Abstract
Individual differences and stability in patterns of salivary cortisol reactivity were examined in 100 African American neonates from low-income environments. A pattern of reactivity was defined by the change from prestressor to poststressor cortisol concentrations and the change following the poststressor during a recovery phase. Cortisol reactivity was measured in response to two stressors: the Neonatal Behavioral Assessment Scale (NBAS; T. B. Brazelton & J. K. Nugent) and the routine hospital heel-stick procedure. The use of two stressors allowed an examination of whether patterns of reactivity to different stimuli vary and whether there is individual stability in patterns of cortisol reactivity. Cortisol concentrations changed significantly across the three time points. The magnitude of change during the recovery period differed across stressors. Prestressor cortisol values were associated with cortisol reactivity. Both prestressor cortisol concentrations and pattern of cortisol response were significantly associated within individuals.
Keywords: neonates, African Americans, cortisol, individual differences
Two areas of research on cortisol response in children have been conducted simultaneously, but have not yet fully converged. The first is the role of neuroendocrine functioning on infant regulation. In particular, changes in cortisol levels have been used as an index of stress reactivity in infants. Much of the research on infant cortisol response has been on documenting normative patterns of maturation of the hypothalamic–pituitary–adrenocortical (HPA) system. To date, research by a number of investigators has led to significant contributions toward the understanding of the effects of environmental and birth conditions (Gunnar, Isensee, & Fust, 1987; Gunnar, Larson, Hertsgaard, Harris, & Brodersen, 1992; Larson, Gunnar & Hertsgaard, 1991), diurnal variation (Lewis & Thomas, 1990), and developmental changes (Gunnar, Broderson, Krueger, & Rigatuso, 1996; Lewis & Ramsay, 1995a; Ramsay & Lewis, 1994) on the infant's baseline cortisol and cortisol response to stress.
Concurrently, developmental psychopathologists have focused on the association between certain types of psychopathology and individual differences in cortisol, both resting levels and reactivity. In some studies, low resting cortisol is associated with conduct problems whereas high resting cortisol is associated with depression and anxiety, with children with comorbid anxiety and conduct disorder having the highest resting cortisol (Granger, Stansbury, & Henker, 1994; McBurnett et al., 1991; McBurnett, Lahey, Rathouz, & Loeben, 2000). Because psychopathology and cortisol were measured concurrently in the majority of studies, hypotheses regarding the relation between this potential biological marker and atypical behavior have not been tested. HPA activity early in life may be a marker for psychopathology in later childhood. Alternatively, atypical HPA functioning may emerge concurrently with behavioral and emotional problems, or may even emerge after the onset of behavioral and emotional problems.
The point at which these two literatures need to converge is at the level of individual differences in cortisol response very early in life, before the onset of psychopathology. One possible developmental trajectory to psychopathology is via early deficits in emotional regulation. Patterns of cortisol response may be indicative of such deficits. Data that support an association between dysfunction in the brain structures involved in emotion regulation and psychopathology are beginning to emerge (Davidson, Putnam, & Larson, 2000). Examining individual differences in patterns of cortisol reactivity very early in life may reveal a mechanism by which children at risk for later psychopathology can be identified. Thus, testing the predictive utility of individual differences in makers such as cortisol in infants is a logical extension of the current work on psychopathology in older children and the literature on normal developmental changes in cortisol reactivity in infants.
Before such hypotheses can be tested, however, the current research on infant cortisol reactivity may benefit from attention to a number of potentially important factors. First, the quality of the stressor in terms of the acuteness and duration may influence the type of response (Cacioppo, 1994), and thereby affect the validity and utility of stress reactivity as a marker for individual difference or later adjustment problems. Only a few studies have systematically tested differences in the quality of the stressors in infants. Ramsay and Lewis (1994) reported no difference in cortisol concentrations following one versus two inoculations in 2- and 6-month-old infants. Larson and colleagues (1991) assessed patterns of cortisol response in three different paradigms in 9-month old infants: waking from a nap, riding in a car, and being separated from mother. Napping and riding in a car resulted in decreases in cortisol, and separation resulted in increases in cortisol. In all three paradigms, the magnitude of the response was small (~.10–.25), but the variation in time of day of sampling made it difficult to assess similarities or differences in the magnitude of the response across paradigms.
Second, little attention has been paid to the stability of individual response patterns in cortisol. Individual stability of response across paradigms and over time also may be an important dimension to consider in evaluating the association with behavioral measures and the predictive validity of cortisol reactivity. Individuals who consistently respond to stress with significant increases or decreases in cortisol may show greater association with behavioral indices of stress reactivity and also may be more likely to continue to demonstrate problems with emotional regulation. Few studies have examined individual stability within or across developmental periods early in life, and the results from these are inconsistent. Spangler and Scheubeck (1993) administered the Neonatal Behavioral Assessment Scale (NBAS; Brazelton & Nugent, 1995) to neonates on two occasions (within and between days). Baseline saliva was collected at three time points: before the NBAS (baseline), at the start of the NBAS, and 15 min after the completion of the NBAS. They reported significant associations between individuals’ baseline cortisol concentrations within and between days, but no association between an individual's cortisol reactivity in response to the NBAS. Lewis and Ramsay (1995b) reported significant correlations between cortisol responses to inoculation at 6 and 18 months of age, but no association between the 2- or 4-month cortisol response to inoculation and the 18-month cortisol score. Gunnar, Hertsgaard, Larson, and Rigatuso (1992) examined the stability of neonatal response to either two heel-stick procedures or two physical exams, which were administered across 2 successive days. There was no evidence of association in rank ordering of the cortisol response between day 1 and day 2. The lack of stability of response may have reflected the infant's ability to habituate to the stressor.
