SUMMARY
RIG-I and MDA5 have emerged as key cytosolic sensors for the detection of RNA viruses, leading to antiviral interferon (IFN) production. Recent studies highlighted the importance of posttranslational modification for controlling RIG-I antiviral activity. However, the regulation of MDA5 signal-transducing ability remains unclear. Here we show that MDA5 signaling activity is regulated by a dynamic balance between phosphorylation and dephosphorylation of its CARD domains. Employing a phosphatome RNAi screen, we identified PP1α and PP1γ as primary phosphatases responsible for MDA5 and RIG-I dephosphorylation, leading to their activation. Silencing of PP1α and PP1γ enhanced RIG-I and MDA5 CARD phosphorylation and reduced antiviral IFN-β production. PP1α and PP1γ depleted cells were impaired in their ability to induce interferon-stimulated gene expression, which resulted in enhanced RNA virus replication. This work identifies PP1α and PP1γ as regulators of antiviral innate immune responses to various RNA viruses, including influenza virus, paramyxovirus, dengue virus and picornavirus.
INTRODUCTION
RNA helicases of the RIG-I-like receptor family play key roles in innate immunity to invading viruses. Specifically, RIG-I and MDA5 sense viral RNA in the cytosol and subsequently bind through their N-terminal caspase recruitment domains (CARDs) to the signaling adaptor MAVS (also called VISA, IPS-1, CARDIF), which triggers antiviral innate immune responses, resulting in the production of type-I interferons (IFN) and proinflammatory cytokines (Barbalat et al., 2011; Loo and Gale, 2011; Meylan et al., 2006). Despite their highly homologous structure, comprising two N-terminal CARDs, a DExD/H box RNA helicase, and a C-terminal domain (CTD), RIG-I and MDA5 sense distinct subsets of RNA viruses (Nakhaei et al., 2009). Whereas RIG-I’s antiviral activity is directed against paramyxoviruses, influenza virus, vesicular stomatitis virus, and Hepatitis C virus, MDA5 primarily senses picornaviruses (Kato et al., 2006; Loo et al., 2008; Sumpter et al., 2005). In addition, it has been recently shown that MDA5 is crucial for the host defense to paramyxoviruses in vivo (Gitlin et al., 2010). Furthermore, both RIG-I and MDA5 function redundantly in the detection of dengue virus and West Nile virus (Loo et al., 2008).
As a premature or overzealous innate immune response would be detrimental to the host, innate immune sensing and antiviral signal transduction must be tightly controlled. On the other hand, upon viral infection the rapid upregulation of IFN and proinflammatory cytokines is crucial for an effective antiviral response. For controlling RIG-I innate immune signaling, several positive and negative regulatory mechanisms have been described. RIG-I expression levels are rapidly increased upon exposure to IFN, providing a positive feedback mechanism for the amplification of the innate immune response upon virus infection. On the other hand, virus-induced up-regulation of a short RIG-I splice variant, that acts dominant-negative, provides a negative feedback mechanism of RIG-I antiviral signaling (Gack et al., 2008). Posttranslational modifications add another level of complexity to the regulation of RIG-I. RIG-I activity is negatively regulated by K48-linked ubiquitination, thereby inducing degradation of RIG-I by the proteasome (Arimoto et al., 2007). In contrast, K63-linked ubiquitination of the RIG-I CARDs as well as its CTD is critical for RIG-I to elicit antiviral activity (Gack et al., 2007; Oshiumi et al., 2009). Specifically, the ubiquitin E3 ligase TRIM25 ubiquitinates K172 in the CARD2 of RIG-I, which is essential for the efficient interaction of RIG-I with MAVS and thereby for antiviral signal transduction (Gack et al., 2007). Notably, influenza virus employs its non-structural protein 1 (NS1) to subvert RIG-I ubiquitination-dependent signaling by binding to and inhibiting TRIM25 (Gack et al., 2009). Furthermore, we have recently shown that Ser-Thr phosphorylation of the RIG-I tandem CARD keeps RIG-I inactive, thereby preventing downstream signaling prior to virus infection. In uninfected cells, RIG-I is robustly phosphorylated at S8 and T170, which inhibits RIG-I binding to TRIM25 and thereby RIG-I CARD ubiquitination, MAVS binding, and type-I IFN induction (Gack et al., 2010; Maharaj et al., 2012). In striking contrast to RIG-I, the regulatory mechanisms for the control of MDA5 signaling activity remain elusive.
