Abstract
We prospectively evaluated the efficacy and safety of imatinib plus hydroxyurea in patients with progressive/recurrent meningioma. A total of 21 patients with progressive/recurrent meningioma were enrolled in this dual center, single-arm, phase II trial. All patients received 500 mg of hydroxyurea twice a day. Imatinib was administered at 400 mg/day for patients not on CYP3A enzyme inducing anti-epileptic drugs (EIAEDs) and at 500 mg twice a day for patients on EIAEDs. The primary endpoint was progression-free survival at 6 months (PFS-6) and secondary endpoints were safety, radiographic response rate, and overall survival (OS). Best radiographic response was stable disease and was observed in 14 patients (67%). PFS-6 for all patients, those with grade I tumors (n = 8) and those with grade II or III tumors (n = 13) was 61.9, 87.5 and 46.2%, respectively. Patients with grade II or III tumors had poorer PFS and OS than those with grade I tumors, (P = 0.025 and P = 0.018) respectively. The only grade 3 or greater adverse event occurring in ≥10% of patients was anemia (10%). Imatinib plus hydroxyurea is well tolerated among patients with meningioma but has modest anti-tumor activity for this indication.
Keywords: Meningioma, Platelet-derived growth factor receptor, Imatinib mesylate, Hydroxyurea
Introduction
Meningiomas, account for 34% of primary brain tumors and are estimated to occur with a frequency of 12.8/1,00,000 [1]. Meningiomas are classified by histopathologic grade into three main subtypes [2]. The majority of meningiomas are benign (World Health Organization [WHO] grade I). This subtype typically grows indolently and is often effectively treated with surgery. However, a subset of grade I meningiomas can transform to high-grade upon recurrence [3]. High-grade subtypes are either atypical (WHO grade II) or anaplastic (WHO grade III). In addition, the 2007 WHO Classification of central nervous system (CNS) tumors specifies that any grade I meningioma exhibiting brain invasion should be reclassified as grade II due to recurrence and mortality rates that are similar to those of atypical meningiomas [4]. Taking this into account, 20–30% of meningiomas are now classified as grade II [5, 6], while grade III lesions are rare and account for only 1–2% of all meningiomas [7]. High-grade meningiomas frequently recur despite surgery and radiotherapy and are therefore associated with poor overall survival (OS) [8].
Effective therapies for meningiomas that recur after radiotherapy have not been well identified. Protracted daily temozolomide was inactive in a small series of recurrent patients after surgery and radiotherapy [9]. Other small single-arm series have reported low rates of radiographic response and variable progression-free survival (PFS) among recurrent/progressive patients treated with systemic cytotoxic and hormonal agents including irinotecan [10], octreotide acetate [11, 12], interferon-α [13], mifepristone [14, 15] and an epidermal growth factor receptor inhibitor [16]. Hydroxyurea, an oral ribonucleoside reductase inhibitor, has been evaluated in several small series and has demonstrated radiographic response rates of approximately 6% and median times to progression of 44–176 weeks [17–24].
An attractive alternative therapeutic approach is the inhibition of key mediators of dysregulated cell signaling that underlie meningioma cell proliferation, survival, invasion and angiogenesis [2, 25]. Meningiomas frequently express platelet derived growth factor receptor (PDGFR)- α and PDGFR-β, independent of histologic grade, while a percentage of meningiomas also express platelet derived growth factor (PDGF), suggesting possible autocrine or paracrine loops of signaling [26–34]. PDGFR activation has been linked with aberrant cell signaling through either the phosphatidylinositol-3-kinase (PI3K) AKT or rasmitogen activated protein kinase (ras-MAPK) pathways [35, 36]. We conducted this phase II study to evaluate the anti-tumor activity of imatinib mesylate (Gleevec; Novartis Pharmaceuticals, East Hanover, New Jersey), an oral inhibitor of PDGFR-α and PDGFR-β, as well as other kinases including Bcr-Abl, c-KIT and c-Fms in combination with hydroxyurea. At the time this study was designed and implemented, no data regarding the anti-tumor activity of imatinib or other molecular targeted therapies had been described for patients with recurrent/progressive meningioma. Our results demonstrate that imatinib plus hydroxy-urea is well tolerated, but is associated with minimal anti-tumor activity.