Third, measures of cortisol have usually been limited to a baseline sample and a sample measuring the “peak” response, typically collected approximately 20 min after the administration of a stressor. However, another potentially important dimension is recovery from stress. In the context of research in developmental psychopathology, an individual's ability to recover from stressful events is related to the risk for and stability of behavioral and emotional problems. For example, temperamental dimensions that include difficulty being soothed as an infant have demonstrated predictive utility to later behavioral and emotional problems in childhood (Keenan, Shaw, Delliquadri, Giovannelli, & Walsh, 1998). Thus, measuring the rapidity of the cortisol response to return to baseline early in life may prove to be a useful biological indicator of an important aspect of emotion regulation.
A final limitation of much of the research in infant cortisol is the inclusion of predominantly Caucasian infants from middle-income environments in samples. There are only a few studies of cortisol reactivity in minority infants (Davis & Emory, 1995; Jacobson, Bihun, & Chiodo, 1999). Within that literature, the focus has often been on the effects of prenatal exposure to alcohol and illicit substances (e.g., Jacobson et al., 1999) rather than the effect of social risk factors. Patterns of stress reactivity as measured by cortisol may differ across ethnic groups, and families living in low-income environments may be exposed to chronic and acute stressors that affect regulation of stress responses (McLoyd, 1990). Young children growing up in such environments are at higher risk for developing behavioral and emotional problems (Adams, Hillman, & Gaydos, 1994), but most ethnic minority children who live in low-income, inner cities do not develop psychopathology. Research that has the potential to generate risk profiles that identify individual characteristics that lead to young children's vulnerability to environmental risk factors is especially needed to determine which children in high-risk environments are going to develop behavioral and emotional problems.
The scientific investigation of biobehavioral markers in infancy of later behavioral and emotional problems may lead to the identification of the developmental origins of some forms of psychopathology (Keenan, 2000). Therefore, it seems appropriate to provide additional data on the patterns of reactivity to different types of stimuli. The purpose of the present study is to examine individual differences and stability in patterns of cortisol reactivity in African-American neonates whose families reside in low-income environments. A pattern of reactivity is defined as changes from baseline to the peak cortisol response, and changes following the peak response during a phase of recovery. Cortisol reactivity in response to two paradigms is measured. The two paradigms allow an examination of whether patterns of reactivity vary across different types of stimuli and whether there is individual stability in cortisol reactivity across stimuli.
METHODS
Subjects
As part of an ongoing longitudinal study, full-term, healthy neonates and their mothers were recruited from the General Care Nursery (GCN) at the University of Chicago. The GCN serves predominately healthy African American women and their newborns. The majority of families have incomes below the poverty level. Neonates requiring admission to the Intensive Care Unit immediately following delivery, who had obvious congenital and chromosomal abnormalities, or who tested positive for cocaine or opiod exposure were excluded because they are likely to have serious deficits in neurobiological functioning. All mothers who did not receive prenatal care from the University of Chicago were screened for drug use by urine toxicology upon admission to the nursery. Mothers also were asked about their use of illegal and legal substances during pregnancy. Mothers who agreed to participate but who reported drug use during pregnancy or who were found to have used illegal substances by urine toxicology were excluded.
Mothers of infants who met the inclusion criteria were approached by one of the project staff. The study was described as one assessing early child development that required visits to the laboratory over the next 2 years. All mothers were asked to sign a consent form in which the risks, benefits, cost, and payments were explained. A total of 155 mothers and infants met inclusion criteria for the study, and 113 (73%) agreed to participate. Thirteen (8%) of those who agreed to participate were unable to because of technical problems including heel sticks conducted at the wrong time or feedings that interfered with saliva collection. Thus, a total of 100 mothers and infants were enrolled in the study and completed the assessment.
Procedures
Data on cortisol reactivity to two stimuli were gathered within 48 hr of birth. The NBAS was conducted approximately 1 hr after a feeding. Blood drawing from the heel (heel stick), a required procedure, was typically conducted approximately 24 hr after birth by one of approximately five technicians. Saliva was collected pre and 20-min and 45-min post-NBAS and heel stick. The prestressor samples were collected on average 9 min before the start of the NBAS and 24 min before the heel stick. The 20-min poststressor saliva sample was designed to represent the peak stress response, and the 45-min sample represented the recovery response. In nearly all participants (80%), the heel-stick procedure was conducted prior to the NBAS. The average interval between the two procedures was 13 hr.
There are important differences between these two stress paradigms. The heel stick is a painful procedure that begins with lancing the heel and is followed by repeatedly squeezing the heel (on average 30 times) to generate enough blood for medical tests. The NBAS is a behavioral exam that includes several different components including habituation to visual and auditory stimuli, assessment of reflexes and head control, and orienting tasks. Thus, there is a significant amount of handling and changing of positions that can be stressful for the infant. The advantage of using two different stressors as opposed to the same stressor twice is that habituation or sensitization of response to a single repeated stressor, which has been demonstrated in neonates (Gunnar, Connors, & Isensee, 1989), is potentially less likely to interfere with the examination of stability of the cortisol response across stressors.
In addition, mothers completed questionnaires including a demographic and pregnancy questionnaires and a measure of obsterical complications. The medical record also was used for generating information on the delivery and the immediate postpartum period.
Measures
The Demographic and Pregnancy questionnaire included questions about family structure (e.g., marital status, number of people in the home), history of pregnancies (e.g., age at first birth, number of abortions), involvement with protective services, parental education, and family income. This is an unpublished questionnaire that was designed for the present study. (Copies are available from the first author.)
Neonatal birth characteristics, including birth weight and gestational age, were collected from the medical record.
