realm of research to clinical care, the simplest answer to the question of “who should be screened?” is to use the NLST criteria for enrollment. The answer should be more complicated than that. The participants for the NLST were not selected on the basis of airway obstruction and presence of chronic obstructive pulmonary disease (COPD), which increases the risk for lung cancer 4-6 times. In a high risk cohort, presence of COPD and CT evidence of emphysema were independent and additive predictors for those who developed lung cancer [32]. Risk due to a first degree relative with lung cancer was also not taken into account by the NLST. Screening patients at comparable or higher risk of lung cancer, as those who were enrolled in the NLST, would appear appropriate and has been recommended in the National Comprehensive Cancer Network (NCCN) guidelines [33]. The American College of Chest Physicians and the American Society of Clinical Oncology recommendation is that “for smokers and former smokers aged 55 to 74 years who have smoked for 30 pack-years or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with low-dose CT should be offered”, but screening is not recommended for those who do not fit these criteria [34]. The American Society of Thoracic Surgery Guideline recommends annual screening for those who fit the NLST criteria and that “annual low-dose computed tomography lung cancer screening should be offered starting at age 50 years with a 20 pack-year history if there is an additional cumulative risk of developing lung cancer of 5% or greater over the following 5 years” [35]. The American Lung Association recommends screening for those who fit the NLST criteria as does the American Cancer Society [36,37].
In addition to CT screening, methods of assessing blood, sputum, mucosa, and breath analysis may provide means to help identify which patients are at highest risk, and which of those with CT abnormalities have lung cancer, or may provide an alternative means to screen other than CT. Research is underway to evaluate biomarkers in airway epithelial cells, sputum, blood, breath, and urine for early diagnosis and prediction of high risk. For example, using visually normal airway epithelial cells, a gene biomarker was able to distinguish smokers with and without lung cancer with an accuracy of 83% [38]. Autoantibodies to tumor antigens have been reported to precede the diagnosis of cancer by as much as 3-5 years, and a panel of autoantibodies has been shown to detect lung cancer with a sensitivity of around 40% and specificity of 90% [39]. Exhaled breath contains volatile organic compounds that can be measured and use of a colorimetric sensor array to identify these compounds was able to predict lung cancer from normals with an overall accuracy of 81%. [40].
Proving that we can save lives with CT lung cancer screening was just the first step, now we need to better identify who needs to be screened, when to start, how often and how to better separate the benign from the malignant abnormality seen on CT. The goal of a CT screening program is to detect early lung cancer and facilitate curative treatment; however, the overall goal of medicine should be to reduce deaths from lung cancer. CT screening may be a part of this effort, yet we must remain mindful that primary prevention though smoking cessation or never starting is the best means to accomplish this goal. Whether or not lung cancer screening becomes widely applied in the US population at risk will likely be heavily influenced by the cost and who pays. Currently, CT screening is being covered by WellPoint and several state Blue Cross Blue Shield Association members. In the NLST screening, 320 participants resulted in one life saved from lung cancer [16]. Approximately 1 in 6 life-long smokers will die from lung cancer, and death from premature heart disease is about 1 in 2 [41,42]. The quality adjusted life year for smoking cessation is less than $1500 [43]. If the cost of CT screening high-risk, current smokers is prohibitive, then it may be appropriate, in regard of cost effectiveness, to require smoking cessation prior to the pursuit of CT screening. The math is pretty simple; smoking cessation and avoidance are the best means to save lives.
Disclosures
The author has nothing to disclose.