Patients and methods
Study design and treatment
We conducted an open-label, dual center, single-arm, phase II trial of continuous daily imatinib and hydroxyurea for patients with recurrent/progressive meningioma. The primary end point was progression-free survival at 6 months (PFS-6) while secondary end points were OS, PFS, objective radiographic response rate and safety. The dose of imatinib differed to account for the effect of CYP3A-inducing anti-epileptic drugs (EIAEDs) on imatinib metabolism [37, 38]: patients not on EIAEDs received 400 mg once a day, while those on EIAEDs received 500 mg twice a day. All patients received 500 mg of hydroxyurea twice a day. Medically appropriate efforts were used to maintain study-specific EIAED exposure for all patients, however, imatinib dosing was changed in accordance with treatment parameters outlined above for patients who required a change in EIAED use.
Patients were assessed every 8 weeks and remained on study unless they withdrew consent, developed tumor progression or unacceptable toxicity.
Patient eligibility
Adult patients (≥18 years of age) were required to have histologically confirmed meningioma that was radiographically progressive or recurrent after prior surgical resection. Measurable disease was required for all patients. Satisfactory hematologic (hemoglobin ≥10 g/dL, absolute neutrophil count (ANC) >1,500 cells/L, platelets >1,00,0 00 cells/L), biochemical (serum creatinine\1.5 mg/dL, BUN\25 mg/dL; AST and bilirubin\1.5× upper limit of normal) and performance status (Karnofsky ≥60%) parameters were also required. Patients were required to be at least 2 weeks from prior surgery and at least 4 weeks from prior radiation or chemotherapy. All patients provided informed consent.
Key exclusion criteria included: prior imatinib or PDGFR-directed therapy; prior progressive disease or ≥3 toxicity to hydroxyurea treatment; grade ≥2 peripheral edema; pulmonary, pericardial or peritoneal effusions of any grade; patients with an excessive risk of an intracranial hemorrhage, or who had evidence of hemorrhage on pre-treatment MRI unless it was stable and grade 1; and concurrent warfarin administration. There was no limit on the number of prior therapies or episodes of progressive disease.
Assessments
Disease status was assessed using the Macdonald criteria [39], that incorporated neurologic, corticosteroid and radiologic changes.
Safety assessments included weekly complete blood counts and monthly chemistry profiles. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Treatment modifications
Initiation of each 28-day treatment cycle required an ANC ≥ 1,000/mm3, a platelet count of ≥1,00,000/mm3, SGOT ≤ 2.5 times the upper institutional norm, and a creatinine ≤1.5 times the upper institutional normal. Dose modifications were implemented for unacceptable toxicity defined as grade 4 neutropenia, grade ≥3 thrombocytopenia, grade ≥3 non-hematologic toxicity not attributable to underlying disease, concurrent medication or comorbid event, or any toxicity requiring >2 weeks to resolve to grade 1 or retreatment criteria. Hydroxyurea was decreased to 500 mg once a day for the first episode of unacceptable hematologic toxicity. Thereafter, additional episodes of unacceptable hematologic toxicity were treated with a decrease in imatinib by 100 mg/day for patients not on EIAEDs and 200 mg/day for patients on EIAEDs. For other unacceptable toxicities, the hydroxyurea dose was decreased by 250 mg/day and the dose of imatinib was decreased by 100 mg/day for patients not on EIAEDs and 200 mg/day for patients on EIAEDs.
