Abstract
Mycobacterium tuberculosis (Mtb) can persist in hostile intracellular microenvironments evading immune cells and drug treatment. However, the protective cellular niches where Mtb persists remain unclear. We report that Mtb may maintain long-term intracellular viability in a human bone marrow (BM)–derived CD271+/CD45− mesenchymal stem cell (BM-MSC) population in vitro. We also report that Mtb resides in an equivalent population of BM-MSCs in a mouse model of dormant tuberculosis infection. Viable Mtb was detected in CD271+/CD45− BM-MSCs isolated from individuals who had successfully completed months of anti-Mtb drug treatment. These results suggest that CD271+ BM-MSCs may provide a long-term protective intracellular niche in the host in which dormant Mtb can reside.
INTRODUCTION
Tuberculosis (TB) infects nearly 2.2 billion people worldwide (1, 2). Effective drugs that can target replicating Mycobacterium tuberculosis (Mtb) have been available for 50 years. Yet, the eradication of this disease remains elusive. This has been largely attributed to the ability of Mtb to maintain a latent or dormant infection in a host despite the evidence for a vigorous host immune response (1, 2).
Dormant Mtb may remain in a nonreplicating state during asymptomatic infection (1). In addition, dormant Mtb can tolerate the extreme hypoxic environment present in the tuberculous granulomas in lung tissue (3, 4). Dormant Mtb remains sensitive to antitubercular drugs like rifampicin (4, 5), as illustrated by their reduced viability in dormant, fibrotic granulomas after drug treatment (6). However, it is not yet clear how Mtb retains viability during the asymptomatic infection phase as well as during post-chemotherapy dormancy, such that it can be reactivated causing clinical disease (7).
Nonreplicating Mtb may reside in a protective intracellular niche to maintain viability (1). Therefore, identification of the protective intracellular niches that enable Mtb to remain in a viable nonreplicating dormant state is urgently needed to understand the pathogenesis of this disease and to enhance our ability to develop better drugs and vaccines.
Macrophages and dendritic cells have been known for decades to serve as host cells for Mtb growth (8). However, the viability of Mtb in these intracellular niches is poor (9), and no evidence exists indicating that these cells can maintain live nonreplicating Mtb. Other cell types such as epithelial cells, fibrocytes, adipocytes, and endothelial cells distributed in pulmonary and extrapulmonary niches have been described as possible host cells for nonreplicating Mtb (1, 10, 11). These possibilities have been suggested on the basis of human autopsy studies that demonstrated the presence of Mtb DNA in these cell types (10, 11). However, viable nonreplicating Mtb in these cell types during infection in vivo have not been demonstrated. Hence, to date, although Mtb is known to infect many cell types, the evidence that any of these host cells may serve as a reservoir of live nonreplicating Mtb in vivo has as yet not been shown.
We postulated that bone marrow stem cells (BMSCs), comprising both hematopoietic and mesenchymal stem cells (MSCs), might provide an ideal protective niche for nonreplicating Mtb because these cells have several properties that are ideal for the pathogen’s long-term persistence and survival. First, these cells are present in the TB granulomas of infected mouse and human lung tissue (12). Second, they have the capacity for self-renewal (13–15). Third, they express drug efflux pumps such as ABCG2 that could contribute to drug evasion by Mtb (16). Fourth, stem cells produce only low levels of endogenous reactive oxygen species, which might benefit the viability of nonreplicating Mtb. Fifth, they are relatively quiescent (17) and reside in the immune-privileged niche in the BM (18, 19). Therefore, we set out to examine whether BMSCs could be a host cell for the long-term persistence of viable nonreplicating Mtb.
Here, we report that Mtb may persist in a mesenchymal subpopulation of human BMSCs from patients previously treated for pulmonary TB and of mouse BMSCs from a mouse model of nonreplicating Mtb infection. Our results suggest that a BMSC subpopulation, CD271+/CD45− mesenchymal BMSCs (19–25), may provide an intracellular niche for Mtb persistence. CD271+/CD45− cells purified from the BM of mice infected with nonreplicating Mtb maintained the ability to reseed infection when injected into healthy mice. Viable Mtb could be recovered from theCD271+/CD45− cells obtained from individuals who had completed antitubercular treatment. Our observations suggest that a BM cellular niche may be important for the maintenance of the nonreplicating phase of the Mtb life cycle.
RESULTS
Mtb infects and survives in human CD271+/CD133+ BMSCs
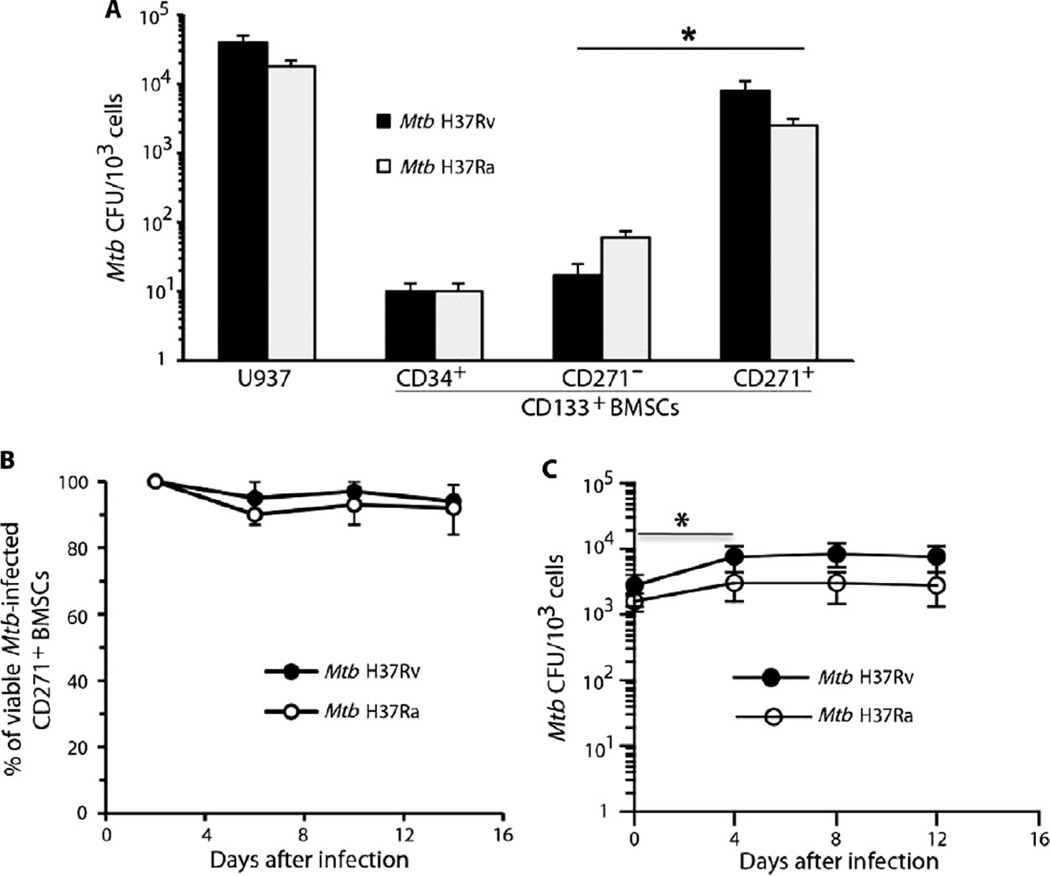
The general stem cell marker CD133 of BMSCs provided the starting point for further subfractionation of human BM-derived stem cells. To determine whether Mtb can infect specific CD133+ BMSC populations, we isolated the CD271+/CD133+, CD271−/CD133+, and CD34+/CD133+ populations from healthy human donors by magnetic sorting (20). The cells were then cultured in vitro in serum-free medium containing growth factors that can maintain CD133+ BMSCs in their undifferentiated state (table S1) (14, 26). Next, these purified subpopulations were exposed to either the virulent strain of Mtb H37Rv or the avirulent strain of Mtb H37Ra. The Mtb-exposed cells were then briefly treated with amikacin to kill residual extracellular bacteria. Subsequently, viable intracellular Mtb were measured by colony-forming unit (CFU) assay using Middlebrook 7H11 agar plates (27). The CD271+/CD133+ BMSC fraction exhibited higher Mtb CFUs than did the other BMSC fractions exposed to either Mtb H37Rv or H37Ra (P < 0.05; Fig. 1A). These results indicated that BMSCs can be infected in vitro with Mtb and that the CD271+/CD133+ BMSC fraction was the most permissive for Mtb infection.
Fig. 1.