The Rochester Research Obstetrical Scale (ROS; Sameroff, Seifer, & Zax, 1982) contains 27 complication items covering three categories: antepartum, intrapartum, and neonate. The scale has shown prediction to both neonatal and infant developmental functioning (Molfese & Thomson, 1985). Although the scale covers the use of chronic medications, it does not contain items pertaining to other substance use. Therefore, an addendum to the ROS, which was designed for the present study, was administered to the mother. The addendum covered frequency and duration of cigarette, alcohol, and drug use during pregnancy, and duration of exposure to cigarette smoke during pregnancy.
The NBAS assesses the infant's response to his or her environment. A broad range of behaviors is sampled including reflexes, state changes, attention, arousal, and regulatory capacities. The NBAS was designed for use with healthy, full-term infants. High interrater reliability has been established through certified training programs. In the present study, the principal investigator was trained and certified by The Brazelton Institute and trained the second rater. Interrater reliability was assessed on 25% of the participants, resulting in Spearman correlation coefficients ranging from .80 to .99.
Salivary Cortisol
To collect saliva samples, an absorbent dental roll (unflavored salivette) was applied to the tongue, cheek, and gums of the infant for 2 to 5 min. The dental roll then was returned to the plastic tube and labeled. Samples were immediately transferred to a freezer and stored at –20° C until assayed. On the day of testing, samples were thawed, centrifuged at 3,000 rpm for 10 min, and a clear sample was pipetted into appropriate test wells. All samples from each subject were assayed in the same assay batch to minimize variability, and all samples were assayed with reagents from the same lot. Samples were assayed in duplicate using the Salimetrics HS Salivary Cortisol EIA Kit for unbound cortisol. This assay has a lower limit of sensitivity from 0.007 to 1.2 μg/dl. The average between-assay variance is 3.9 and 7.1%, and the average within-assay variance is 6.7 and 6.9% for high and low concentrations, respectively. The correlation between saliva and serum using the salimetrics HS Salivary Cortisol EIA Kit and the Coat-a-Count Serum Cortisol RIA kit was .96, p<.0001. Typically, salivary cortisol values generated by the RIA kits are slightly higher than those generated by the EIA kit. Unlike the RIA kits, external controls, with known cortisol values, are included in the EIA kit. Thus, an assessment of the accuracy of the assay is systematically evaluated, the results of which support the validity of the cortisol values returned by the EIA protocol (D. Granger, personal communication, November 20, 2001).
In the present study, the average of the duplicate tests was used in the analyses: Approximately 10% of the samples did not have sufficient quantity to run duplicate assays. The within-assay variance in the present sample was 3.2%.
Of the 100 participants recruited for the study, 83 had three (i.e., baseline, 20-, and 45-min poststressor samples) sufficient saliva samples for at least one of the stressors (i.e., NBAS or heel stick). A total of 62 participants had complete cortisol data for the NBAS paradigm, and 64 participants had complete data for the heel-stick paradigm: the overlap between these two groups resulted in the 83 subjects with complete data for at least one of the two stressors. Stimulants, such as Kool-Aid crystals, were not used in the present study because of the variable effect on cortisol assays (Schwartz, Granger, Susman, Gunnar, & Larid, 1998). Although newborns have minimal saliva output, sufficient amounts were collected for most of the sample. Although in some cases the amount of saliva collected was overestimated, the majority of inadequate volumes was the result of lower saliva production by the neonates. Since our sample consisted of healthy neonates of average gestational age and weight, one could conceptualize the amount of missing cortisol data as a reflection of normative variation in saliva production. On the other hand, individual differences in saliva production may reflect more systematic differences in stress regulation. In an effort to preliminarily address this issue, we compared infants who had sufficient saliva output in response to at least one stressor versus those who did not. There were no significant differences between these two groups in pregnancy complications, birth weight, or sex. There also were no differences in cortisol values between those with complete and those with incomplete data.
RESULTS
Demographic characteristics of the sample are presented in Table 1. The goal of recruiting participants from the general care nursery was to generate a relatively homogenous group with regard to sociodemographic and prenatal factors. All mothers were African-American and received some type of public aid. On average, the mothers in the present study were in their early twenties, had graduated from high school, were single, and had family incomes of about $600 per month. About a third of the births were first births. Mothers in this sample were generally healthy during pregnancy, received regular prenatal care, with relatively few mothers reporting use of cigarettes or alcohol during pregnancy. The newborns were full term and of average weight. Almost half of the sample (47%) was male, and all but 4 (8.5%) of the males were circumcised.
Table 1.
Demographic Characteristics of Sample (N = 100)
| M | SD | Median | Range | |
|---|---|---|---|---|
| Family characteristics | ||||
| Maternal age | 22.8 | 4.5 | 21.5 | 17–41 |
| Maternal age at first pregnancy | 17.8 | 2.5 | 18.0 | 13–29 |
| Family income (monthly) | 604.3 | 610.6 | 414.0 | 0–2,800 |
| % | ||||
| Mothers’ with high school degree | 70.0 | |||
| Married/coresiding with father | 30.0 | |||
| Pregnancy characteristics | % | |||
| Primiparous | 34.0 | |||
| Births by Caesarean sectiona | 21.0 | |||
| Cigarette use during pregnancyb | 14.0 | |||
| Alcohol use during pregnancyc | 4.0 | |||
| Infant characteristics | ||||
| Gestational age | 39.1 | 1.3 | 39.0 | 36–42 |
| Birth weight | 3,220.3 | 398.3 | 3,137.5 | 2,500–4,560 |
| % | ||||
| Males | 46.0 |
12 (57%) of the 21 births by Caesarean section were planned.
Range from one cigarette to one half-pack per day.
