Abstract
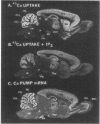
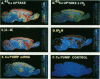
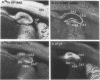
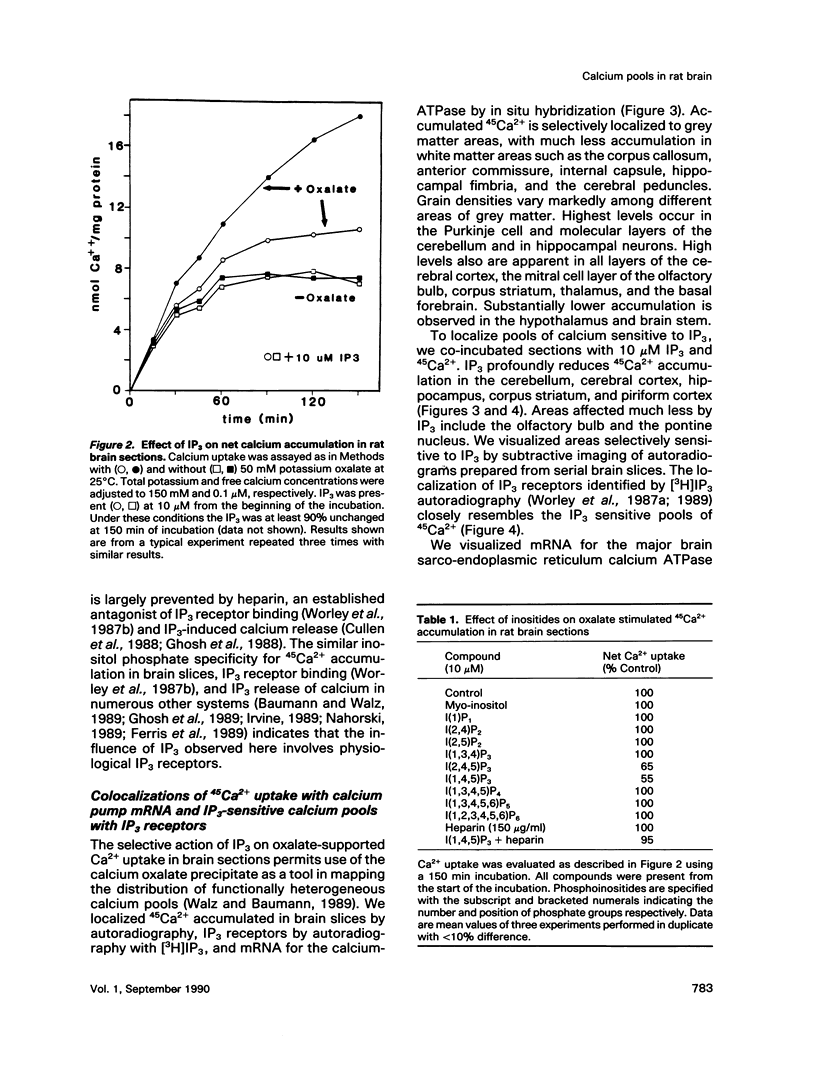
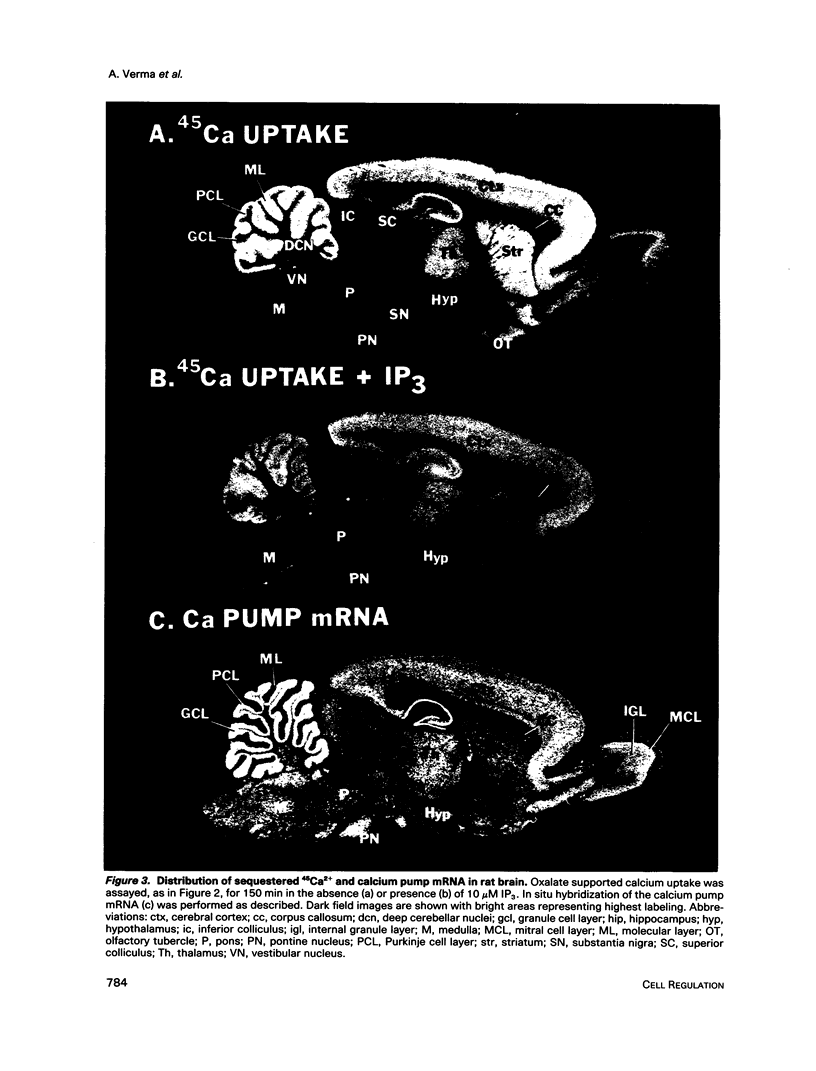
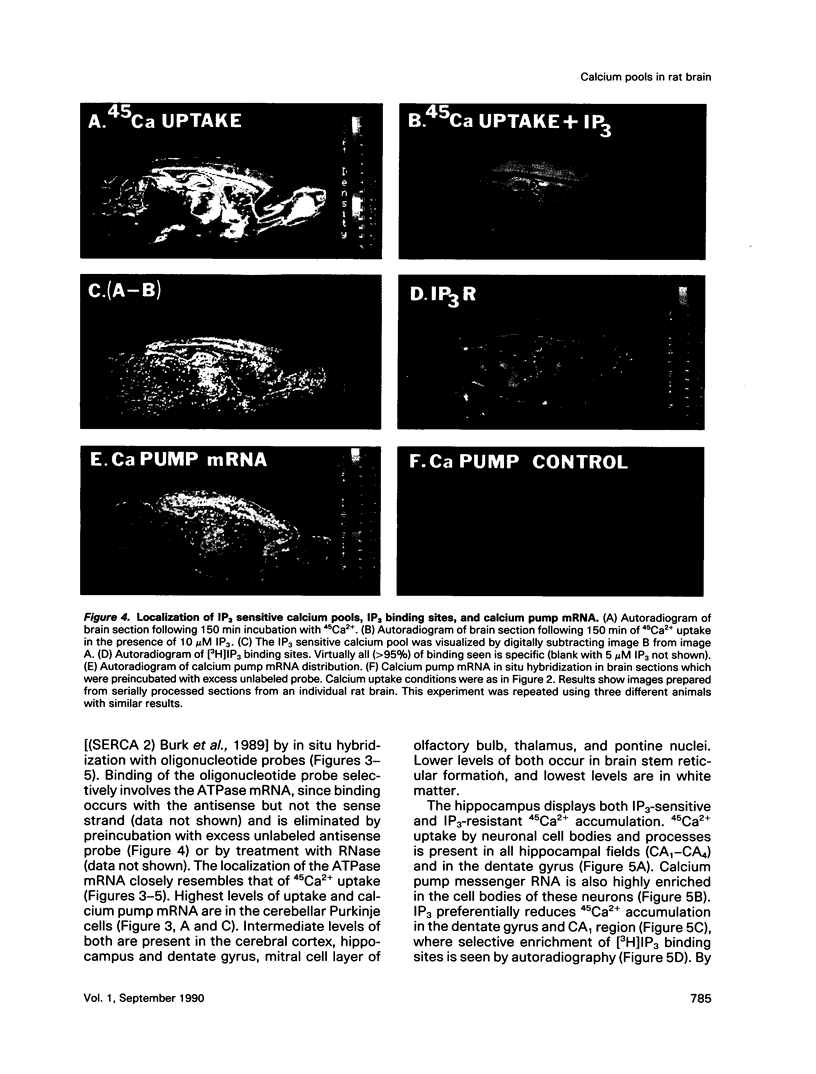
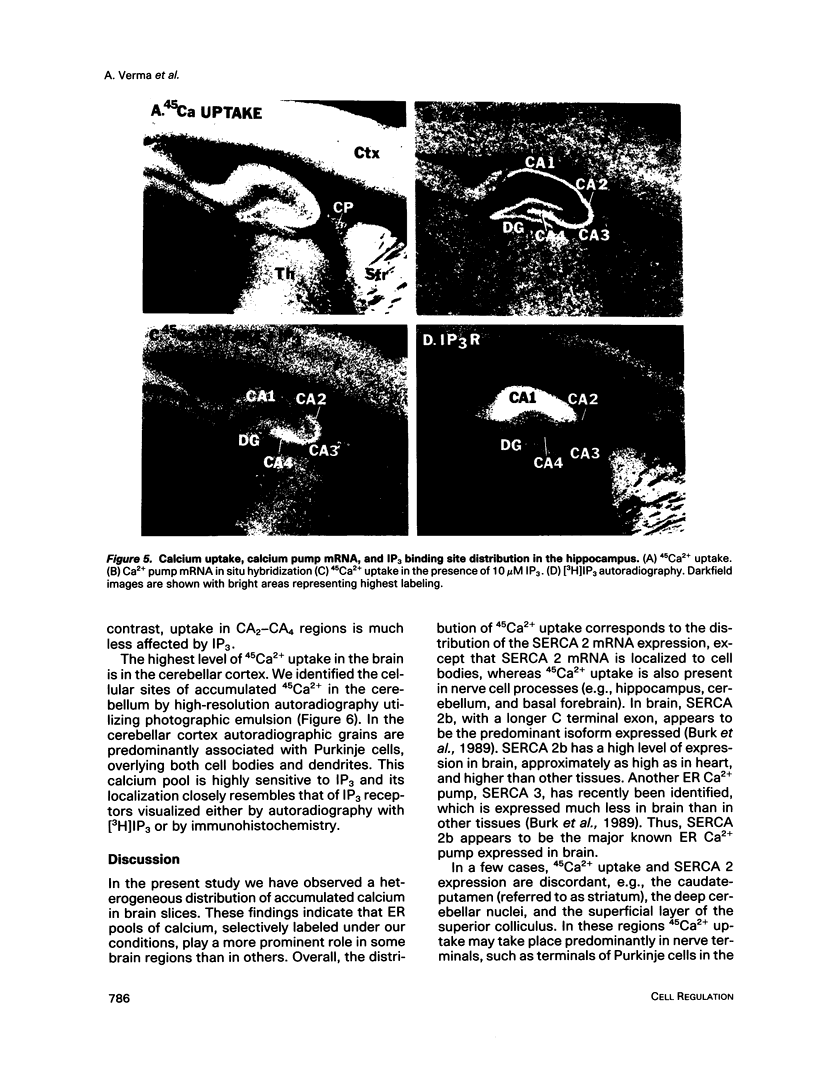
Autoradiographic imaging can localize 45Ca2+ selectively accumulated via the Ca2+, Mg2(+)-ATPase into endoplasmic reticulum stores in rat brain slices. 45Ca2+ accumulation is markedly stimulated by oxalate and displays a heterogeneous distribution which resembles the mRNA distribution for a sarcoendoplasmic reticulum Ca2+, Mg2(+)-ATPase. Inositol 1,4,5-triphosphate [I(1,4,5)P3] inhibits 45Ca2+ accumulation selectively into regions corresponding to those enriched in I(1,4,5)P3 receptor-binding sites and in Ca2+, -Mg2(+)-ATPase mRNA. Thus rat brain endoplasmic reticulum calcium stores are anatomically and functionally differentiated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Cullen