Mtb infects human CD271+/CD133+ BMSCs. (A) In vitro infection of human CD271+/CD133+ BMSCs with Mtb H37Ra or Mtb H37Rv mycobacterial strains resulted in highest CFU production in the CD271+ fraction of CD133+ BMSCs. Infection of U937 cells was the control. The cells were infected with Mtb [multiplicity of infection (MOI), 5:1], and CFUs were counted after 4 days of in vitro culture. (B) Mtb infection did not reduce CD271+/CD133+ BMSC viability as revealed by the Alamar blue assay (28). (C) Mtb CFUs derived from infected CD271+/CD133+ BMSCs cultured in serum-free medium for 2 weeks (table S1). *P < 0.05, Student’s t test; n = 4 independent experiments.
In the next step, the long-term viability of both the host cells and Mtb from Mtb-infected CD271+/CD133+ BMSCs was measured after 2 weeks of in vitro culture (Fig. 1B). The infection of CD271+/CD133+ human BMSCs with either Mtb H37Ra or Mtb H37Rv did not change the viability of the host cells as measured by Alamar blue assay (28). The number of Mtb CFUs increased only two- to threefold between 0 and 4 days of infection (P < 0.05; Fig. 1C) and then remained unchanged. These results suggest that the internalized and viable avirulent and virulent Mtb strains can replicate, albeit slowly, without impeding the growth of CD271+/CD133+ BMSCs.
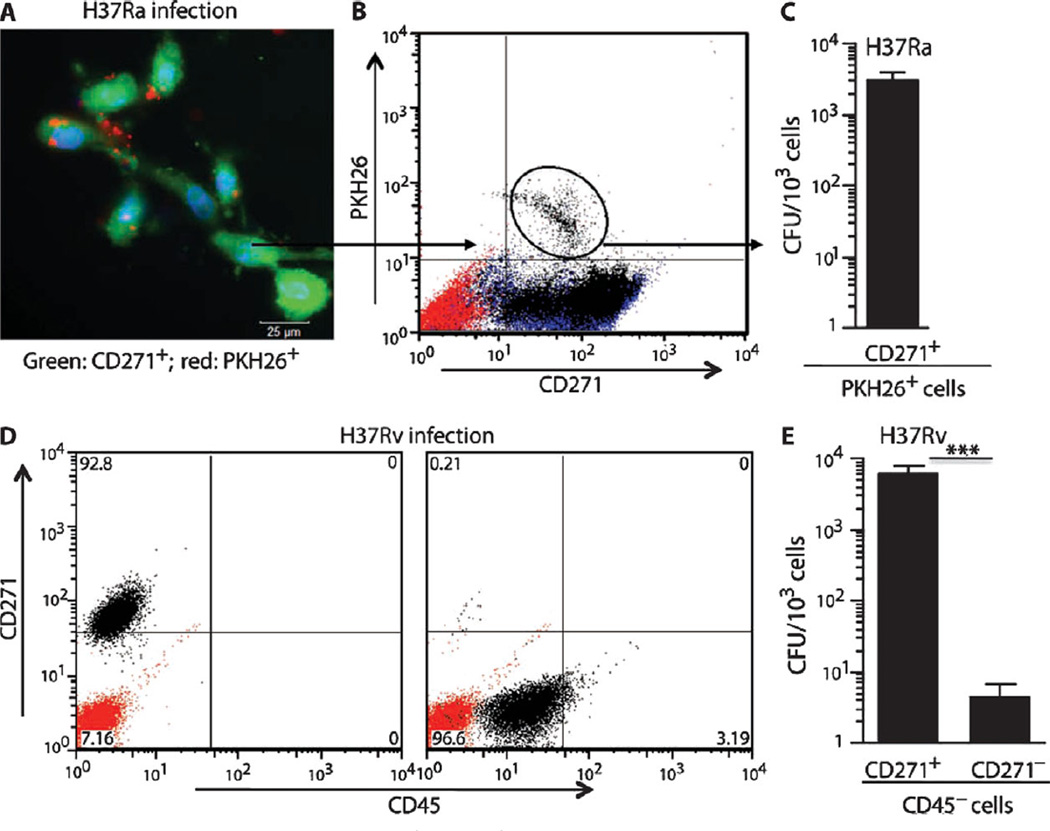
To further examine and quantify Mtb infection of CD271+/CD133+ human BMSCs, we used PKH26-labeled Mtb (27) to visualize internalized mycobacteria by fluorescence microscopy (Fig. 2A) and to isolate them by fluorescence-activated cell sorting (FACS). Internalized H37Ra Mtb were clearly visible (Fig. 2A), and viable Mtb could be isolated from the FACS-sorted CD271+/PKH26+ cells (Fig. 2, B and C). As a control, labeled heat-killed Mtb or rifampicin-killed Mtb were confirmed to show markedly reduced intracellular internalization with fewer Mtb DNA copies associated with CD271+/CD133+ BMSCs (fig. S1). Thus, viable Mtb H37Ra can be internalized and retained in CD271+/CD133+ BMSCs.
Fig. 2.
Mtb resides inside human CD271+/CD133+ BMSCs. (A) Epifluorescence image showing amikacin-treated CD271+/CD133+ BMSCs (green) containing intracellular PKH26-labeled Mtb H37Ra strain (red). (B and C) Flow cytometry analysis of infected CD271+/CD133+ BMSCs, and CFUs of PKH26-labeled Mtb H37Ra bacteria derived from infected CD271+/CD133+ BMSCs. (D) Purity of the magnetically sorted CD271+/CD45− and CD271−/CD45− cells obtained from H37Rv-infected CD271+/CD133+ BMSCs. (E) Mtb H37Rv CFUs obtained from both of the cell populations depicted in (D). The CD271+/CD133+ BMSCs were infected with Mtb (MOI, 5:1) and grown for 2 weeks in serum-free medium (table S1) before FACS analysis (B) or immunomagnetic sorting (D). The red-stained population in flow cytometry panels (B and D) represents isotype controls. Before harvesting for the Mtb CFU study, infected cells were treated with amikacin (200 µg/ml) for 3 hours to kill extracellular Mtb. *P < 0.05, Student’s t test; n = 4 independent experiments.
CD271+/CD133+ BMSCs contain both hematopoietic stem cells (HSCs; CD34+/CD45+) and MSCs (CD271+/CD45−) (20–22). To define which subpopulation was preferentially infected by Mtb, we examined FACS-sorted CD271+/PKH26+ cells for HSC and MSC markers by real-time quantitative polymerase chain reaction (qPCR) gene expression and FACS analysis. Gene expression analysis revealed that the Mtb-infected cells expressed MSC markers CD105, CD73, and CD90, whereas the hematopoietic markers CD45 and CD11b were not expressed (fig. S2A).Moreover, expression of the HSC marker CD34 and endothelial progenitor marker VEGFR2 (vascular endothelial growth factor receptor 2) was negligible in the CD271+/PKH26+ cells (fig. S2A). Also, the CD271+/PKH26+ cells expressed ABCG2 (fig. S2A), a drug efflux pump expressed in the undifferentiated CD271+/CD133+ cells (table S1) (20). The FACS analysis of the CD271+/PKH26+ cells confirmed the expression of the MSC markers CD105, CD73, and CD90, and the absence of the hematopoietic marker CD45 (fig. S2B). We conclude that Mtb preferentially infects CD271+/CD45− BM MSCs (henceforth called CD271+ BM-MSCs).
Finally, we examined if Mtb H37Rv also preferentially infects the CD271+ BM-MSCs. Magnetic sorting was performed to isolate the CD271+/CD45− fraction from the infected CD271+/CD133+ BMSCs grown in serum-free culture for 2 weeks (see Supplementary Materials and Methods). Purity of the CD271+/CD45− cells was confirmed (89.2 ± 4.4%; n = 4), as shown in Fig. 2D. Most of the Mtb H37Rv CFUs were obtained from the CD271+/CD45− versus CD271−/CD45− fraction (P < 0.0001; Fig. 2E). We conclude that Mtb preferentially infects CD271+ BM-MSCs.