Here, we not only show that MDA5-mediated signaling is regulated by a dynamic balance between phosphorylation and dephosphorylation of its N-terminal CARDs, but also identify the phosphatases PP1α and PP1γ as essential activators for both RIG-I and MDA5 signal transduction.
RESULTS
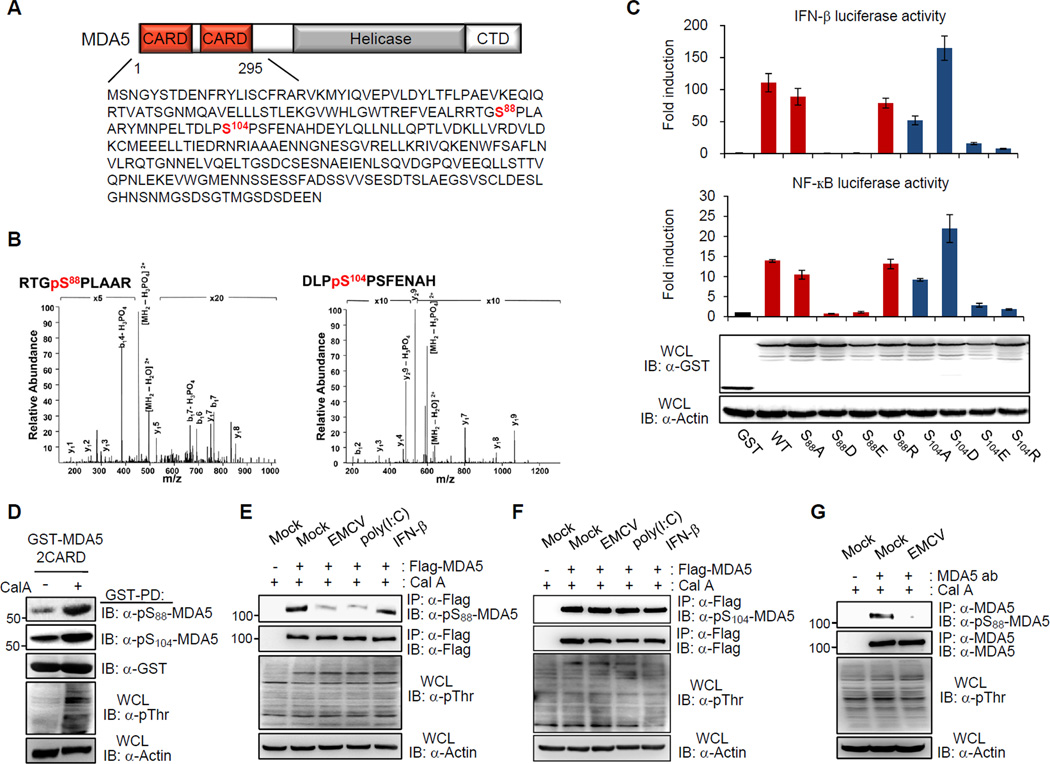
The N-terminal CARDs of MDA5 are phosphorylated
To define the molecular mechanisms that regulate MDA5 signal-transducing ability, we sought to identify posttranslational modifications of the N-terminal 2CARD of MDA5 using a mammalian glutathione S-transferase (GST) fusion construct. Purified GST-MDA5(2CARD) was then analyzed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) (Figures 1A and S1A). This showed that the MDA5(2CARD) carried phosphorylations at Ser-88 (S88) and Ser-104 (S104), located in the 1st CARD and short CARD-CARD linker region, respectively (Figures 1A, 1B, S1B and S1C). To determine the role of S88 and S104 phosphorylation in the regulation of MDA5 signaling activity, S88 and S104 were replaced individually with alanine or arginine (S88A/R or S104A/R) to mimic non-phosphorylation, or with aspartic or glutamic acid (S88D/E or S104D/E) to mimic constitutive phosphorylation (Figure 1C). GST-MDA5(2CARD) wild-type (WT) and mutants were then tested for their signal-transducing abilities in an IFN-β and NF-κB promoter activation assay. As shown in Figure 1C, no phosphorylation state-dependent effect was observed for the GST-MDA5(2CARD) S104 mutants: while S104A, S104E and S104R exhibited reduced abilities to induce IFN-β or NF-κB promoter activation compared with WT, the S104D mutant showed an increase in signaling activity. Strikingly, GST-MDA5(2CARD) phospho-mimetic S88D and S88E mutants exhibited a near-complete loss of downstream signaling compared with MDA5(2CARD) WT, whereas GST-MDA5(2CARD) S88A and S88R activated the IFN-β and NF-κB promoter at similar levels to WT (Figure 1C).
Figure 1. Phosphorylation of the MDA5 CARDs.