Statistical considerations
The primary endpoint of the current study was PFS-6. Secondary endpoints included time to progression, OS, objective response rate as well as safety and tolerability. Given the lack of an established treatment option for patients with recurrent/progressive meningioma, sample size calculations were empirically set. Specifically, the null hypothesis indicated that PFS-6 is ≤15%. In order to justify further evaluation of the study regimen, we specified that imatinib plus hydroxyurea would need to increase PFS-6 to ≥40%. A two-stage design with 21 patients (10 patients in first-stage and 11 patients in second stage) was chosen to allow 88% power to differentiate between PFS-6 rates of 15 and 40% with a type I error rate of 7.7%. Accrual was to be discontinued after 10 patients if fewer than two of these patients remained progression-free at 6 months. If 6 or more of the 21 patients were progression-free at 6 months, the treatment regimen would be considered worthy of further scientific investigation.
“Stopping rules” for toxicity were incorporated for this study. Specifically, if at any point, 4 or more patients were observed to experience unacceptable toxicity as defined above, further accrual would be suspended. PFS and OS were measured from the cycle 1 start date and summarized using Kaplan–Meier estimator including 95% CIs. A log-rank test was used to test for survival differences between patients with grade I tumors and those with grade II or III tumors.
Results
Patient characteristics
Twenty-one patients were enrolled between August 2005 and September 2008. Characteristics of patients at enrollment are summarized in Table 1. Twelve patients were female (57%) and the median age was 51.0 years (range 28.0–85.3 years). Nine patients (43%) had a KPS 90–100. Eight patients (38%) had grade I meningioma while 9 (43%) and 4 (19%) had grade II and III meningiomas, respectively. Eight patients (38%) had only 1 lesion while 6 patients (29%) had four or more lesions at study enrollment. Only one patient (10%) had neurofibromatosis (type 2). Seven patients (33%) had received prior conformal radiotherapy while 7 (33%) had received prior stereotactic radiosurgery and only 2 (10%) had received prior chemotherapy. Only three patients (14%) were on EIAEDs.
Table 1.
Patient characteristics
| Age (years) | |
| Median | 51.0 |
| Range | 28.0–85.3 |
| Gender | |
| Male | 9 (43) |
| Female | 12 (57) |
| KPS | |
| 90–100 | 9 (43) |
| 80 | 10 (48) |
| 70 | 2 (10) |
| Histology | |
| Benign (WHO grade I) | 8 (38) |
| Atypical (WHO grade II) | 9 (43) |
| Anaplastic (WHO grade III) | 4 (19) |
| Time from Dx (years) | |
| Median | 6.33 |
| Range | 1.21–23.94 |
| Prior treatment | |
| Surgery only | 9 (43) |
| XRT (conformal) | 7 (33) |
| SRS | 7 (33) |
| Chemotherapy | 2 (10) |
| EIAED | |
| Yes | 3 (14) |
| No | 18 (86) |
| Neurofibromatosis | |
| Yes | 1 (5) |
| No | 20 (95) |
| Number lesions | |
| 1 | 8 (38) |
| 2–3 | 7 (33) |
| 4–6 | 4 (19) |
| > 6 | 2 (10) |
| Number prior PD | |
| 1 | 4 (19) |
| 2 | 10 (48) |
| 3 | 4 (19) |
| ≥4 | 3 (14) |
PD progressive disease, EIAED CYP3A enzyme inducing anti-epileptic drugs, SRS stereotactic radiosurgery, XRT external beam radiotherapy, Dx diagnoses, KPS Karnofsky performance status
Outcome
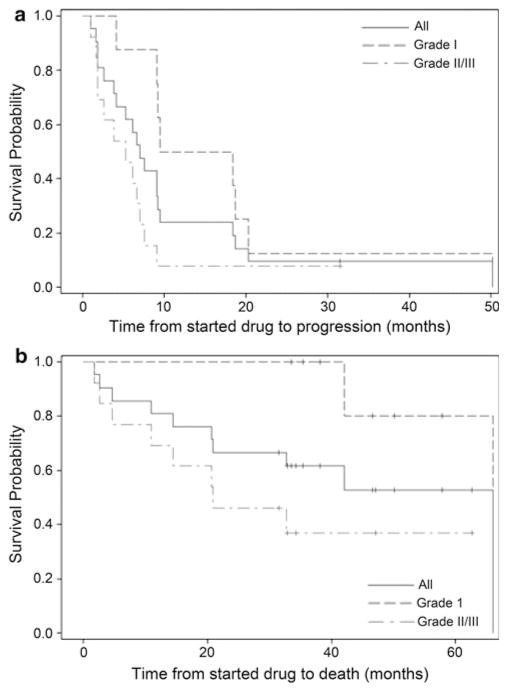
Table 2 and Fig. 1 summarizes study outcome. As of April 1, 2011, median follow-up for all patients was 46.6 months (95% CI 33.5, 57.8) and all patients have discontinued study therapy. Eight patients (38%) elected to discontinue study therapy with stable disease after 1–23 cycles of therapy, while 13 patients (62%) came off study due to progressive disease. No patients discontinued study therapy due to toxicity.