Undifferentiated CD271+ BM-MSCs are needed to maintain Mtb viability
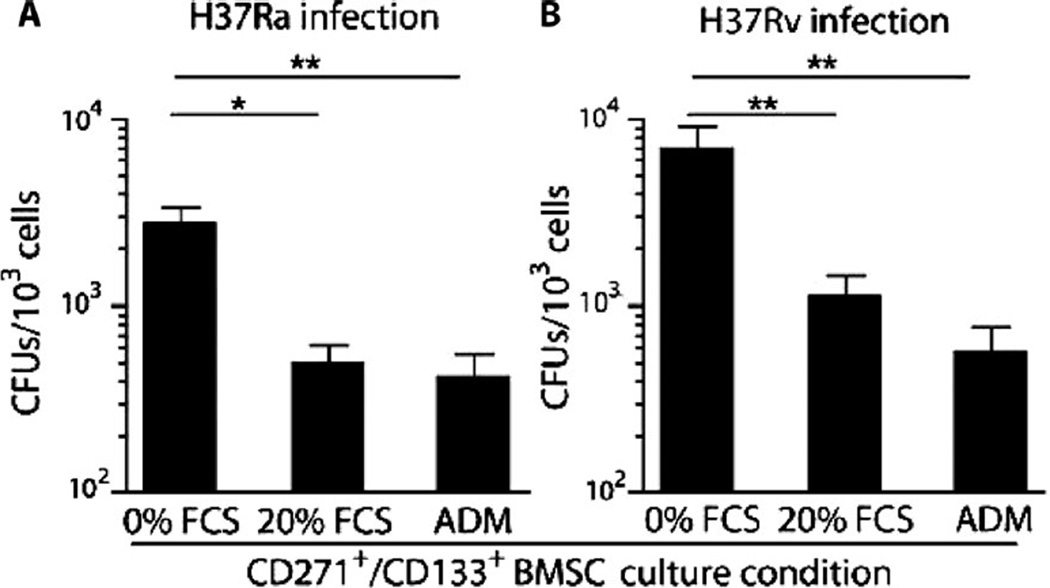
Next, we investigated whether the undifferentiated state of CD271+ BM-MSCs (20) is required to maintain the viability of intracellular Mtb. FACS-sorted CD271+/PKH26+ cells were cultured in a serum-rich medium that induces their differentiation as illustrated by the loss of the CD271/CD133 markers (table S1) (20). Mtb-infected CD271+ BMMSCs were treated with adipogenic differentiation medium to induce their differentiation from BM-MSCs to adipocytes (28). The results (Fig. 3A) illustrate that Mtb viability in the differentiated CD271+ BM-MSCs was reduced by fourfold (P < 0.05). Similar findings were obtained with Mtb H37Rv infection of undifferentiated versus serum-rich and adipogenic differentiation medium–treated CD271+ BM-MSCs (P < 0.001; Fig. 3B). Hence, Mtb viability appears to require the undifferentiated state of CD271+ BM-MSCs.
Fig. 3.
Viable Mtb reside in undifferentiated CD271+/CD133+/CD45− BM-MSCs. The PKH26+/CD271+ (Fig. 2B) or CD271+/CD45− BM-MSCs, which were obtained from CD271+/CD133+ BMSCs (Fig. 2D), were cultured in vitro in high-serum medium [fetal calf serum (FCS); table S1] or adipogenic differentiation medium (ADM) and cultured for 2 weeks before harvesting. Shown are the numbers of Mtb CFUs grown from each cell population. *P < 0.05, **P < 0.001, Student’s t test; n = 5 independent experiments.
Dissemination of Mtb H37Rv from lungs to BM in mice infected with aerosolized bacteria
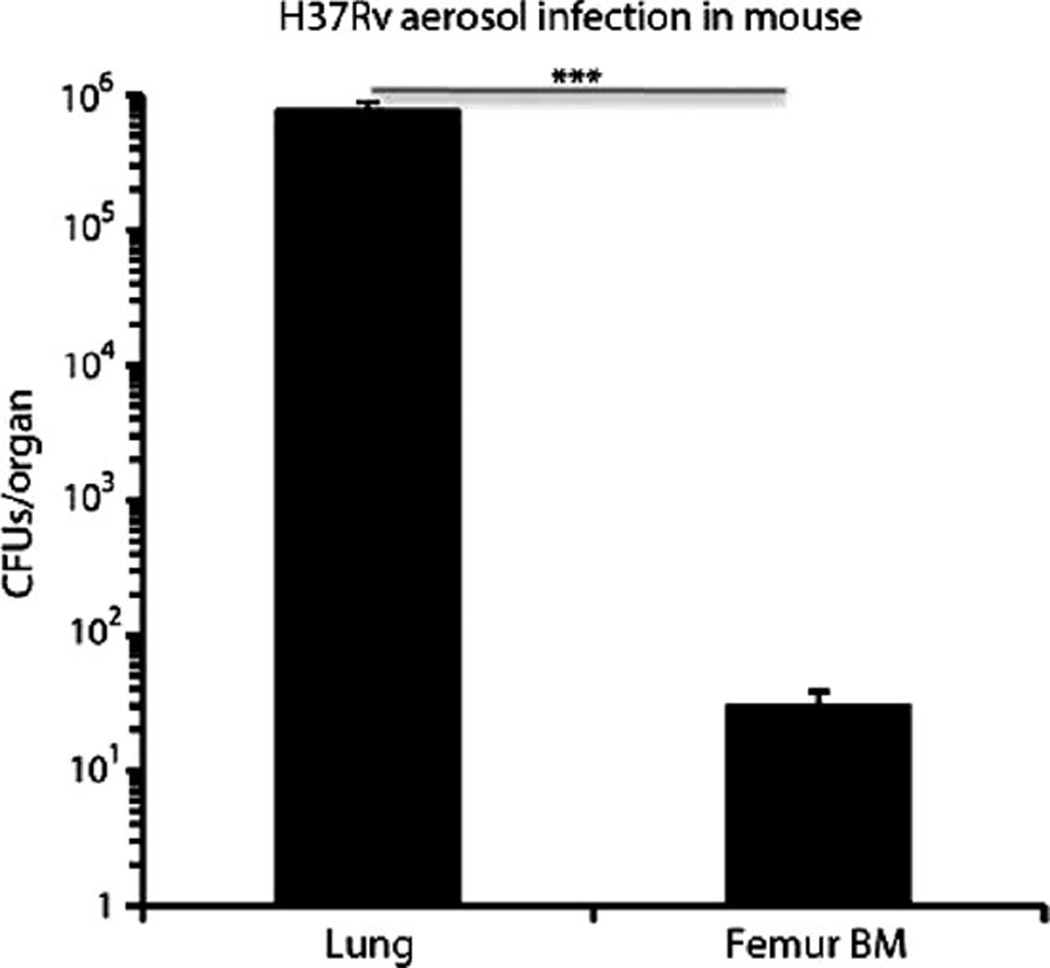
To translate our in vitro results to an in vivo infectious process, we used a well-established mouse model of TB. In this model, mice are infected via the respiratory route with a low number of aerosolized virulent Mtb (~100 CFUs per mouse) (29). Mycobacterium grows in the murine lungs and subsequently is disseminated to organs such as spleen and liver. To evaluate whether the infectious process would also enable dissemination of Mtb from the lung to BM, we infected mice with aerosolized virulent Mtb (strain H37Rv), and 4 weeks later, the animals were sacrificed and the presence of Mtb in the animals’ lungs and BM (femur) was enumerated using Middlebrook 7H11 agar plates to grow the mycobacteria. Mtb was found in mouse BM and lung (Fig. 4). Our results support the possibility that BM cells can be a target of Mtb infection in vivo as well as in vitro.
Fig. 4.
Dissemination of Mtb H37Rv to BM in a murine aerosolized model of infection. BM cells (107 mononuclear cells) obtained from mice (female BALB/c) infected with Mtb H37Rv (~100 CFUs) for 4 weeks contained viable Mtb. Shown are numbers of Mtb CFUs grown from mouse femur BM and from lung as a positive control. P < 0.0001, Student’s t test; n = 5 independent experiments.
Viable nonreplicating Mtb resides in lung and BM MSCs of mice
We then evaluated whether mouse MSCs present in lung and BM could maintain the viability of nonreplicating Mtb. To perform these experiments, we used a unique mouse model of nonreplicating Mtb infection. In this model, an auxotrophic mutant of Mtb (strain 18b) replicates only in the presence of streptomycin (30–32). After streptomycin withdrawal, the mutant Mtb persists in a nonreplicating viable state, and a small number of viable Mtb CFUs can be obtained from the mouse lung and spleen even after 6 months after infection (31). Hence, this model provided the opportunity to test whether nonreplicating Mtb retained viability in MSCs in vivo.