Abbreviations
- CT
computer tomography
- ELCAP
Early Lung Cancer Action Project
- NLST
The National Lung Screening Study
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/5/12
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989834
- 3.Frost JK, Ball WC, Levin ML, Tockman MS, Baker RR, Carter D, Eggleston JC, Erozan YS, Gupta PK, Khouri NF. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130:549–54. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 4.Flehinger BJ, Melamed MR, Zaman MB, Heelan RT, Perchick WB, Martini N. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan-Kettering study. Am Rev Respir Dis. 1984;130:555–60. doi: 10.1164/arrd.1984.130.4.555. [DOI] [PubMed] [Google Scholar]
- 5.Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR, Bernatz PE, Payne WS, Pairolero PC, Bergstralh EJ. Screening for lung cancer. A critique of the Mayo Lung Project. Cancer. 1991;67:1155–64. doi: 10.1002/1097-0142(19910215)67:4+<1155::AID-CNCR2820671509>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Flehinger BJ, Melamed MR. Current status of screening for lung cancer. Chest Surg Clin N Am. 1994;4:1–15. [PubMed] [Google Scholar]
- 7.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, Crawford ED, Fouad MN, Isaacs C, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Ragard LR, Rathmell JM, Riley TL, Wright P, Caparaso N, Hu P, Izmirlian G, Pinsky PF, Prorok PC, Kramer BS, Miller AB, Gohagan JK, Berg CD. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–73. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/13827959
- 8.Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasu K, Moriyama N. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology. 1996;201:798–802. doi: 10.1148/radiology.201.3.8939234. [DOI] [PubMed] [Google Scholar]
- 9.Sone S, Takashima S, Li F, Yang Z, Honda T, Maruyama Y, Hasegawa M, Yamanda T, Kubo K, Hanamura K, Asakura K. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351:1242–5. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 10.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, Smith JP. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989835
- 11.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby D, Pasmantier M, Koizumi J, Altorki N, Smith JP. Early lung cancer action project: a summary of the findings on baseline screening. Oncologist. 2001;6:147–52. doi: 10.1634/theoncologist.6-2-147. [DOI] [PubMed] [Google Scholar]
- 12.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, Sykes A, Aughenbaugh GL, Bungum AO, Allen KL. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 13.Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, Heindel W. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222:773–81. doi: 10.1148/radiol.2223010490. [DOI] [PubMed] [Google Scholar]
- 14.Pastorino U, Bellomi M, Landoni C, Fiori E de, Arnaldi P, Picchio M, Pelosi G, Boyle P, Fazio F. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–7. doi: 10.1016/S0140-6736(03)14188-8. [DOI] [PubMed] [Google Scholar]
- 15.Sobue T, Moriyama N, Kaneko M, Kusumoto M, Kobayashi T, Tsuchiya R, Kakinuma R, Ohmatsu H, Nagai K, Nishiyama H, Matsui E, Eguchi K. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol. 2002;20:911–20. doi: 10.1200/JCO.20.4.911. [DOI] [PubMed] [Google Scholar]
- 16.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717989836
- 17.National Lung Screening Trial Research Team. Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, Gareen IF, Gatsonis C, Goldin J, Gohagan JK, Hillman B, Jaffe C, Kramer BS, Lynch D, Marcus PM, Schnall M, Sullivan DC, Sullivan D, Zylak CJ. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717989837
- 18.Richardson A. Screening and the number needed to treat. J Med Screen. 2001;8:125–7. doi: 10.1136/jms.8.3.125. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989841
- 19.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, van Iersel CA, van den Bergh KAM, van Westeinde S 't, van der Aalst C, Thunnissen E, Xu DM, Wang Y, Zhao Y, Gietema HA, Hoop B de, Groen HJM, Bock GH de, van Ooijen P, Weenink C, Verschakelen J, Lammers JJ, Timens W, Willebrand D, Vink A, Mali W, Koning HJ de. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989844
- 20.Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, Spinelli C, Falaschi F, Grazzini M, Innocenti F, Ronchi C, Paci E. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer. 2009;64:34–40. doi: 10.1016/j.lungcan.2008.07.003. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989846
- 21.Blanchon T, Bréchot J, Grenier PA, Ferretti GR, Lemarié E, Milleron B, Chagué D, Laurent F, Martinet Y, Beigelman-Aubry C, Blanchon F, Revel M, Friard S, Rémy-Jardin M, Vasile M, Santelmo N, Lecalier A, Lefébure P, Moro-Sibilot D, Breton J, Carette M, Brambilla C, Fournel F, Kieffer A, Frija G, Flahault A. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR) Lung Cancer. 2007;58:50–8. doi: 10.1016/j.lungcan.2007.05.009. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989848