Table 2.
Survival estimates for all patients
| Group | Total | Number of failed | Median survival in months (95% CI) | 6-month survival (95% CI) | 12-month survival (95% CI) | 24-month survival (95% CI) | Logrank P-value | |
|---|---|---|---|---|---|---|---|---|
| OS | All | 21 | 10 | 66 (20.7, 66) | 85.7 (62, 95.2) | 81 (56.9, 92.4) | 66.7 (42.5, 82.5) | |
| Grade I | 8 | 2 | 66.0 (42, 66) | 100 | 100 | 100 | 0.018 | |
| Grade II/III | 13 | 8 | 20.9 (4.6, ∞) | 76.9 (44.2, 91.9) | 69.2 (37.3, 87.2) | 46.2 (19.2, 69.6) | ||
| PFS | All | 21 | 20 | 7 (3.8, 9.2) | 61.9 (38.1, 78.8) | 23.8 (8.7, 43.1) | 9.5 (1.6, 26.1) | |
| Grade I | 8 | 8 | 13.9 (4.1, 20.3) | 87.5 (38.7, 98.1) | 50 (15.2, 77.5) | 12.5 (0.7, 42.3) | 0.025 | |
| Grade II/III | 13 | 12 | 5.3 (1.8, 7) | 46.2 (19.2, 69.6) | 7.7 (0.5, 29.2) | 7.7 (0.5, 29.2) |
OS overall survival, PFS progression-free survival, CI confidence interval
Fig. 1.
Kaplan–Meier curves of profession-free survival (a) and overall survival (b)
Median PFS and PFS-6 for all patients were 7.0 months (95% CI 3.8, 9.2) and 61.9% (95% CI 38.1, 78.8). Median OS was 66.0 months (95% CI 20.7, 66.0). Best radiographic response was stable disease and none of the patients achieved a radiographic response. Patients with grade II or III tumors had poorer PFS (P = 0.025) and OS (P = 0.018) compared to patients with grade I tumors.
Toxicity
One-hundred and fifty-eight cycles of therapy were administered. The adverse events seen in the study were as expected for this population and these agents. They were mostly mild and transient and gave no indication of target organ toxicity. Table 3 summarizes grade ≥2 adverse events felt to be at least possibly attributable to the study regimen. Of note, the frequency and severity of adverse events did not differ between patients on and not on EIAEDs. Most adverse events were grade 2. The sole grade 4 event was reversible neutropenia which developed in a heavily pretreated patient. Among grade 3 events, two patients developed grade 3 anemia, while single patients experienced edema, fatigue, and hypoalbuminemia. There were no grade 5 attributable adverse events. Only one patient required dose modification and none of the patients discontinued therapy due to toxicity.
Table 3.