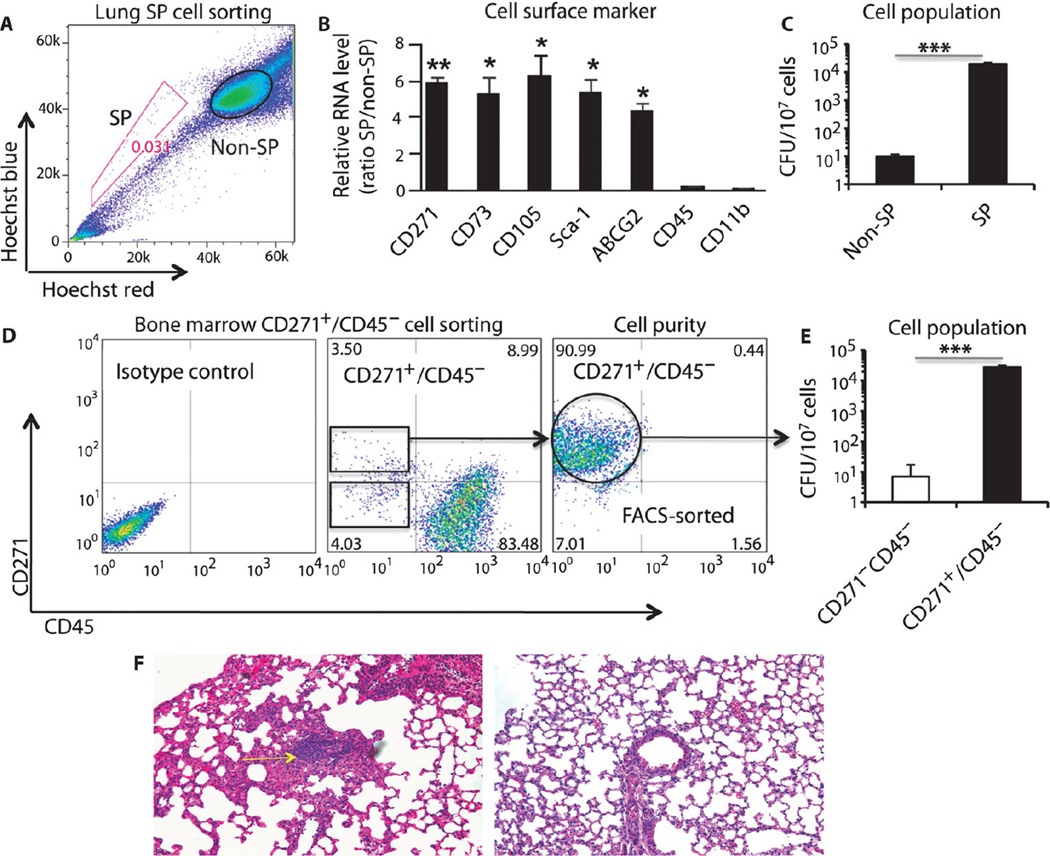
Using this model, we measured the recovery of viable Mtb from the MSC fractions in the lung and BM. In the mouse lung, the MSCs are enriched in a population of cells, the side population (SP) (33), which is defined by their ability to exclude Hoechst 33342 dye as detected by FACS gating and by their expression of the drug efflux pump ABCG2 (16). As depicted in Fig. 5A, using Hoechst dye exclusion (34) and FACS (Fig. 5A), we could purify a population that did not retain the Hoechst dye (SP) as well as a population that retained the dye (non-SP fraction). The SP fraction was positive for ABCG2 and showed an increase in expression of MSC markers Sca-1, CD105, CD44, and CD271 (more than fourfold) in the SP versus the non-SP fraction (Fig. 5B). In contrast, neither CD45 nor the macrophage-associated CD11b cell markers were detected in the SP fraction (Fig. 5B). Hence, lung SP cells express CD271 and are enriched in MSCs, as has been suggested previously (33). In the mouse BM, MSCs are enriched in CD271+/CD45− cells (fig. S3). Therefore, we used lung SP cells and BM CD271+/CD45− cells of infected mice (6 months after streptomycin withdrawal) to measure the recovery of viable nonreplicating Mtb strain 18b.
Fig. 5.
Recovery of viable nonreplicating Mtb from MSCs of infected mice. (A) Flow cytometry profile of infected lung SP and non-SP cells after staining with Hoechst 33342 dye. SP gatingwas performed with verapamil treatment. (B and C) Expression ofMSC markers (B) and detection of viable Mtb CFUs (C) in the FACS-sorted SP and non-SP cellpopulations from infected lungs. (D) Flow cytometry profiles of BM CD271+/CD45− cells from infectedmice. The far right flow cytometry panel represents the purity of the FACS-sorted CD271+/CD45− mouse BM-MSCs. (E) Viable Mtb CFUs were detected in mouse BM-MSC CD271+/CD45− cells (see fig. S3 for characteristics of the CD271+/CD45− BM-MSCs). (F) Granuloma development in the lungs of mice injected with CD271+ BM-MSCs [see (D)] harboring viable dormant Mtb (infected lung, left panel, yellow arrow; the right panel depicts normal lung tissue). Hematoxylin and eosin section was prepared as described (30, 31). Magnification, ×20. *P < 0.05, **P < 0.001, Student’s t test; n = 3 independent experiments.
First, we examined the lung SP and non-SP cells for viable Mtb by culturing lysates of these cells in Middlebrook agar plates containing streptomycin. Viable Mtb strain 18b could be retrieved from the lung SP fraction even after spending a prolonged period of time in a non-replicating state (Fig. 5C).When the infected SP cells (5 × 104 SP cells, containing on average 70 Mtb CFUs, n = 4) were injected by tail vein injection into recipient mice (female C57BL/6 mice, 6 to 8 weeks old), the mice developed lung granulomas. Viable CFUs (1000 ± 430 per lung, n = 4) were obtained after 4 weeks of infection, suggesting that Mtb in the SP cells maintained their ability to reinfect host lung tissue. In contrast, few viable Mtb were recovered from the non-SP population of cells. Thus, nonreplicating Mtb sequestered in mouse MSCs in the lung SP population retain in vivo viability and reinfection capabilities.
Second, in examining the BM fraction, we observed that viable Mtb could be recovered from CD271+ BM-MSCs (Fig. 5, D and E). Figure 5E shows that on average 32,182 viable CFUs were recovered per 107 CD271+/CD45− cells. This implies that ~0.32% of CD271+/CD45− cells contained viable and nonreplicating Mtb. These intracellular non-replicating Mtb maintain reinfection capabilities similar to those of lung SP cells. Similar to lung SP cells, the tail vein injection of Mtb-infected 5 × 104 CD271+ BM-MSCs (Fig. 5D) led to lung granuloma development in the recipient mice 4 weeks after injection (Fig. 5, E and F). Notably, CD271+ BM-MSCs recovered from the recipient mice contained Mtb CFUs (Fig. 5E). Thus, viable nonreplicating Mtb could be obtained from both BM and lung MSCs of mice infected with Mtb strain 18b.
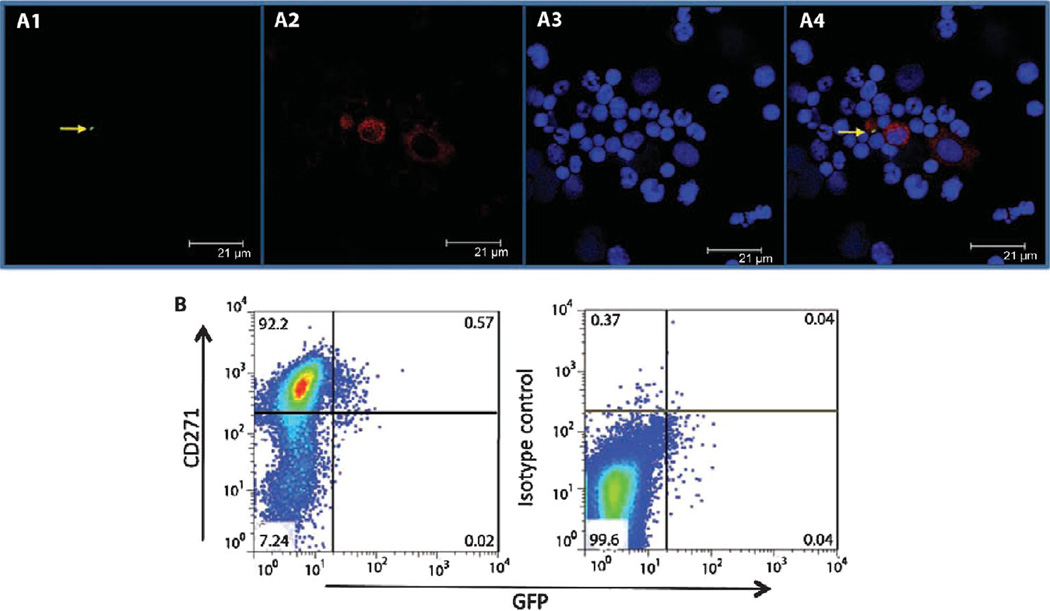
To directly examine if CD271+ mouse BM-MSCs can serve as a host of infectious Mtb, the auxotrophic mutant Mtb strain 18b was labeled with the green fluorescent protein (GFP)–expressing plasmid GFPpUV15+(35). The GFPpUV15+-transfected Mtb strain 18b (GFP-Mtb 18b) maintained its infective ability as assessed in vitro using the mouse macrophage RAW cell line (fig. S4). Mice were then infected with the GFP-Mtb 18b followed by daily administration of streptomycin sulfate for 3 weeks. Mice were sacrificed, and BM cells were obtained and analyzed by both confocal microscopy and multicolor flow cytometry. By confocal microscopy examination, we observed the presence of intracellular GFP-labeled Mtb strain 18b in CD271+ cells among the heterogeneous BM mononuclear cell population (Fig. 6A). By FACS analysis, 0.6 ± 0.2% (P < 0.02; n = 5) of the CD271+ cells stained double positive for both the CD271 MSC marker and GFP (Fig. 6B). Our results further suggest that CD271+ BM-MSCs can be infected with Mtb strain 18b in vivo.