All 4 mothers reported drinking one to two times per month during the first trimester.
Distribution of Cortisol Values
The first step was to examine the descriptive statistics for the cortisol samples. Historgrams were computed to assess skewness. Prestressor cortisol values were highly skewed (1.6 for pre-heel stick and 1.1 for pre-NBAS). Based on these results, log10 tranformations of all cortisol values were computed. The results that follow are based on these log10-transformed values.
Descriptive statistics for the baseline, peak, and recovery cortisol values and the length and time of day of each stressor are in Table 2. The average baseline cortisol value was statistically higher in the heel-stick paradigm than in the NBAS paradigm (–.40 vs. –.53, t = 2.39, p<.05). The heel stick also occurred somewhat later in the day (mean = 15:06) than the NBAS (mean = 13:52); however, this difference in timing of stimulus was not statistically significant. Moreover, the lack of an established circadian rhythm in the neonate reduces the possibility of effects of time of day of the saliva sampling (Lewis & Ramsay, 1995a). The NBAS differed from the heel stick in terms of the average duration of the stressor (20 vs. 5 min, respectively).
Table 2.
Descriptive Statistics for Cortisol (log10) and Stressors
| M | SD | Median | Range | |
|---|---|---|---|---|
| NBAS (n = 62) | ||||
| Prestressor cortisol | –0.53 | 0.37 | –0.57 | –2.0–.51 |
| 20-min poststressor cortisol | –0.42 | 0.43 | –0.46 | –2.0–.45 |
| 45-min poststressor cortisol | –0.48 | 0.39 | –0.57 | –1.52–.35 |
| Length of NBAS (min) | 20.54 | 5.48 | 20.00 | 12–32 |
| Time of day of NBAS | 13:52 | 2:48 | 13:47 | 9:35–19:51 |
| Heel stick (n = 64) | ||||
| Prestressor cortisol | –0.40 | 0.39 | –0.47 | –1.10–.56 |
| 20-min poststressor cortisol | –0.24 | 0.38 | –0.36 | –.97–.60 |
| 45-min poststressor cortisol | –0.40 | 0.42 | –0.46 | –1.10–.42 |
| Length of heel stick (min) | 5.14 | 3.40 | 4.00 | 01–17 |
| Time of day of heel stick | 15:06 | 3:17 | 14:43 | 9:44–20:39 |
NBAS = Neonatal Behavioral Assessment Scale.
The effect of the ordering of the stressors on cortisol response was examined by including all subjects irrespective of the completeness of the cortisol data. This allowed us maximum power to test for ordering effects. For these analyses, the ns ranged from 76 to 86. The heel-stick procedure occurred first for 80% of the participants. Analyses of variance between those who received the heel stick first versus those who received the heel stick second were computed within each stressor, for each sample (pre-, 20- and 45-min post). There were no significant differences in any of the cortisol values based on the order of the stressors.
Change in Cortisol Level
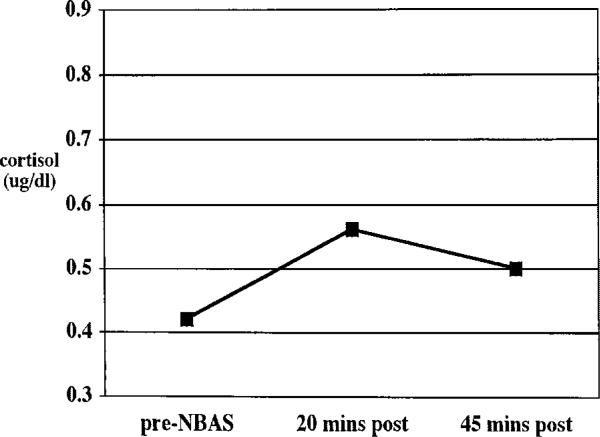
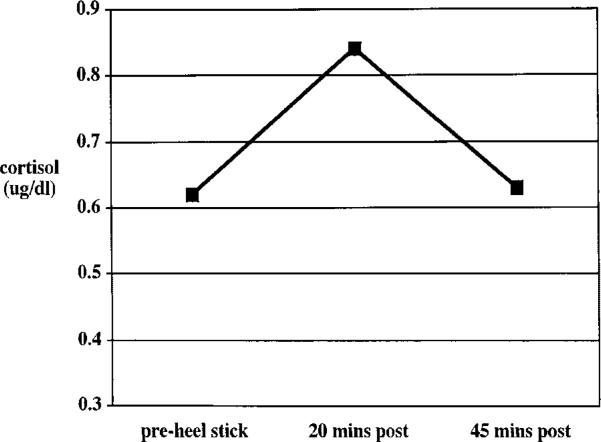
Friedman two-way ANOVA for related samples revealed a significant effect of time in cortisol values in response to both the NBAS (chi-square 8.8, df = 2, p<.05) and the heel stick (chi-square = 21.5, df = 2, p<.001) Figures 1 and 2 depict the average cortisol level across time using nontransformed cortisol values for ease of explication. To test whether these patterns of cortisol response were significantly different across the two paradigms, paired t-tests were conducted on the change scores from pre- to 20-min post-, 20-min post- to 45-min post-, and pre- to 45-min poststressor for the heel stick versus the NBAS. For these sets of analyses, only subjects who had complete data for both the heel stick and the NBAS were included (n = 41). The difference between the change from 20-min to 45-min poststressor for the NBAS (M = –.07) and the heel stick (M = –.17) was significant (t = 2.19, df = 40, p<.05). The other two comparisons were not significantly different.
FIGURE 1.
Average cortisol response to the Neonatal Behavioral Assessment Scale administered within 48 hr of birth. Log10 cortisol values were converted back to raw scores for ease of explication. NBAS = Neonatal Behavioral Assessment Scale, N = 62.