Summary of treatment toxicity
| Toxicity | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Anemia | 2 (10) | ||
| Constipation | 2 (10) | ||
| Edema | 1 (5) | 1 (5) | |
| Fatigue | 2 (10) | 1 (5) | |
| Hypoalbuminemia | 1 (5) | ||
| Hypophosphatemia | 1 (5) | ||
| Infection | 1 (5) | ||
| Nausea | 3 (14) | ||
| Neutropenia | 1 (5) | 1 (5) | |
| Rash | 1 (5) | ||
| Thrombocytopenia | 1 (5) |
Numbers in parentheses indicate percentage of treated patients
Discussion
Treatment of patients with recurrent/progressive meningioma, particularly after surgical and radiotherapy options are depleted, remains an unanswered challenge. Most series evaluating systemic therapies such as chemotherapy or hormonal agents demonstrate poor activity although are limited by small sample size and/or retrospective design. Hydroxyurea, a ribonucleoside diphosphate reductase inhibitor, exerts a cell-cycle specific effect during the S-phase of mitosis, thereby blocking DNA synthesis. Radiographic responses, initially reported in two of four patients treated with hydroxyurea monotherapy [23], have proven to be rare in subsequent series. In addition, subsequent studies document that hydroxyurea is associated with variable durability of stable disease in a subset of patients [17–24]. Although, these series report prolonged disease stabilization in some patients, the significance of this finding requires cautious assessment given the slow growth rate of many meningiomas, particularly the benign subtype. In addition, in a recent retrospective review of 60 patients with recurrent meningioma following surgery and radiotherapy, 35% of patients treated with hydroxyurea achieved stable disease, however, the median duration was only 4 months and none of the patients achieved a radiographic response [40].
At the time this study was designed and implemented, combination trials with hydroxyurea for recurrent/progressive meningioma patients had not been reported. We therefore developed the current clinical trial to evaluate hydroxyurea combined with imatinib mesylate. The primary rationale for adding imatinib in this study is based on its inhibitory capacity against PDGFR. Meningiomas frequently express PDGFR while some tumors also express PDGF implicating a potential autocrine and/or paracrine loop of dysregulated signaling via the PI3/AKT and ras/MAPK growth/survival pathways [26–36].
Despite its ability to inhibit PDGFR, a recent study demonstrated negligible activity of imatinib among a series of 23 heavily pretreated patients with recurrent/progressive meningioma [41]. Among five patients with adequate archival tumor material available for immunohistochemistry in this study, all of the tumors demonstrated expression of both PDGFR-α and PDGFR-β in >90% of tumor cells. Nonetheless, no radiographic responses to imatinib monotherapy were observed and PFS-6 was only 29.4%. For the current study, we hypothesized that the addition of hydroxyurea as a cytotoxic agent to imatinib may result in additive if not synergistic anti-tumor activity, with modest toxicity due to the overall good tolerance of each agent.
Indeed our study findings confirm that the combination of imatinib and hydroxyurea is well tolerated among recurrent/progressive meningioma patients. Most adverse events were limited to grade 2, and none of the patients discontinued study therapy due to toxicity. However, overall activity of this regimen was modest at best. Specifically, we failed to observe any radiographic responses and prolonged stable disease was primarily limited to patients with grade I lesions. PFS and OS were significantly better among patients with grade I tumors compared to those with grade II or III tumors. However, given the inherent slow growth of these tumors in general, durable stable disease must be interpreted cautiously particularly for benign meningioma patients. Four out of thirteen patients with grade II/III meningiomas (31%) achieved stable disease for at least 6 months (range 7–18 months), suggesting that this regimen may have modest benefit for some of high-grade patients. Further study of PDGFR inhibitors for grade II/III recurrent/progressive meningioma patients should be considered, particularly given the advent of more potent and specific inhibitors. A significant deficiency of this study was the lack of archival tumor analysis for PDGF/PDGFR expression. Future studies of targeted therapeutics for this challenging patient population should optimally include analysis of target expression among treated patients.