(A) MDA5 domain structure and amino acid sequence of the CARDs. Phosphorylation sites identified by MS are highlighted in red. CTD, C-terminal domain. (B) MS/MS spectra of tryptic phosphopeptides RTGpSPLAAR and DLPpSPSFENAH, which identified phosphorylation sites at S88 and S104, respectively. p, phosphorylation. b- and y-ion designations are shown (Aebersold and Goodlett, 2001). (C) GST, GST-MDA5(2CARD) WT or the indicated mutants were expressed together with IFN-β or NF-κB luciferase and constitutive β-gal expressing pGK-β-gal in HEK293T cells. At 48 h posttransfection, values for luciferase and β-galactosidase were determined. The results are expressed as means +/− s.d. (n=3). Whole-cell lysates (WCLs) were used for immunoblotting (IB) with anti-GST and anti-Actin antibodies. (D) Detection of S88 and S104 phosphorylations of MDA5 in vivo by using phospho-specific pS88-MDA5 and pS104-MDA5 antibodies. At 48 h posttransfection with GST-MDA5(2CARD), HEK293T cells were mock-treated or treated with Calyculin A (Cal A). WCLs were subjected to GST pull-down (GST-PD), followed by IB with the indicated antibodies. WCLs were further used for IB with anti-phosphothreonine (anti-pThr) and anti-Actin antibodies. (E and F) HEK293T cells transfected with Flag-MDA5, were either mock-treated, infected with EMCV (MOI 0.5) for 3 h, transfected with 5 µg/ml poly(I:C) or treated with 1,000 U/ml IFN-β for 20 h. WCLs were subjected to immunoprecipitation (IP) with anti-Flag antibody, followed by IB with anti-pS88-MDA5 (E) or anti-pS104-MDA5 (F), and anti-Flag antibodies (E and F). (G) HEK293T cells were either mock-infected or infected with EMCV (MOI 0.5) for 3 h. WCLs were used for IP with anti-MDA5 antibody, followed by IB. See also Figure S1.
To demonstrate the in vivo phosphorylations of MDA5 at S88 and S104, the 15-aminoacid peptides [80VEALRRTG(p)SPLAARY94] and [98ELTDLP(p)SPSFENAHD112] containing phosphorylated S88 or S104, respectively, were used to generate the phosphorylation state-specific pSer-88-MDA5 and pSer-104-MDA5 antibodies. Both antibodies strongly and specifically reacted with GST-MDA5(2CARD) WT, but not with the S88A or S104A mutant (Figures S1D and S1E). Furthermore, while both S88 and S104 phosphorylations of GST-MDA5(2CARD) and Flag-MDA5 were readily detectable in mock-treated cells, treatment with the Ser-Thr phosphatase inhibitor calyculin A (Cal A) further enhanced the S88 phosphorylation levels, but had only a minimal effect on the S104 phosphorylation of MDA5 (Figures 1D and S1F). This suggests that MDA5 phosphorylation at S88, but not S104, is tightly controlled by phosphatase-dependent dephosphorylation.