- 22.Infante M, Chiesa G, Solomon D, Morenghi E, Passera E, Lutman FR, Bottoni E, Cariboni U, Errico V, Voulaz E, Ferraroli G, Testori A, Inzirillo F, Chiarenza M, Roncalli M, Cavuto S, Chiti A, Alloisio M, Ravasi G. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. J Thorac Oncol. 2011;6:327–35. doi: 10.1097/JTO.0b013e318200f523. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989850
- 23.Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, Døssing M, Hansen H, Kofoed KF, Larsen KR, Mortensen J, Rasmussen JF, Seersholm N, Skov BG, Thorsen H, Tønnesen P, Pedersen JH. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296–301. doi: 10.1136/thoraxjnl-2011-200736. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989854
- 24.Strauss GM. Screening for lung cancer: An evidence-based synthesis. Surg Oncol Clin N Am. 1999;8:747–74. viii. [PubMed] [Google Scholar]; http://f1000.com/prime/717989856
- 25.Crestanello JA, Allen MS, Jett JR, Cassivi SD, Nichols FC, Swensen SJ, Deschamps C, Pairolero PC. Thoracic surgical operations in patients enrolled in a computed tomographic screening trial. J Thorac Cardiovasc Surg. 2004;128:254–9. doi: 10.1016/j.jtcvs.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Nakata H, Watanabe H, Nakamura K, Kasai T, Hashimoto H, Yasumoto K, Kido M. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol. 2000;174:763–8. doi: 10.2214/ajr.174.3.1740763. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989858
- 27.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD, Mandrekar JN. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242:555–62. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1069821
- 28.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989859
- 29.McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009;47:27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyenson BS, Sander MS, Jiang Y, Kahn H, Mulshine JL. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff (Millwood) 2012;31:770–9. doi: 10.1377/hlthaff.2011.0814. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717965016
- 31.McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, Johnson BE, Weeks JC, Gazelle GS. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–8. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, Sciurba FC. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–44. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1123226
- 33.www.nccn.org NCCN Guidelines.
- 34.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, Smith-Bindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–29. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717447884
- 35.Jaklitsch MT, Jacobson FL, Austin JHM, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, Swanson SJ, Travis WD, Sugarbaker DJ. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–8. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717989862
- 36.http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/
- 37.Wender R, Fontham ETH, Barrera E, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Scott Gazelle G, Kelsey DK, Lamonte SJ, Michaelson JS, Oeffinger KC, Shih YT, Sullivan DC, Travis W, Walter L, Wolf AMD, Brawley OW, Smith RA. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:106–17. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717990370
- 38.Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas Y, Calner P, Sebastiani P, Sridhar S, Beamis J, Lamb C, Anderson T, Gerry N, Keane J, Lenburg ME, Brody JS. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13:361–6. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1083784
- 39.Lam S, Boyle P, Healey GF, Maddison P, Peek L, Murray A, Chapman CJ, Allen J, Wood WC, Sewell HF, Robertson JFR. EarlyCDT-Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila) 2011;4:1126–34. doi: 10.1158/1940-6207.CAPR-10-0328. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717990374
- 40.Mazzone PJ, Wang X, Xu Y, Mekhail T, Beukemann MC, Na J, Kemling JW, Suslick KS, Sasidhar M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J Thorac Oncol. 2012;7:137–42. doi: 10.1097/JTO.0b013e318233d80f. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717990368
- 41.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717990369
- 42.Gellert C, Schöttker B, Brenner H. Smoking and All-Cause Mortality in Older People: Systematic Review and Meta-analysis Smoking and All-Cause Mortality in Older People. Arch Intern Med. 2012;172:837–44. doi: 10.1001/archinternmed.2012.1397. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717447809
- 43.Javitz HS, Zbikowski SM, Deprey M, McAfee TA, McClure JB, Richards J, Catz SL, Jack LM, Swan GE. Cost-effectiveness of varenicline and three different behavioral treatment formats for smoking cessation. Transl Behav Med. 2011;1:182–90. doi: 10.1007/s13142-010-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