P. J., Comerford J. G., Dawson A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-induced Ca2+ release from rat liver microsomes. FEBS Lett. 1988 Feb 8;228(1):57–59. doi: 10.1016/0014-5793(88)80584-2. [DOI] [PubMed] [Google Scholar]
- Daniell L. C., Harris R. A. Ethanol and inositol 1,4,5-trisphosphate release calcium from separate stores of brain microsomes. J Pharmacol Exp Ther. 1989 Sep;250(3):875–881. [PubMed] [Google Scholar]
- Delfert D. M., Hill S., Pershadsingh H. A., Sherman W. R., McDonald J. M. myo-Inositol 1,4,5-trisphosphate mobilizes Ca2+ from isolated adipocyte endoplasmic reticulum but not from plasma membranes. Biochem J. 1986 May 15;236(1):37–44. doi: 10.1042/bj2360037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Chueh S. H., Whitlow C. L. Functional importance of the synaptic plasma membrane calcium pump and sodium-calcium exchanger. J Biol Chem. 1984 Sep 10;259(17):10807–10813. [PubMed] [Google Scholar]
- Grover A. K. Ca-pumps in smooth muscle: one in plasma membrane and another in endoplasmic reticulum. Cell Calcium. 1985 Jun;6(3):227–236. doi: 10.1016/0143-4160(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Henne V., Piiper A., Söling H. D. Inositol 1,4,5-trisphosphate and 5'-GTP induce calcium release from different intracellular pools. FEBS Lett. 1987 Jun 22;218(1):153–158. doi: 10.1016/0014-5793(87)81037-2. [DOI] [PubMed] [Google Scholar]
- Henne V., Söling H. D. Guanosine 5'-triphosphate releases calcium from rat liver and guinea pig parotid gland endoplasmic reticulum independently of inositol 1,4,5-trisphosphate. FEBS Lett. 1986 Jul 7;202(2):267–273. doi: 10.1016/0014-5793(86)80699-8. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. How do inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate regulate intracellular Ca2+? Biochem Soc Trans. 1989 Feb;17(1):6–9. doi: 10.1042/bst0170006. [DOI] [PubMed] [Google Scholar]
- Leslie B. A., Burgess G. M., Putney J. W., Jr Persistent inhibition by inositol 1,4,5-trisphosphate of oxalate-dependent 45calcium accumulation in permeable guinea-pig hepatocytes. Cell Calcium. 1988 Feb;9(1):9–16. doi: 10.1016/0143-4160(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Harris G. D., Lovenberg W. Characterization of inositol 1,4,5-trisphosphate-stimulated calcium release from rat cerebellar microsomal fractions. Comparison with [3H]inositol 1,4,5-trisphosphate binding. Biochem J. 1988 Oct 15;255(2):677–683. [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Walz B., Baumann O. Calcium-sequestering cell organelles: in situ localization, morphological and functional characterization. Prog Histochem Cytochem. 1989;20(2):1–47. doi: 10.1016/s0079-6336(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Snyder S. H. Inositol 1,4,5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989 Jan;9(1):339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986 Dec;387(3):231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]