Fig. 6.
Mtb strain 18b resides in the CD271+/CD45− BM-MSCs of infected mice. (A) Confocal image of flow cytometry–sorted mouse CD271+ BM-MSCs depicting (A1) GFP-tagged Mtb strain 18b (green; indicated by the yellow arrow), (A2) BM cells stained with anti-CD271 monoclonal antibody (red), and (A3) BM nuclei stained with Draq5, a nuclear staining dye. A4, merged image. Images are representative of a typical experiment (n = 3). (B) FACS analysis of the CD271+ BM-MSC population in (A). Representative data are shown for cells staining double positive for CD271 and GFP. Five independent experiments were performed to obtain the percentage of CD271+/GFP+ cells.
Viable Mtb persist in CD271+/CD45− BM-MSCs of treated TB patients
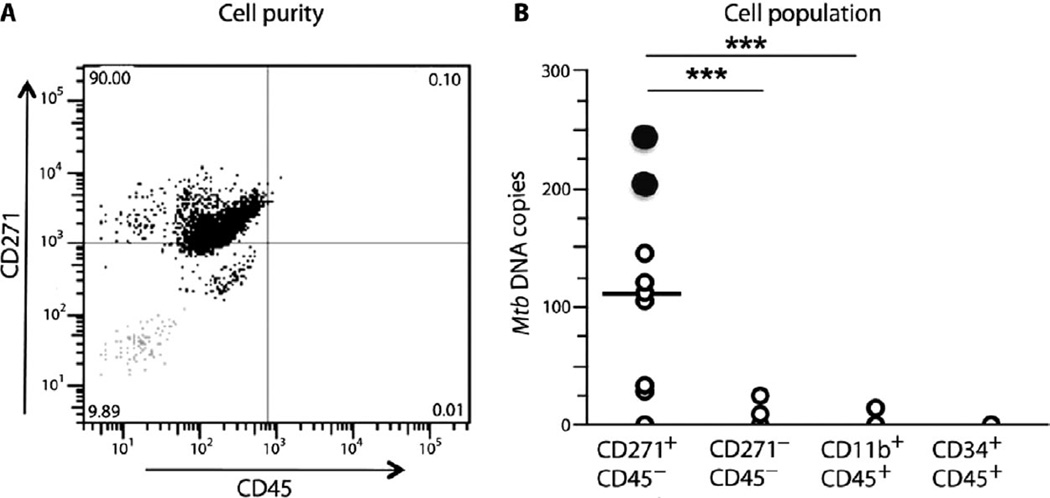
To examine whether Mtb could infect human CD271+ BM-MSCs and maintain Mtb long-term viability, we obtained BM cells from individuals previously treated for pulmonary TB. Nine subjects who had under-gone complete Directly Observed Treatment Short-Course (DOTS) were studied. After completion of treatment, these individuals had negative sputum for Mtb culture and Mtb DNA, which is consistent with a therapeutically successful treatment. However, such individuals are known to potentially still harbor residual Mtb infection (1, 7, 36, 37). CD271+ BM-MSCs were obtained from BM biopsies from these individuals by an immunomagnetic cell sorting procedure (20–22). The purity and phenotype were evaluated by performing FACS analysis, as well as gene expression analysis as described (20–22). Using an immunomagnetic technique, we could obtain an 89.2 ± 5.8% (n = 2) pure population of CD271+ BM-MSCs (3.2 ± 2 × 104 CD271+/CD45− cells from 5 × 107 total BM mononuclear cells) as confirmed by FACS analysis (Fig. 7A). From these sorted CD271+ BM-MSCs, the presence of Mtb DNA was investigated by qPCR using Mtb-specific primers (38). As a negative control, DNA was obtained from CD271+ BM-MSCs and CD271−/CD45− cells of healthy volunteers (n = 6) from nonendemic areas (ABC026F, Stem Cell Technologies; details in the Supplementary Materials and Methods). We detected on average 110 Mtb DNA copies per 104 cells in eight of nine subjects (P < 0.0001; Fig. 7B). In two individuals, Mtb DNA was found in the CD271−CD45− cell population (25 and 10 copies, respectively, per 106 cells). In one individual, Mtb DNA was found in the CD11b+/CD45+ cell population (15 copies per 107 cells). No measurable Mtb DNA was associated with CD34+/CD45+ cells. The DNA obtained from CD271+/CD45− cells of healthy volunteers from nonendemic areas did not exhibit measurable Mtb DNA. Although these results do not exclude other cell types as potential hosts for dormant Mtb, they do suggest that Mtb can infect and persist in human CD271+ BM-MSCs.
Fig. 7.
Viable Mtb persist in the CD271+/CD45− BM-MSCs of treated TB patients. (A) Representative flow cytometry panel showing the purity of the magnetically sorted CD271+/CD45− cells obtained from fresh human BM from subjects previously treated for pulmonary TB. The gray dots (bottom left square) represent the isotype control. (B) CD271+/CD45− BM-MSCs were obtained from previously treated TB patients (DOTS II) who did not show evidence of pulmonary TB. This cell population is enriched for Mtb DNA in eight of nine subjects. The black circles denote two subjects for which viable Mtb could be cultured from their BM-MSCs. A total of 11 post-therapy TB patients were studied. BM from two subjects was verified for enrichment by magnetic sorting (A). The nine other subjects were examined for Mtb DNA and for viable Mtb mycobacteria. Mtb DNA results were analyzed by Student’s t test; ***P < 0.0001.
To examine the presence of viable Mtb in the sorted CD271+/CD45− cells from these human subjects, we used a liquid culture method that facilitates the replication of “difficult to culture” Mtb (9, 39). We identified two positive Mtb cultures (black circles, Fig. 7B) of eight Mtb DNA–positive subjects. Notably, positive cultures were obtained from individuals with a higher copy number of Mtb DNA (245 and 206 Mtb DNA copies per 104 cells; Fig. 7B). In contrast, the microbial cultures from the CD271−/CD45− and CD11b+/CD45+ subpopulations were all negative. Thus, our observations suggest that viable Mtb could persist in CD271+/CD45− BM-MSCs obtained from TB patients who have completed anti-Mtb therapy.
DISCUSSION
The protective cellular niche where Mtb persists to maintain long-term viability is not yet clearly defined, complicating the development of effective vaccines and therapeutic strategies to eliminate TB (1). Here, we demonstrate in vitro and in vivo in human subjects previously treated for TB and in a mouse model of Mtb infection that Mtb can infect and persist in CD271+ BM-MSCs.
Specifically, we identified that in vitro human CD271+ BM-MSCs can be infected and maintain viable Mtb. Then, using an in vivo mouse model designed to study nonreplicating Mtb, we found that CD271+ BM-MSCs purified from the BM of Mtb-infected mice maintain viable nonreplicating Mtb with reinfection capabilities. In humans, we identified Mtb DNA as well as viable Mtb in the CD271+ BM-MSCs from patients who had successfully completed antitubercular therapy. Our results suggest that CD271+ BM-MSCs are a possible reservoir for nonreplicating live Mtb that potentially is a source for reinfection of the host.