Acknowledgments
This study was financially supported by grants P50 NS20023 and R37 CA011898 from NIH, Department of Health and Human Services, Bethesda, Maryland and a research grant from Novartis Pharmaceuticals. The investigators wish to thank Wendy Gentry for her assistance in the preparation of this manuscript.
Abbreviations
- OS
Overall survival
- PFS
Progression-free survival
- mg
Milligram
- EIAEDs
CYP3A enzyme inducing anti-epileptic drugs
- KPS
Karnofsky performance status
- PD
Progressive disease
- STR
Stereotactic radiosurgery
- XRT
External beam radiotherapy
- CNS
Central nervous system
- PDGFR
Platelet derived growth factor receptor
- STR
Subtotal resection
- SRS
Stereotactic radiosurgery
Contributor Information
David A. Reardon, Email: reard003@mc.duke.edu, Duke University Medical Center, Box 3624, Durham, NC 27710, USA. Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA. Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
Andrew D. Norden, The Center for Neuro-Oncology, Dana Farber Cancer Institute, 44 Binney Street, Boston, MA 02115, USA
Annick Desjardins, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA. Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA.
James J. Vredenburgh, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA. Department of Medicine, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
James E. Herndon, II, Department of Biostatistics, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA.
April Coan, Department of Biostatistics, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA.
John H. Sampson, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
Sridharan Gururangan, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA. Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA.
Katherine B. Peters, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA. Department of Medicine, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
Roger E. McLendon, Departments of Neurobiology and Pathology, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
Julie A. Norfleet, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
Eric S. Lipp, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
Jan Drappatz, The Center for Neuro-Oncology, Dana Farber Cancer Institute, 44 Binney Street, Boston, MA 02115, USA.
Patrick Y. Wen, The Center for Neuro-Oncology, Dana Farber Cancer Institute, 44 Binney Street, Boston, MA 02115, USA
Henry S. Friedman, Department of Surgery, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA. Department of Pediatrics, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA
References
- 1.CBTRUS (2004–2006) Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. Central Brain Tumor Registry of the United States; 2010. [Google Scholar]
- 2.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–391. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101:210–218. doi: 10.3171/jns.2004.101.2.0210. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer (IARC); Lyon: 2007. [Google Scholar]
- 5.U.S. Department of Health and Human Services CfDE, and Research FaDa. FDA project on cancer drug approval endpoint. 2006. [Google Scholar]
- 6.Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99:393–405. doi: 10.1007/s11060-010-0343-1. [DOI] [PubMed] [Google Scholar]
- 7.Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol. 2010;99:433–443. doi: 10.1007/s11060-010-0348-9. [DOI] [PubMed] [Google Scholar]