FIGURE 2.
Average cortisol response to a heel stick administered within 48 hr of birth. Log10 cortisol values were converted back to raw scores for ease of explication. N = 64.
Correlates of Baseline Cortisol and Reactivity
A number of child and environmental factors were examined with respect to their association with baseline cortisol, change from baseline to peak response, and change from peak response to recovery. Gender, gestational age, birth weight, and total number of obstetrical complications, as measured by the ROS, were not significantly correlated with any cortisol value. Duration and time of day of stressor were not associated with baseline cortisol or change scores. Given the small number of boys who were not circumcised (n = 4), we were unable to compare cortisol response for circumcised and uncircumcised boys.
The effect of state at the onset of the stressor was tested. Infants were classified as being in any stage of sleep (deep, light, or semidozing) or wakefulness (alert, active, or crying). Infant state prior to the heel stick was coded from videotape. As part of the standard NBAS protocol, the examiner coded the infant's state prior to beginning the NBAS. Although there were some differences in handling prior to each stressor (e.g., application of a heel warmer prior to the heel stick), there were no differences in the proportion of awake or asleep infants across stressor. Prior to the administration of the NBAS, 41 (66%) of the infants with complete data were in sleep states, and 13 (21%) were in wake states (eight subjects were missing data). Prior to the onset of the heel stick, 46 (75%) infants were in a sleep state, and 13 (21%) were in a wake state (two subjects were missing data). State of infant prior to the heel stick and the NBAS was not associated with cortisol values pre-or poststressor.
Quality of Initial Response
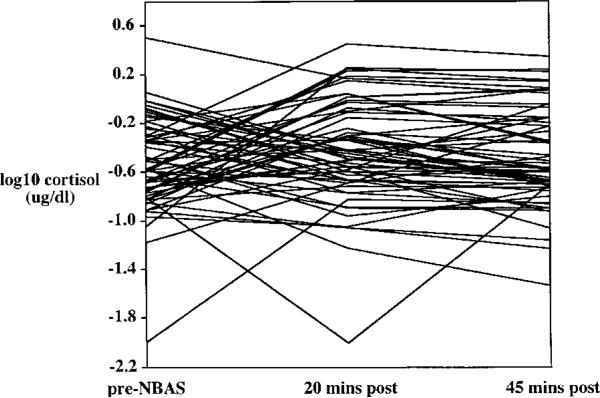
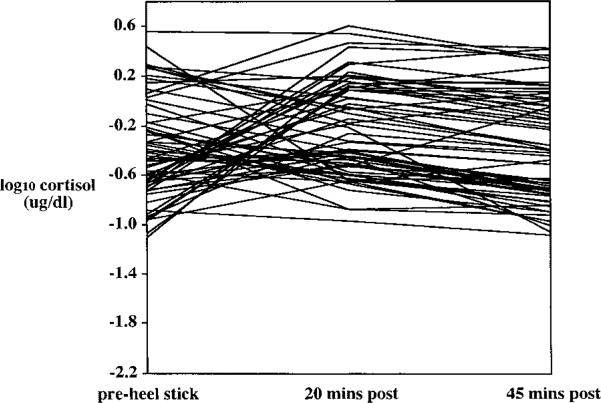
It was of interest to examine individual patterns of cortisol reactivity to the two stressors. Each infant's cortisol level at pre-, 20-min post-, and 45-min post-stressor was plotted for the two stressors (Figures 3 and 4). These plots provided evidence for three qualitatively different subgroups: infants whose cortisol level increases following a stressor, infants whose cortisol level decreases following a stressor, and infants who show relatively little change in cortisol.
FIGURE 3.
Individual differences in cortisol response to the Neonatal Behavioral Assessment Scale. Cortisol was assayed from saliva collected prior to the onset of the stressor (pre) and 20 and 45 min after the stressor ended. NBAS = Neonatal Behavioral Assessment Scale, N = 62.
FIGURE 4.
Individual differences in cortisol response to a heel stick. Cortisol was assayed from saliva collected prior to the onset of the stressor (pre) and 20 and 45 min after the stressor ended. N = 64.
To classify these responses into subgroups, an increase of at least .5 of 1 SD of the delta value from pre- to 20-min poststressor defined the increasers, a decrease of at least .5 of 1 SD defined the decreasers, and the remaining infants were defined as no change. Using these definitions, the following groups were generated: 6 (9.4%) decreasing values, 40 (62.5%) no-change values, and 18 (28.1%) increasing values in response to the heel stick. The corresponding distribution in response to the NBAS was 16 (25.8%) decreasing values, 23 (37.1%) no-change values, and 23 (37.1%) increasing values.
Associations between the three groupings based on quality of cortisol response and individual infant characteristics including gestational age, birth weight, obstetrical complications, and gender were tested by chi-square for categorical data and ANOVA for continuous data; none were significant. Duration of stressor and infant state prior to stressor were not associated with quality of response.
The effect of baseline values on the quality of the initial response was tested by ANOVA, in which the baseline values among the three groups were compared. Post hoc tests using the Scheffe test revealed that infants with decreasing cortisol values had significantly higher prestressor cortisol than either the no-change group or the increasing group in response to the NBAS (M = –.19 vs. M = –.55 and M –.74, respectively, overall F(2, 59) = 15.34, p<.0001). Infants with increasing cortisol values had significantly lower prestressor cortisol than either the no-change group or the decreasing group in response to the heel stick (M = –.67 vs. M = –.34 and M = –.00, respectively, overall F(2, 61) = 10.08, p<.001).