Although BM-MSCs have been studied for several decades in an in vitro setup by culture expansion and selection methods (40), only recently has it been possible to study this cell type in their micro-environment in vivo (15, 17, 24, 40). This success is mainly attributable to the identification of markers that define BM-MSCs including CD271, CD146, Stro-1, and SSEA-4 (19–25, 40). This has enabled direct isolation and in vivo study of primary CD271+ BM-MSCs including their self-renewal and differentiation into multiple lineages (19–25). In addition, CD271+ BM-MSCs express the drug efflux pump ABCG2 (20) and exhibit immunosuppressive activity (21). In mouse BM, we found a CD271+/CD45− BM cell population that expresses Sca-1+ and exhibits an MSC phenotype. Others have reported that BM Sca-1+ cells are enriched in MSCs (41, 42). However, it is not yet clear whether the mouse and human CD271+ BM-MSCs are necessarily equivalent. Nevertheless, we were able to isolate viable Mtb from both human and mouse CD271+ BM-MSCs. Thus, recent advances in the characterization of primary BM-MSCs enabled us to identify the CD271+ BM-MSCs as a host cell type that can maintain viable Mtb. Our work suggests that BM-MSCs, identified many decades ago (43), could be involved in the pathogenesis of an infectious disease.
Our results introduce a new possible route for TB pathogenesis (fig. S5), whereby a subpopulation of BM cells, the CD271+ BM-MSCs, could be responsible for themaintenance of viable nonreplicating Mtb. The CD271+ BM-MSCs are localized in the BM niche (19). The BM niche (including both calvarial and trabecular BM sites) is a niche that provides immune privilege to stem cells and protects stem cells from immune attack (17, 18). Therefore, this niche is an attractive site where Mtb could persist in CD271+ BM-MSC host cells while evading the host immune response.
For several reasons, host cells have been thought to be important in the dormant infection phase of TB pathogenesis (1). First, primary granulomas of asymptomatic subjects are mostly sterile, whereas viable Mtb has been recovered from the normal part of lung (44–46), indicating the potential existence of an extragranuloma niche for Mtb. Second, human autopsy findings report Mtb DNA within macrophages, epithelial cells, and fibroblasts distributed throughout normal lung tissues without any sign of visible granuloma lesions (10). Mtb DNA has also been detected in adipocyte-like cells of extrapulmonary tissues (11). Thus, it appears that Mtb may reside within a variety of host cells during the dormant infection phase. However, the data supporting epithelial cells and fibroblasts as host cells of Mtb infection are based solely on the detection of Mtb DNA in these cells (10). Therefore, it is not clear whether these various cells could maintain viable nonreplicating Mtb for a prolonged period of time such as would occur during the dormant infection phase.
Our in vitro experiments suggest that Mtb can infect, multiply, and maintain viability intracellularly in undifferentiated human CD271+ BM-MSCs. To further evaluate whether Mtb could infect MSCs in vivo, we used a mouse model of nonreplicating dormant Mtb. First, we showed that viable Mtb could be obtained in the BM of mice infected with a low dose of aerosolized virulent Mtb H37Rv (~100 CFUs). However, technical restrictions imposed by working with virulent organisms inside BSL-3 facilities precluded us from performing flow cytometry characterization of the target BM cells infected by the virulent Mtb. Magnetic sorting, which can be done in a BSL-3 facility, did not work to isolate murine CD271+ BM-MSCs. Briefly, the anti-CD271 magnetic sorting antibody was not able to enrich the population of mouse CD271+ BM-MSCs. There is no other available mouse anti-CD271 magnetic sorting antibody. Therefore, to investigate the BM target cells infected by Mtb in vivo, we opted to use a less virulent streptomycin auxotrophic mutant of Mtb that has been used to study the persistence of nonreplicating Mtb in mice and guinea pigs. In this model, which we (30, 31) and others (32) have previously characterized, inoculation of mice with this unique streptomycin auxotrophic mutant of Mtb recapitulates several features of a dormant or latent infectious process. Although in this model the bacilli stop replicating because of a lack of streptomycin rather than in response to host defenses, this model has the advantage that it can be manipulated to stringently ensure that the microorganisms remain in a dormant nonreplicating state for long periods during the infectious process (31). This is an important condition enabling demonstration that infection of the CD271+ BM-MSCs with Mtb is due to long-term persistence and not due to recent infection caused by replicating organisms. Hence, data from the mouse model demonstrate that the nonreplicating infection with viable Mtb strain 18b is due to dormant Mtb (32).
Using this mouse model, we showed that dormant Mtb could be isolated from the MSC populations present in both lungs and BM of infected mice that had not been treated with streptomycin for ~6 months. In BM, viable Mtb were obtained directly from the CD271+ BM-MSCs. Also, we confirmed the infection of this cell type by using GFP-labeled Mtb. In lung, viable Mtb was obtained from the SP cell population that is enriched in MSCs. We found that when these infected MSCs were reinjected into healthy mice, the animals developed lung granulomas, suggesting that the nonreplicating Mtb maintained intracellularly in the MSCs had reinfection capabilities. At this time, it is not clear to what extent mouse lung CD271+ SP cells and BM-derived CD271+ BM-MSCs are phenotypically equivalent. Nevertheless, our data are consistent with our in vitro findings that Mtb specifically infected and remained viable in an undifferentiated CD271+ BM-MSC population.
Our observations suggest the possibility that CD271+ BM-MSCs may be an alternative host cell population for supporting the long-lasting nonreplicating phase of the Mtb life cycle. It is intriguing that CD271+ BM-MSCs, members of the heterogeneous MSC population, do have features that could maintain the nonreplicating Mtb for a prolonged period of time. In particular, adult undifferentiated stem cells including MSCs are characterized by their self-renewal and quiescent properties (15–19). In contrast, quiescent, but differentiated, cells including macrophages, endothelial cells, and fibroblast are characterized by a shorter life span. Our results from differentiating MSCs suggest that such differentiated cells do not support the persistence of viable Mtb. Nonreplicating Mtb seems to persist for long periods of time in CD271+ BM-MSCs without being compromised or needing to change their host cells.
Validation of these findings in mice was obtained using BM cells from subjects previously treated for pulmonary TB, who did not exhibit evidence of active pulmonary TB (Supplementary Materials and Methods). We found that specific Mtb DNA was present or associated with the CD271+ BM-MSCs from most of these individuals. We confirmed the presence of viable Mtb in the CD271+ BM-MSCs of two of eight subjects having Mtb DNA copies. The negative culture results in the other Mtb DNA–positive subjects are likely in part due to the limitation of the current culture methods for growth of dormant or nonreplicating Mtb.
Epidemiological studies indicate that disease recurrence after drug treatment for TB remains a major challenge (36, 37). TB recurrence could occur due to either endogenous reactivation of persistent Mtb infection or exogenous reinfection with a new Mtb strain (47). Several studies indicate that reactivation could be a significant source of recurrent TB (48–50). Therefore, prevention and management of the reactivation process could reduce the incidence of recurrent TB (36, 49). However, the sources and mechanisms of reactivation are not clearly known (1, 7), which compromises our ability to eradicate TB (7, 51). Although our results do not yet address whether Mtb-infected BM-MSCs are a major source of TB reactivation, our findings suggest a new potential route for TB reactivation that should be studied further.
Our results from in vitro studies of human BMSCs, an in vivo mouse model of nonreplicating Mtb infection, and the analysis of human CD271+ BM-MSCs from patients with pulmonary TB suggest that CD271+ BM-MSCs are a cellular niche that may be important for the maintenance of the nonreplicating phase of Mtb in humans. CD271+ BM-MSCs have many features that could be potentially advantageous for Mtb persistence including their ability to self-renew, expression of the ABCG2 drug efflux pump, and the immune-privileged nature of the BM stem cell niche (19–25). We suggest that human BM-derived MSCs may participate in TB pathogenesis with potentially important clinical implications.
MATERIALS AND METHODS
Stem cell sorting and culture
All studies conducted in the various institutions were approved by Stanford Stem Cell Research Oversight Panel (protocol number 289; principal investigator: B.D.). Human BM–derived CD133+ (ABC026F) and CD34+ (ABM016F) were cultured in serum-free medium with appropriate growth factors, and CD271+/CD133+ cells were obtained from the CD133+ BM cells with the EasySep Human CD271 Positive Selection kit (18659). The sorted cells were cultured in the serum-free medium with the growth factor cocktails (TPO, SCF, and Flt3 ligand) to maintain the CD271+/CD133+ phenotype (Supplementary Materials and Methods), and used for Mtb infection and growth. The purity and the phenotype of the sorted cells are described in table S1.
Mycobacterium strains, growth conditions, and stem cell infections
The Mtb strains H37Ra [American Type Culture Collection (ATCC) 25177] and H37Rv (ATCC 27294) and a streptomycin-auxotrophic mutant Mtb strain 18b (provided by S. Cole, Global Health Institute, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland) were grown and maintained in BBL Middlebrook 7H9 broth with glycerol (BD Biosciences, 221832) at 37°C and 5% CO2. Single-cell suspension was obtained to infect stem cells with standard protocol used to infect macrophages with an MOI of five bacteria per cell for 8 hours. For the immunofluorescence visualization and flow cytometry sorting of Mtb-infected stem cells, Mtb was tagged with PKH26 red dye (PKH26 red fluorescent cell linker mini kit, Sigma) (27) and subsequently used for stem cell infection. GFP-tagged Mtb strain 18b was obtained as described (35). The viable intracellular Mtb from the Mtb-infected stem cells was measured by Mtb CFU assay (27).