We next monitored the S88 and S104 phosphorylation levels of exogenous MDA5 under normal conditions, or in cells either infected with encephalomyocarditis virus (EMCV), a picornavirus sensed by MDA5, or transfected with the dsRNA analog polyinosine-polycytidylic acid [poly(I:C)] (Figures 1E and 1F). This showed that both S88 and S104 phosphorylations of MDA5 were readily detectable under normal conditions. Remarkably, while the phosphorylation levels of MDA5 at S104 did not change upon EMCV infection or poly(I:C) transfection, the MDA5 S88 phosphorylation markedly decreased upon virus infection or dsRNA stimulation (Figures 1E and 1F). This decline of S88 phosphorylation was specific for MDA5 stimulation by virus infection or dsRNA, as IFN-β treatment did not affect the S88 phosphorylation levels of MDA5 (Figure 1E). Consistent with our data on exogenously expressed MDA5, endogenous MDA5 was robustly phosphorylated at S88 in uninfected cells, and this phosphorylation markedly declined upon EMCV infection (Figure 1G). Taken together, these results indicate that MDA5 undergoes robust phosphorylation in its N-terminal CARDs under normal conditions, and that specifically phosphorylation and dephosphorylation at S88 are critical for regulating MDA5 signaling activity.
Phosphorylation of MDA5 at S88 inhibits its downstream signaling
To corroborate the S88 phosphorylation of the 2CARD of MDA5, the basal and poly(I:C)-stimulated activity of full-length MDA5 WT and S88 mutants were tested in an IFN-β promoter activation assay (Figure 2A). Consistent with previous reports (Andrejeva et al., 2004; Saito et al., 2007), ectopic MDA5 WT readily activated the IFN-β promoter in mock-treated cells, and its activation was further enhanced by poly(I:C) stimulation. While MDA5 S88A mutant exhibited an IFN-β promoter activation stronger than WT MDA5, the phospho-mimetic MDA5 S88E and S88D mutants did not activate the IFN-β promoter with or without poly(I:C) stimulation (Figure 2A). MDA5 binding to the CARD of the downstream adapter MAVS (also known as VISA, IPS-1, or CARDIF) is essential for MDA5 to elicit an IFN response (Kawai et al., 2005; Meylan et al., 2005; Seth et al., 2005; Xu et al., 2005). Thus, we next tested GST-MDA5(2CARD) WT or S88 mutants for their binding to the CARD of MAVS (Figure 2B). Whereas GST-MDA5(2CARD) WT and S88A mutant bound strongly to Flag-tagged MAVS-CARD-proline-rich-domain (PRD), the S88D and S88E mutants showed a complete loss of MAVS-CARD interaction (Figure 2B). Furthermore, these mutants were tested for their ability to induce the dimerization and nuclear translocation of IFN regulatory factor 3 (IRF3), an essential transcription factor for type-I IFN induction (Honda et al., 2006) (Figures 2C and 2D). GST-MDA5(2CARD) WT and S88A mutant efficiently induced IRF3 dimerization and nuclear translocation; in contrast, no IRF3 dimerization or nuclear localization was detected in cells transfected with the phospho-mimetic mutants S88D and S88E (Figures 2C and 2D). Moreover, increasing amounts of Flag-MDA5 S88D mutant potently suppressed the poly(I:C)-induced IFN-β promoter activation in a dominant-negative manner, whereas Flag-MDA5 S88A mutant robustly increased the IFN-β gene expression induced by poly(I:C) (Figure 2E). Finally, correlated with their signal-transducing abilities in HEK293T cells, ectopically expressed MDA5 WT and S88A mutant in Mda5-deficient (Ifih1−/−) mouse embryonic fibroblasts (MEFs) detectably induced IFN-β production in mock-treated cells, whereas MDA5 S88E or S88D mutant did not induce any IFN-β (Figure 2F). Furthermore, poly(I:C) transfection of MDA5 WT or S88A-expressing MEFs, but not of cells reconstituted with MDA5 S88E or S88D, enhanced the IFN-β production to significantly higher levels than vector-complemented MEFs (Figure 2F). These results indicate that while phosphorylation of MDA5 at S88 suppresses the MDA5 interaction with MAVS and thereby MDA5 downstream signaling, phosphatase-dependent dephosphorylation of MDA5 at S88 is necessary for its ability to induce an antiviral IFN response.