Quality of Recovery
The degree to which individuals’ cortisol changed from 20- to 45-min poststressor was categorized using the same definition described earlier. Thus, infants whose delta cortisol values from 20- to 45-min posts-tressor had decreased at least .5 of 1 SD were classified as decreasers, individuals whose delta cortisol values were greater than .5 of 1 SD were classified as increasers, and the remainder was classified as demonstrating no change. This definition resulted in the following distribution of scores: 24 (38.7%) decreasers, 29 (46.8%) no change, and 9 (14.5%) increasers in response to the NBAS, and 32 (50.0%) decreasers, 30 (46.9%) no change, and 2 (3.1%) increasers in response to the heel stick.
Associations between these three groupings and infant characteristics revealed no significant associations. The effect of baseline values on the degree of change during the recovery response was tested by ANOVA. None of the comparisons were significant.
Individual Stability Across Stressors
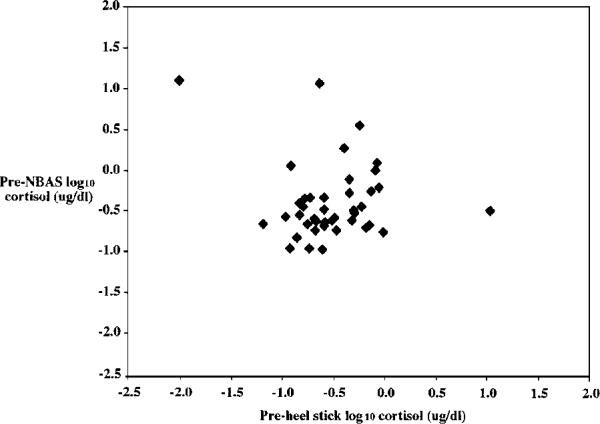
The next question posed was whether individual differences in cortisol were stable across paradigms (NBAS and heel stick). Prestressor cortisol values were significantly correlated across the two paradigms (r =.41, p<.01) (Figure 5). Change scores from 20- to 45-min poststressor also were significantly correlated across the two stressors (r=.32, p<.05). Change scores reflecting initial response from pre- to 20-min poststressor were not significantly correlated.
FIGURE 5.
Stability of prestressor cortisol values. Pearson correlation coefficient = .42, p<.01, N = 41.
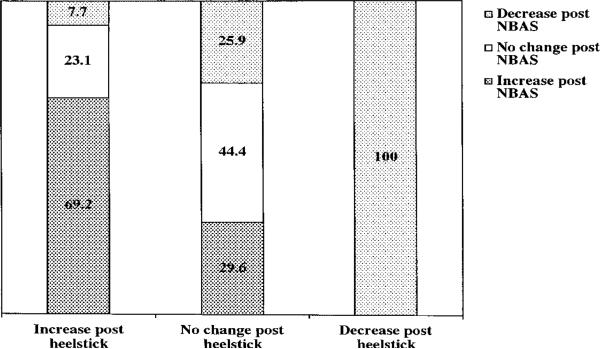
Finally, stability of cortisol response across the two stressors was tested using the quality of response categorical variable (i.e., decrease, no change, and increase in cortisol from baseline to 20-min poststressor). A 3×3 chi-square analysis was then computed to test whether participants were more likely than chance to demonstrate the same pattern of increasing and decreasing cortisol values across both stressors. The stability of classification as a decreaser, no change, or increaser in cortisol reactivity was statistically significant (Mantel Haenszel = 6.91, df=1, p<.01). The results are presented in Figure 6. Of the 13 infants who were classified as increasers in response to the heel stick, 9 (69.2%) were classified as increasers, 3 (23.1%) were classified as stable, and 1 (7.7%) was classified as a decreaser in response to the NBAS. Of the 27 infants classified as no change in response to the heel stick, 12 (44.4%) showed no change in response to the NBAS, 8 (29.6%) were classified as increasers, and 7 (25.9%) were classified as decreasers. Finally, only 1 infant was classified as a decreaser to the heel stick. That infant also was a decreaser in response to the NBAS (Figure 6).
FIGURE 6.
Stability of the pattern of cortisol response across two stressors. NBAS = Neonatal Behavioral Assessment Scale, N = 41. An increase of at least .5 of 1 SD of the delta value from pre-to 20-min poststressor defined the increasers, a decrease of at least. 5 of 1 SD defined the decreasers, and the remaining infants were defined as no change. Note that only 1 subject demonstrated a decrease in cortisol in response to the heel stick. Mantel-Haenszel test for linear association = 6.9, df = 1, p<.01.
The stability of classification based on degree of change from 25 to 45 min also was tested by chi-square analysis, but was not significant.
DISCUSSION
The purpose of this study was to expand upon the existing literature on infant cortisol reactivity by examining patterns of reactivity using two poststressor samples, and individual stability in prestressor and cortisol reactivity across two different paradigms. Unlike previous studies, cortisol response was examined in a sample of African American neonates whose families live in low-income environments. In general, correlations were small to moderate in this study. Thus, replication of findings on larger, but similar samples with regard to sociodemographic characteristics and ethnicity are needed. It is useful, however, to generate data that are relevant to a population of children who have been underrepresented in the literature to date and for whom identifying markers of sensitivity to stress may be particularly meaningful. Despite the small sample size, many significant relations were detected that further our understanding individual differences in and factors related to cortisol reactivity.