Mesenchymal characterization of human and mouse CD271+/CD45− BM cells
The phenotypic characterization of the human and mouse CD271+/CD45+ BM cells was performed as described (28).
mRNA and DNA extraction, and qPCR assay
To isolate mammalian mRNA and bacterial DNA from the infected cells, we used the µMACS technology (Miltenyi Biotec). Methods and various primers used in the article are described in the Supplementary Materials and Methods.
Mice infection with Mtb
Six- to 8-week-old female C57BL/6 mice were obtained from Charles River Laboratories. The streptomycin-auxotrophic mutant 18b strain was delivered intravenously at 2 × 106 CFUs per mouse (31), and after 24 weeks of streptomycin starvation, the CD271+/CD45− cells were obtained by FACS. The lung SP and non-SP cells were isolated by dissociating the lung tissue (33), followed by FACS as described (34). The FACS-sorted cells from lung and BM were subjected to Mtb CFU assay. A portion of the sorted cells was subjected to qPCR study by the µMACS technique described above. For the aerosolized TB infection, phosphate-buffered saline/Tween–suspended Mtb H37Rv was delivered to mice (~100 CFUs per mouse; n = 5) with an aerosol generation device (Inhalation Exposure System; Glas-Col).
Clinical study
The clinical study was approved by Stanford Stem Cell Research Oversight Panel, Stanford University (protocol number 289; principal investigator: B.D.), and the government of Arunachal Pradesh, India (MSTB/STDC/IRL/20/2010). Subjects were recruited from the Idu-Mishmi tribe living in a remote mountain range of Roing, Arunachal Pradesh, India. The population represents an extremely homogeneous background (Supplementary Materials and Methods). Previously treated subjects (DOTS II) were prospectively recruited on the basis of the local TB control program data and chest x-ray findings. After proper consent, 6 to 7 ml of BM were taken from the posterior superior iliac crest, and then, the immunomagnetically sorted cells were lysed and subjected to mRNA and Mtb DNA isolation by qMACS technology as described above. The magnetically sorted cells obtained from healthy volunteers (n = 6) were subjected to both FACS and qPCR analysis to confirm the purity and phenotype of the CD271+ BM-MSCs, and also served as a negative control for Mtb DNA. Part of the lysate was subjected to liquid culture to obtain viable Mtb, as described (9, 39), with modifications.
Statistics
Mean values ± SEM are shown. Student’s t test was used for comparisons (GraphPad Prism version 4.0a for Macintosh). Data are expressed as means ± SEM; *P < 0.05, **P < 0.001, ***P < 0.0001.
Supplementary Material
Materials and Methods
Fig. S1. Internalization and retention of dead H37Ra in the CD271+ BMSCs.
Fig. S2. The PKH26+/CD271+ cells expressed MSC markers.
Fig. S3. The CD271+/CD45− BM cells obtained from Mtb strain 18b–infected C57BL/6 mouse is enriched in MSCs.
Fig. S4. Confocal microscopy image of GFPpUV15–expressing Mtb strain 18b.
Fig. S5. A hypothetical model of CD271+ BM-MSCs’ role in tubercular dormancy and reactivation.
Table S1. Serum-free culture system maintains the primary MSC phenotype of CD271+ BMSCs.
Acknowledgments
We thank the Idu-Mishmi people, volunteers, and the government of Arunachal Pradesh, India, for allowing us to conduct the clinical study in the Idu-Mishmi land. Correspondence for this clinical study should be addressed to B.D. at bikuldas@stanford.edu. We also thank L. Qin and M. Bigos for flow cytometry assay and analysis.
Funding: The work was funded by a grant from the Bill & Melinda Gates Foundation through the “Grand Challenges Exploration Initiative” (B.D.). Additional funding was obtained from the Canadian Cancer Society (B.D.); the KaviKrishna Foundation, Sualkuchi, Assam, India (D.K. and I.P.); Laurel Foundation (B.D. and D.W.F.); NIH grants R01AI076425 (A.C.-N.), R01 CA105102, CA89305-0351, and CA112973; and Department of Defense grant PR080163 (D.W.F.).
Footnotes
Author contributions: B.D. initiated, designed, and supervised the study. B.D. and S.S.K. performed the in vitro and in vivo experiments. B.D., D.K., V.S., and I.P. performed the clinical study. H.Y. contributed reagents/analytic tools. B.D., A.C.-N., and D.W.F. analyzed the data. B.D., H.Y., A.C.-N., and D.W.F. wrote the paper.
Competing interests: B.D. is a founding director and now the honorary director of KaviKrishna Foundation. The Foundation is a not-for-profit, nongovernmental organization based in Assam, India. The Foundation is involved in the clinical study described in the manuscript. The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17:183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., III Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippini P, Iona E, Piccaro G, Peyron P, Neyrolles O, Fattorini L. Activity of drug combinations against dormant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2010;54:2712–2715. doi: 10.1128/AAC.01736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandiviere HM, Loring WE, Melvin I, Willis S. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am. J. Med. Sci. 1956;232:30–37. doi: 10.1097/00000441-195607000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Bloom BR, McKinney JD. The death and resurrection of tuberculosis. Nat. Med. 1999;5:872–874. doi: 10.1038/11309. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460:98–102. doi: 10.1038/nature08123. [DOI] [PubMed] [Google Scholar]
- 9.Biketov S, Mukamolova GV, Potapov V, Gilenkov E, Vostroknutova G, Kell DB, Young M, Kaprelyants AS. Culturability of Mycobacterium tuberculosis cells isolated from murine macrophages: A bacterial growth factor promotes recovery. FEMS Immunol. Med. Microbiol. 2000;29:233–240. doi: 10.1111/j.1574-695X.2000.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, Harboe M, Rook GA, Bjune G. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–2138. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- 11.Neyrolles O, Hernández-Pando R, Pietri-Rouxel F, Fornès P, Tailleux L, Barrios Payán JA, Pivert E, Bordat Y, Aguilar D, Prévost MC, Petit C, Gicquel B. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghuvanshi S, Sharma P, Singh S, Van Kaer L, Das G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21653–21658. doi: 10.1073/pnas.1007967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34−Lin− and CD34+Lin− hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000;95:2813–2820. [PubMed] [Google Scholar]
- 15.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 17.Aria F, Suda T. Quiescent stem cells in the niche, in StemBook. Cambridge, MA: Harvard Stem Cell Institute; 2008. [PubMed] [Google Scholar]
- 18.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, Sato T, Côté D, Sykes M, Strom TB, Scadden DT, Lin CP. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, Gregory CA, Spees JL. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol. Ther. 2009;17:1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuçi S, Kuçi Z, Kreyenberg H, Deak E, Pütsch K, Huenecke S, Amara C, Koller S, Rettinger E, Grez M, Koehl U, Latifi-Pupovci H, Henschler R, Tonn T, von Laer D, Klingebiel T, Bader P. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 23.Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann. N. Y. Acad. Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 24.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 25.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J. Biol. Chem. 2012;287:25795–25807. doi: 10.1074/jbc.M112.339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baksh D, Davies JE, Zandstra PW. Soluble factor cross-talk between human bone marrow-derived hematopoietic and mesenchymal cells enhances in vitro CFU-F and CFU-O growth and reveals heterogeneity in the mesenchymal progenitor cell compartment. Blood. 2005;106:3012–3019. doi: 10.1182/blood-2005-01-0433. [DOI] [PubMed] [Google Scholar]
- 27.Kelly DM, ten Bokum AM, O’Leary SM, O’Sullivan MP, Keane J. Bystander macrophage apoptosis after Mycobacterium tuberculosis H37Ra infection. Infect. Immun. 2008;76:351–360. doi: 10.1128/IAI.00614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das B, Antoon R, Tsuchida R, Lotfi S, Morozova O, Farhat W, Malkin D, Koren G, Yeger H, Baruchel S. Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth. Neoplasia. 2008;10:1105–1119. doi: 10.1593/neo.08466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orme I, Gonzalez-Juarrero M. Animal models of M. tuberculosis infection. Chapter 10, Unit 10A.5. Curr. Protoc. Microbiol. 2007 doi: 10.1002/9780471729259.mc10a05s7. [DOI] [PubMed] [Google Scholar]