Figure 2. MDA5 phosphorylation at S88 inhibits its downstream signaling, whereas S88 dephosphorylation is required for optimal MDA5 activity.
(A) Vector, Myc-tagged MDA5 WT or mutants together with IFN-β luciferase and pGK-β-gal were transfected into HEK293T cells. 24 h after transfection, cells were either mock-treated or transfected with 0.1 µg/ml poly(I:C). 20 h later, luciferase and β-galactosidase activity were determined. The results are expressed as means +/− s.d. (n=3). Protein expressions were determined by IB. (B) HEK293T cells were transfected with MAVS-CARD-PRD-Flag together with GST or GST-MDA5(2CARD) constructs. WCLs were subjected to GST-PD. (C) 48 h after transfection with Flag-IRF3 and GST or GST-MDA5(2CARD) fusion constructs, WCLs of HEK293T cells were subjected to native PAGE, followed by IB with anti-Flag antibody. WCLs were also used for SDS-PAGE, followed by IB with anti-GST and anti-Flag antibodies. (D) HEK293T cells were transfected with GST, GST-MDA5(2CARD) WT or the indicated mutants and IRF3-eGFP. 48 h later, cells were stained for GST (red) and imaged by confocal microscopy. (E) MDA5 S88D mutant inhibits the poly(I:C)-induced IFN-β promoter activation. HEK293T were transfected with vector or increasing amounts of Flag-tagged MDA5 S88D or S88A mutant together with IFN-β luciferase and pGK-β-gal. Cells were either mock-treated or co-transfected with 3 µg/ml poly(I:C). 48 h later, luciferase activity was determined as described in Fig.1C. The results, expressed as means +/− s.d. (n=2), are representative of 3 independent experiments. (F) Mda5-deficient (Ifih1−/−) MEFs were transfected with vector, MDA5 WT or mutants. At 24 h posttransfection, cells were either mock-treated or transfected with 0.1 µg/ml poly(I:C). 20 h later, IFN-β production in the supernatant was measured by ELISA. The results are expressed as means +/− s.d. (n=3). ND, non-detectable.
A functional screen identifies PP1α and PP1γ as activators of MDA5 and RIG-I signal transduction
To identify the phosphatase responsible for MDA5 S88 dephosphorylation and hence activation, we undertook a screen utilizing the Dharmacon phosphatome small interfering RNA (siRNA) library comprising siRNA pools targeting 257 human phosphatases, including Ser-Thr phosphatases, Tyr phosphatases, and Ser-Thr-Tyr dual specificity phosphatases. Two parallel screens were performed to determine the impact of phosphatase silencing on the IFN-β promoter activation induced by GST-MDA5(2CARD) or full-length MDA5. Depletion of various phosphatase genes led to a marked decrease in MDA5-induced IFN-β promoter activation compared to non-targeting control siRNA (si.C) (Figure 3A and data not shown), and the nine strongest and/or overlapping hits that suppressed the MDA5(2CARD)- and MDA5 full-length-mediated IFN-β promoter activation were then cloned into an eukaryotic expression vector. The effect of ectopic expression of candidate phosphatases on MDA5(2CARD)- and MDA5 full-length-induced IFN-β promoter activation was then examined by luciferase assay (Figures 3B and S2A). Strikingly, exogenous expression of phosphoprotein phosphatase 1-α (PP1α) and PP1γ specifically and strongly augmented the IFN-β transcriptional activation induced by MDA5 full-length or MDA5(2CARD), while other phosphatases had no or minimal effects (Figures 3B and S2A).