Data from the present study indicated that for most infants, a significant increase in cortisol in response to both the heel stick and the NBAS was captured by sampling 20-min poststressor. Similarly, the 45-min poststressor sample captured a phase of recovery from the stressors for most infants. However, an examination of individual cortisol values revealed that there is a significant amount of variability across individuals that warrants further assessment. For example, 14.5% of infants continued to show an increase in cortisol 45-min post-NBAS. Thus, the recovery period for a substantial group of infants appears to be longer than 45 min. Capturing the peak and recovery phases in cortisol reactivity is likely to be an important aim of studies designed to identify markers for psychopathology. The ability to identify the magnitude of the initial response and the point at which levels start to decline may be particularly useful for identifying infants at risk for problems with emotion regulation. Thus, multiple samples may be needed to capture important individual differences, especially with respect to identifying atypical or maladaptive patterns of stress response.
There is some evidence of individual stability in both baseline cortisol levels and the magnitude of recovery from 20- to 45-min poststressor. Whether an infant responded with a cortisol increase or decrease also was relatively stable across stressors. Slightly more than half of the infants demonstrated the same pattern of initial response across both paradigms. Given that the stressors were quite different in terms of the demands on the infant, the level of discomfort, and the duration, one might hypothesize that there would be more variability than stability in the quality of the cortisol response. On the other hand, baseline cortisol values have a significant impact on the direction of the initial response. For many infants, the effect of baseline values appears strong enough to be maintained even in the context of qualitatively different stressors.
With regard to patterns of cortisol responsiveness, there appear to be three: neonates who respond to a stressor with an increase in cortisol, those who have a minimal or no response, and those who respond with a decrease in cortisol. Increasers and decreasers have been described before (Ramsay & Lewis, 1994), but little data has been presented on the stable-low group in the infant literature. At this stage, it is difficult to know how to conceptualize these groups of infants with regard to regulation of arousal. Many approaches have been used to quantify patterns of responding across development including dividing subjects into increasers versus decreasers (Ramsay & Lewis, 1994), using a change of 1 full SD to identify responders (Gunnar, Connors, Isensee, & Wall, 1988), and using the upper and lower quartiles of the delta distributions (Granger, Weisz, & Kauneckis, 1994). Currently, there is no standard by which to quantify how much of a change is meaningful. In the present study, increasers and decreasers were defined on the basis of a change of 0.5 of 1 SD from baseline to 20-min poststressor, and from 20- to 45-min poststressor. This definition has been used in the literature previously in quantifying cortisol response in elementary-school children (Davis, Donzella, Krueger, & Gunnar, 1999). We focused on the delta values to quantify patterns of response because of the significant effect of baseline values on change scores. The use of 0.5 of 1 SD also allowed us to generate distributions that could vary across stressor, which was an important criterion given that we viewed the two stressors as having the potential to lead to different patterns of cortisol response.
Developing methods for differentiating adaptive versus maladaptive patterns of cortisol response is a critical component to the interface between the study of individual differences in stress reactivity and developmental psychopathology. Most developmental psychologists would agree that a well-modulated response to stimuli is considered adaptive (Keenan, 2000). Definitions of “well-modulated,” however, have not fully emerged from the existing literature. There are a number of ways to begin to address this issue. Additional data on level of arousal at both the biological and behavioral level may be needed to refine the operationalization of change or reactivity. For example, behavioral data on intensity of crying or latency to soothe may be used in conjunction with cortisol levels to indicate whether an increase is meaningful. However, that approach assumes that behavioral and biological responses to stimuli operate in tandem. There is actually little evidence for this (Gottlieb, Johnston and Scoville, 1982; Gunnar, Fisch, & Malone, 1984).
Another approach to examining the importance of the magnitude of response and recovery is testing the predictive utility over time to other measures of emotion dysregulation. Such an approach also will require the assessment of contextual factors (e.g., caregiving) so that stability of cortisol reactivity can be measured in the context of stable and unstable environmental factors. Family context does appear to be related to cortisol reactivity in older children (Flinn & England, 1995; Granger et al., 1998), and maternal responsiveness is related to cortisol reactivity in older infants (Spangler, Schieche, Ilg, Maier, & Ackerman, 1994). Temperamental difficultness may explain some of the variance in cortisol response to different caregiving contexts (Gunnar et al., 1992).
There also is evidence to suggest that cortisol increases in response to stressors during infancy are a sign of robustness, which is associated with better temperamental outcomes (Gunnar, Porter, Wolf, Rigatuso, & Larson, 1995). However, infants in these types of studies are typically growing up in environments with adequate levels of resources. Thus, it remains to be seen whether cortisol reactivity to stress results in positive developmental outcomes for other groups of children.
Finally, we limited the measure of stress reactivity to changes in cortisol concentration. Yet, there are other hormones involved in stress regulation as well as other biological systems. Cacioppo (1994) reported that adults who were characterized as high heart-rate reactors showed higher stress-related levels of cortisol. Thus, studies that examine whether an individual's pattern of stress regulation varies or remains consistent across stressors and biological systems are likely to be the most useful in clarifying how this complex process of regulation of arousal is achieved.
In summary, two dimensions of cortisol reactivity are in need of continued investigation: individual stability and patterns of responsiveness, including recovery from stress. Both of these dimensions have the potential to contribute to our understanding of how deficits in regulatory skills early in life may be related to later problems in behavioral and emotional functioning. Using biological markers to inform our understanding and prediction of behavioral and emotional processes is complex. The assessment of a system such as the HPA system needs to be dynamic and comprehensive. By examining cortisol reactivity and other systems involved in the regulation of arousal in response to qualitatively different stressors, meaningful differences in individual patterns of response may emerge.
Acknowledgments
This study was supported by Grant K01 MH-01484 from the National Institute of Mental Health to K.K., the Walden and Jean Young Shaw Foundation, and the Clinical Research Center at the University of Chicago. We thank the mothers and babies who participated in the Chicago Baby Project and Marguerite Herschel, Director of the General Care Nursery at the University of Chicago. We also thank Nina Perales for assistance with data collection and entry, and Douglas Granger and Marry Curran from the Behavioral Endocrinology Laboratory for assistance with the cortisol assays and interpretation of the cortisol data. Benjamin Lahey and Michael Lewis provided valuable comments on earlier drafts of the article.