- 8.Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, Curry WT, Jr, Barker FG., 2nd Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. doi: 10.1227/01.NEU.0000330399.55586.63 discussion 60. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62:1210–1212. doi: 10.1212/01.wnl.0000118300.82017.f4. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78:271–276. doi: 10.1007/s11060-005-9093-x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, Buckner JC. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011;13:530–535. doi: 10.1093/neuonc/nor044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain MC, Glantz MJ. Interferon-alpha for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer. 2008;113:2146–2151. doi: 10.1002/cncr.23803. [DOI] [PubMed] [Google Scholar]
- 14.Grunberg SM, Weiss MH, Russell CA, Spitz IM, Ahmadi J, Sadun A, Sitruk-Ware R. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006;24:727–733. doi: 10.1080/07. [DOI] [PubMed] [Google Scholar]
- 15.Lamberts SW, Tanghe HL, Avezaat CJ, Braakman R, Wijngaarde R, Koper JW, De Jong H. Mifepristone (RU 486) treatment of meningiomas. J Neurol Neurosurg Psychiatry. 1992;55:486–490. doi: 10.1136/jnnp.55.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norden AD, Raizer J, Abrey L, Lamborn K, Lassman A, Chang SM, Yung WKA, Gilbert M, Fine HA, Mehta M, De Angelis L, Cloughesy T, Robins HI, Aldape K, Dancey J, Prodos M, Lieberman F, Wen PY. Phase II Trials of erlotinb or gefitinib in patients with recurrent meningioma. J Neurooncol. 2009;96(2):211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton HB. Hydroxyurea chemotherapy in the treatment of meningiomas. Neurosurg Focus. 2007;23:E11. doi: 10.3171/FOC-07/10/E11. [DOI] [PubMed] [Google Scholar]
- 18.Newton HB, Scott SR, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18:495–499. doi: 10.1080/02688690400012392. [DOI] [PubMed] [Google Scholar]
- 19.Newton HB, Slivka MA, Stevens C. Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neurooncol. 2000;49:165–170. doi: 10.1023/a:1026770624783. [DOI] [PubMed] [Google Scholar]
- 20.Loven D, Hardoff R, Sever ZB, Steinmetz AP, Gornish M, Rappaport ZH, Fenig E, Ram Z, Sulkes A. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol. 2004;67:221–226. doi: 10.1023/b:neon.0000021827.85754.8e. [DOI] [PubMed] [Google Scholar]
- 21.Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002;97:341–346. doi: 10.3171/jns.2002.97.2.0341. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal MA, Ashley DL, Cher L. Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci. 2002;9:156–158. doi: 10.1054/jocn.2001.1019. [DOI] [PubMed] [Google Scholar]
- 23.Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, Kiesewetter F, Fahlbusch R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840–844. doi: 10.3171/jns.1997.86.5.0840. [DOI] [PubMed] [Google Scholar]
- 24.Hahn BM, Schrell UM, Sauer R, Fahlbusch R, Ganslandt O, Grabenbauer GG. Prolonged oral hydroxyurea and concurrent 3d-conformal radiation in patients with progressive or recurrent meningioma: results of a pilot study. J Neurooncol. 2005;74:157–165. doi: 10.1007/s11060-004-2337-3. [DOI] [PubMed] [Google Scholar]
- 25.Ragel BT, Jensen RL. Aberrant signaling pathways in meningiomas. J Neurooncol. 2010;99:315–324. doi: 10.1007/s11060-010-0381-8. [DOI] [PubMed] [Google Scholar]
- 26.Yang SY, Xu GM. Expression of PDGF and its receptor as well as their relationship to proliferating activity and apoptosis of meningiomas in human meningiomas. J Clin Neurosci. 2001;8(Supplement 1):49–53. doi: 10.1054/jocn.2001.0877. [DOI] [PubMed] [Google Scholar]
- 27.Nagashima G, Asai J, Suzuki R, Fujimoto T. Different distribution of c-myc and MIB-1 positive cells in malignant meningiomas with reference to TGFs, PDGF, and PgR expression. Brain Tumor Pathol. 2001;18:1–5. doi: 10.1007/BF02478918. [DOI] [PubMed] [Google Scholar]
- 28.Todo T, Adams EF, Fahlbusch R, Dingermann T, Werner H. Autocrine growth stimulation of human meningioma cells by platelet-derived growth factor. J Neurosurg. 1996;84:852–858. doi: 10.3171/jns.1996.84.5.0852. discussion 858–859. [DOI] [PubMed] [Google Scholar]