- 30.Kashino SS, Napolitano DR, Skobe Z, Campos-Neto A. Guinea pig model of Mycobacterium tuberculosis latent/dormant infection. Microbes Infect. 2008;10:1469–1476. doi: 10.1016/j.micinf.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashino SS, Ovendale P, Izzo A, Campos-Neto A. Unique model of dormant infection for tuberculosis vaccine development. Clin. Vaccine Immunol. 2006;13:1014–1021. doi: 10.1128/CVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sala C, Dhar N, Hartkoorn RC, Zhang M, Ha YH, Schneider P, Cole ST. Simple model for testing drugs against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2010;54:4150–4158. doi: 10.1128/AAC.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy. 2008;10:140–151. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- 34.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 35.Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat. Med. 2007;13:1515–1520. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: Adequately treated patients are still at high risk. Int. J. Tuberc. Lung Dis. 2007;11:828–837. [PubMed] [Google Scholar]
- 37.Millet JP, Orcau A, de Olalla PG, Casals M, Rius C, Caylà JA. Tuberculosis recurrence and its associated risk factors among successfully treated patients. J. Epidemiol. Community Health. 2009;63:799–804. doi: 10.1136/jech.2008.077560. [DOI] [PubMed] [Google Scholar]
- 38.Zumárraga M, Bigi F, Alito A, Romano MI, Cataldi A. A 12.7 kb fragment of the Mycobacterium tuberculosis genome is not present in Mycobacterium bovis. Microbiology. 1999;145:893–897. doi: 10.1099/13500872-145-4-893. [DOI] [PubMed] [Google Scholar]
- 39.Sun Z, Zhang Y. Spent culture supernatant of Mycobacterium tuberculosis H37Ra improves viability of aged cultures of this strain and allows small inocula to initiate growth. J. Bacteriol. 1999;181:7626–7628. doi: 10.1128/jb.181.24.7626-7628.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, Shimmura S, Miyawaki A, Nakagawa T, Suda T, Okano H, Matsuzaki Y. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vranken I, De Visscher G, Lebacq A, Verbeken E, Flameng W. The recruitment of primitive Lin− Sca-1+, CD34+, c-kit+ and CD271+ cells during the early intraperitoneal foreign body reaction. Biomaterials. 2008;29:797–808. doi: 10.1016/j.biomaterials.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Goujon E. Recherches experimentales sur les proprietes physiologiques de la moelle des os. J. Anat. Physiol. 1869;6:399–412. [Google Scholar]
- 44.Aronson JD, Whitney CE. The types of tubercle bacilli found in tuberculous lesions and in nontuberculous tissue in man. J. Infect. Dis. 1930;47:30–55. [Google Scholar]
- 45.Balasubramanian V, Wiegeshaus EH, Taylor BT, Smith DW. Pathogenesis of tuberculosis: Pathway to apical localization. Tuber. Lung Dis. 1994;75:168–178. doi: 10.1016/0962-8479(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 46.Medlar EM. The pathogenesis of minimal pulmonary tuberculosis; a study of 1,225 necropsies in cases of sudden and unexpected death. Am. Rev. Tuberc. 1948;58:583–611. doi: 10.1164/art.1948.58.6.583. [DOI] [PubMed] [Google Scholar]
- 47.van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, Beyers N, van Helden PD. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 1999;341:1174–1179. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 48.Farnia P, Masjedi MR, Varahram M, Mirsaeidi M, Ahmadi M, Khazampour M, Tabarsi P, Baghei P, Marjane M, Bahadori M, Zarifi AZ, Velayati AA. The recent-transmission of Mycobacterium tuberculosis strains among Iranian and Afghan relapse cases: A DNA-fingerprinting using RFLP and spoligotyping. BMC Infect. Dis. 2008;8:109. doi: 10.1186/1471-2334-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jasmer RM, Bozeman L, Schwartzman K, Cave MD, Saukkonen JJ, Metchock B, Khan A, Burman WJ. Tuberculosis Trials Consortium, Recurrent tuberculosis in the United States and Canada: Relapse or reinfection? Am. J. Respir. Crit. Care Med. 2004;170:1360–1366. doi: 10.1164/rccm.200408-1081OC. [DOI] [PubMed] [Google Scholar]
- 50.Dobler CC, Crawford AB, Jelfs PJ, Gilbert GL, Marks GB. Recurrence of tuberculosis in a low-incidence setting. Eur. Respir. J. 2009;33:160–167. doi: 10.1183/09031936.00104108. [DOI] [PubMed] [Google Scholar]
- 51.Brewer TF, Heymann SJ. To control and beyond: Moving towards eliminating the global tuberculosis threat. J. Epidemiol. Community Health. 2004;58:822–825. doi: 10.1136/jech.2003.008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charrier S, Boiret N, Fouassier M, Berger J, Rapatel C, Pigeon P, Mareynat G, Bonhomme J, Camilleri L, Berger MG. Normal human bone marrow CD34+CD133+ cells contain primitive cells able to produce different categories of colony-forming unit megakaryocytes in vitro. Exp. Hematol. 2002;30:1051–1060. doi: 10.1016/s0301-472x(02)00882-2. [DOI] [PubMed] [Google Scholar]
- 53.Mehta PK, King CH, White EH, Murtagh JJ, Jr, Quinn FD. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect. Immun. 1996;64:2673–2679. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei J, Dahl JL, Moulder JW, Roberts EA, O’Gaora P, Young DB, Friedman RL. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J. Bacteriol. 2000;182:377–384. doi: 10.1128/jb.182.2.377-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takii T, Abe C, Tamura A, Ramayah S, Belisle JT, Brennan PJ, Onozaki K. Interleukin-1 or tumor necrosis factor-α augmented the cytotoxic effect of mycobacteria on human fibroblasts: Application to evaluation of pathogenesis of clinical isolates of Mycobacterium tuberculosis and M. avium complex. J. Interferon Cytokine Res. 2001;21:187–196. doi: 10.1089/107999001750133258. [DOI] [PubMed] [Google Scholar]
- 56.Mack E, Neubauer A, Brendel C. Comparison of RNA yield from small cell populations sorted by flow cytometry applying different isolation procedures. Cytometry A. 2007;71:404–409. doi: 10.1002/cyto.a.20391. [DOI] [PubMed] [Google Scholar]
- 57.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am. J. Respir. Cell Mol. Biol. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kashino SS, Vallerskog T, Martens G, Troudt J, Keyser A, Taylor J, Izzo A, Kornfeld H, Campos-Neto A. Initiation of acquired immunity in the lungs of mice lacking lymph nodes after infection with aerosolized Mycobacterium tuberculosis. Am. J. Pathol. 2010;176:198–204. doi: 10.2353/ajpath.2010.090446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McMurray DN. A coordinated strategy for evaluating new vaccines for human and animal tuberculosis. Tuberculosis. 2001;81:141–146. doi: 10.1054/tube.2000.0265. [DOI] [PubMed] [Google Scholar]
- 60.Arankalle VA, Gandhe SS, Borkakoty BJ, Walimbe AM, Biswas D, Mahanta J. A novel HBV recombinant (genotype I) similar to Vietnam/Laos in a primitive tribe in eastern India. J. Viral Hepat. 2010;17:501–510. doi: 10.1111/j.1365-2893.2009.01206.x. [DOI] [PubMed] [Google Scholar]
- 61.Thomas A, Gopi PG, Santha T, Chandrasekaran V, Subramani R, Selvakumar N, Eusuff SI, Sadacharam K, Narayanan PR. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int. J. Tuberc. Lung Dis. 2005;9:556–561. [PubMed] [Google Scholar]
- 62.Das B, Yeger H, Baruchel H, Freedman MH, Koren G, Baruchel S. In vitro cytoprotective activity of squalene on a bone marrow versus neuroblastoma model of cisplatin-induced toxicity. Implications in cancer chemotherapy. Eur. J. Cancer. 2003;39:2556–2565. doi: 10.1016/j.ejca.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J. Bone Joint Surg. Am. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Fig. S1. Internalization and retention of dead H37Ra in the CD271+ BMSCs.
Fig. S2. The PKH26+/CD271+ cells expressed MSC markers.
Fig. S3. The CD271+/CD45− BM cells obtained from Mtb strain 18b–infected C57BL/6 mouse is enriched in MSCs.
Fig. S4. Confocal microscopy image of GFPpUV15–expressing Mtb strain 18b.
Fig. S5. A hypothetical model of CD271+ BM-MSCs’ role in tubercular dormancy and reactivation.
Table S1. Serum-free culture system maintains the primary MSC phenotype of CD271+ BMSCs.