Contract grant sponsor: NIMH
Contract grant number: K01 MH01484
Contract grant sponsor: Walden and Jean Young Shaw
Contract grant sponsor: University of Chicago Clinical Research Center
REFERENCES
- Adams CD, Hillman N, Gaydos GR. Behavioral difficulties in toddlers: Impact of sociocultural and biological risk factors. Journal of Clinical Child Psychology. 1994;23:373–381. [Google Scholar]
- Brazelton TB, Nugent JK. Neonatal Behavioral Assessment Scale. 3rd ed. Cambridge University Press; New York: 1995. [Google Scholar]
- Cacioppo JT. Social neuroscience: Autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31:113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: Individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology. 1999;35:188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Davis M, Emory E. Sex differences in neonatal stress reactivity. Child Development. 1995;66:14–27. doi: 10.1111/j.1467-8624.1995.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Childhood stress and family environment. Current Anthropology. 1995;36:854–866. [Google Scholar]
- Gottlieb G, Johnston TD, Scoville RP. Conceptions of development and the evolution of behavior. Behavioral and Brain Sciences. 1982;5:284. [Google Scholar]
- Granger DA, Serbin LA, Schwartzman A, Lehoux P, Cooperman J, Ikeda SC. Children's salivary cortisol, internalizing behaviour problems, and family environment: Results from the Concordia Longitudinal Risk Project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Granger DA, Stansbury K, Henker B. Preschoolers’ behavioral and neuroendocrine responses to social challenge. Merrill–Palmer Quarterly. 1994;40:190–211. [Google Scholar]
- Gunnar M, Broderson L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67:877–889. [PubMed] [Google Scholar]
- Gunnar M, Connors J, Isensee J. Lack of stability in neonatal adrenocortical reactivity because of rapid habituation of the adrenocortical response. Developmental Psychobiology. 1989;22:221–233. doi: 10.1002/dev.420220304. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Connors J, Isensee J, Wall L. Adrenocortical activity and behavioral distress in human newborns. Developmental Psychobiology. 1988;21:297–310. doi: 10.1002/dev.420210402. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisch RO, Malone SM. The effects of pacifying stimulus on behavioral and adrenocortical responses to circumcision in the newborn. Journal of the American Academy of Child and Adolescent Psychiatry. 1984;23:34–38. doi: 10.1097/00004583-198401000-00005. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Hertsgaard L, Larson M, Rigatuso J. Cortisol and behavioral responses to repeated stressors in the human newborn. Developmental Psychobiology. 1992;24:487–505. doi: 10.1002/dev.420240704. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Isensee J, Fust LS. Adrenocortical activity and the Brazelton Neonatal Assessment Scale: Moderating effects of the newborn's biomedical status. Child Development. 1987;58:1448–1458. [PubMed] [Google Scholar]
- Gunnar M, Larson MC, Hertsgaard L, Harris ML, Brodersen L. The stressfulness of separation among nine-month-old infants: Effects of social context variables and infant temperament. Child Development. 1992;63:290–303. [PubMed] [Google Scholar]
- Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: Predictions to later emotional temperament. Child Development. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development and Psychopathology. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Keenan K. Emotion dysregulation as a risk factor for psychopathology. Clinical Psychology: Science and Practice. 2000;7:418–434. [Google Scholar]
- Keenan K, Shaw DS, Delliquadri E, Giovannelli J, Walsh B. Evidence for the continuity of early problem behaviors: Application of a developmental model. Journal of Abnormal Child Psychology. 1998;26:443–454. doi: 10.1023/a:1022647717926. [DOI] [PubMed] [Google Scholar]
- Larson MC, Gunnar MR, Hertsgaard L. The effects of morning naps, car trips, and maternal separation on adrenocortical activity in human infants. Child Development. 1991;62:362–372. [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Developmental change in infants’ responses to stress. Child Development. 1995a;66:657–670. doi: 10.1111/j.1467-8624.1995.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Stability and change in cortisol and behavioral response to stress during the first 18 months of life. Developmental Psychobiology. 1995b;28:419–428. doi: 10.1002/dev.420280804. [DOI] [PubMed] [Google Scholar]
- Lewis M, Thomas D. Cortisol release in infants in response to inoculation. Child Development. 1990;61:50–59. [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Christ MAG, Hanson KS. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American AcademyofChildandAdolescentPsychiatry. 1991;30:192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. The impact of economic hardship on Black families and children: Psychological distress, parenting, and socioemotional development. Child Development. 1990;61:311–346. doi: 10.1111/j.1467-8624.1990.tb02781.x. [DOI] [PubMed] [Google Scholar]
- Molfese VJ, Thomson B. Optimality versus complications: Assessing predictive values of perinatal scales. Child Development. 1985;56:810–823. [PubMed] [Google Scholar]
- Ramsay DS, Lewis M. Developmental change in infant cortisol and behavioral response to inoculation. Child Development. 1994;65:1491–1502. doi: 10.1111/j.1467-8624.1994.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Zax M. Early development of children at risk for emotional disorder. Monographs for the Society for Research in Child Development. 1982;47 [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Spangler G, Scheubeck R. Behavioral organization in newborns and its relation to adrenocortical and cardiac activity. Child Development. 1993;64:622–633. [PubMed] [Google Scholar]
- Spangler G, Schieche M, Ilg U, Maier U, Ackerman C. Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology. 1994;27:425–437. doi: 10.1002/dev.420270702. [DOI] [PubMed] [Google Scholar]