- 29.Mauro A, Di Sapio A, Mocellini C, Schiffer D. Control of meningioma cell growth by platelet-derived growth factor (PDGF) J Neurol Sci. 1995;131:135–143. doi: 10.1016/0022-510x(95)00106-c. [DOI] [PubMed] [Google Scholar]
- 30.Schrell UM, Gauer S, Kiesewetter F, Bickel A, Hren J, Adams EF, Fahlbusch R. Inhibition of proliferation of human cerebral meningioma cells by suramin: effects on cell growth, cell cycle phases, extracellular growth factors, and PDGF-BB autocrine growth loop. J Neurosurg. 1995;82:600–607. doi: 10.3171/jns. 1995.82.4.0600. [DOI] [PubMed] [Google Scholar]
- 31.Kuratsu JI, Seto H, Kochi M, Ushio Y. Expression of PDGF, PDGF-receptor, EGF-receptor and sex hormone receptors on meningioma. Acta Neurochir (Wien) 1994;131:289–293. doi: 10.1007/BF01808629. [DOI] [PubMed] [Google Scholar]
- 32.Figarella-Branger D, Vagner-Capodano AM, Bouillot P, Graziani N, Gambarelli D, Devictor B, Zattara-Cannoni H, Bianco N, Grisoli F, Pellissier JF. Platelet-derived growth factor (PDGF) and receptor (PDGFR) expression in human meningiomas: correlations with clinicopathological features and cytogenetic analysis. Neuropathol Appl Neurobiol. 1994;20:439–447. doi: 10.1111/j.1365-2990.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 33.Black PM, Carroll R, Glowacka D, Riley K, Dashner K. Platelet-derived growth factor expression and stimulation in human meningiomas. J Neurosurg. 1994;81:388–393. doi: 10.3171/jns. 1994.81.3.0388. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell M, Galanopoulos T, Hedley-Whyte ET, Black PM, Antoniades HN. Human meningiomas co-express platelet-derived growth factor (PDGF) and PDGF-receptor genes and their protein products. Int J Cancer. 1990;46:16–21. doi: 10.1002/ijc.2910460106. [DOI] [PubMed] [Google Scholar]
- 35.Johnson MD, Okedli E, Woodard A, Toms SA, Allen GS. Evidence for phosphatidylinositol 3-kinase-Akt-p7S6K pathway activation and transduction of mitogenic signals by platelet-derived growth factor in meningioma cells. J Neurosurg. 2002;97:668–675. doi: 10.3171/jns.2002.97.3.0668. [DOI] [PubMed] [Google Scholar]
- 36.Johnson MD, Woodard A, Kim P, Frexes-Steed M. Evidence for mitogen-associated protein kinase activation and transduction of mitogenic signals by platelet-derived growth factor in human meningioma cells. J Neurosurg. 2001;94:293–300. doi: 10.3171/jns.2001.94.2.0293. [DOI] [PubMed] [Google Scholar]
- 37.Reardon DA, Egorin MJ, Quinn JA, Rich JN, Sr, Gururangan I, Vredenburgh JJ, Desjardins A, Sathornsumetee S, Provenzale JM, Herndon JE, 2nd, Dowell JM, Badruddoja MA, McLendon RE, Lagattuta TF, Kicielinski KP, Dresemann G, Sampson JH, Friedman AH, Salvado AJ, Friedman HS. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 38.Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, Abrey LE, Raizer J, Cloughesy TF, Fink K, Gilbert M, Chang S, Junck L, Schiff D, Lieberman F, Fine HA, Mehta M, Robins HI, De Angelis LM, Groves MD, Puduvalli VK, Levin V, Conrad C, Maher EA, Aldape K, Hayes M, Letvak L, Egorin MJ, Capdeville R, Kaplan R, Murgo AJ, Stiles C, Prados MD. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 39.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain MC, Johnston SK. Hydroxyurea for recurrent surgery and radiation refractory meningioma: a retrospective case series. J Neurooncol. 2011;104(3):765–771. doi: 10.1007/s11060-011-0541-5. [DOI] [PubMed] [Google Scholar]
- 41.Wen PY, Yung WK, Lamborn KR, Norden AD, Cloughesy TF, Abrey LE, Fine HA, Chang SM, Robins HI, Fink K, De Angelis LM, Mehta M, Di Tomaso E, Drappatz J, Kesari S, Ligon KL, Aldape K, Jain RK, Stiles CD, Egorin MJ, Prados MD. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01–08) Neuro Oncol. 2009;11:853–860. doi: 10.1215/15228517-2009-010. [DOI] [PMC free article] [PubMed] [Google Scholar]