Figure 3. Identification of PP1α and PP1γ as critical regulators of MDA5 and RIG-I signal transducing activities.
(A) Identified candidate phosphatases using a siRNA-based screen. HEK293T cells were transfected with GST-MDA5(2CARD) together with siRNA pools specific for one of 257 human phosphatases, as well as IFN-β luciferase and pGK-β-gal. 48 h posttransfection, luciferase and β-gal activities were determined. Results of the top 9 siRNA hits as well as of the siRNA specific for IRF3 (positive control) are shown. Activation by GST-MDA5(2CARD) upon co-transfection of a non-targeting control siRNA (si.C) was set to 100%. (B) Flag-MDA5 was co-transfected with the indicated candidate phosphatases together with IFN-β luciferase and pGK-β-gal. Luciferase and β-gal activities were determined 48 h after transfection. The results are expressed as means +/− s.d. (n=3). (C) Flag-MDA5 was transfected together with increasing amounts of HA-tagged PP1α, PP1β, or PP1γ, and IFN-β luciferase and pGK-β-gal. Luciferase and β-gal activities were measured 48 h after transfection. The results, expressed as means +/− s.d. (n=2), are representative of 3 independent experiments. (D and E) HEK293T were transfected with GST or GST-MDA5(2CARD) together with IFN-β or NF-κB luciferase and pGK-β-gal as well as non-targeting control siRNA (si.C), or siRNAs specific for PP1α, PP1β, PP1γ, or IRF3 (control). 48 h later, luciferase and β-gal activities were determined. The results are expressed as means +/− s.d. (n=3). (F and G) HEK293T cells, transfected with IFN-β luciferase, pGK-β-gal, and the indicated siRNAs, were either co-transfected with GST or GST-RIG-I(2CARD) (F), or infected with SeV (5 HA units/ml) for 14 h (G). Luciferase and β-gal activities were determined. The results are expressed as means +/− s.d. (n=3). See also Figure S2.
Mammals encode three PP1 isoforms, PP1α, PP1β and PP1γ, that are ubiquitously expressed and implicated in the regulation of various cellular processes, including glycogen metabolism, brain function, and muscle relaxation (Cohen, 2002). We thus tested the effect of PP1α, PP1β, or PP1γ expression on the MDA5 signal transduction (Figure 3C). Increasing amounts of PP1α or PP1γ, but not PP1β, robustly enhanced the IFN-β gene expression triggered by MDA5 (Figure 3C). Notably, exogenous expression of PP1α or PP1γ did not have any effect on the IFN-β promoter activation induced by exogenous MAVS (Figure S2B), reinforcing that PP1α and PP1γ act specifically at the level of MDA5 and not downstream of it. Conversely, specific silencing of endogenous PP1α or PP1γ, but not of PP1β, strongly inhibited MDA5(2CARD)-mediated IFN-β and NF-κB transcriptional activation, and depletion of endogenous PP1α and PP1γ was confirmed by Western blotting (Figures 3D, 3E, S2C, and S2D). To exclude off-target effects, the four unique siRNAs from each pool as well as one additional siRNA targeting specifically PP1α or PP1γ were retested separately (Figures S2E and S2F). This showed that all five distinct siRNAs targeting PP1α or PP1γ reduced the MDA5(2CARD)-induced IFN-β promoter activation, with the levels of depletion correlating with their suppressive effect on the IFN-β promoter (Figures S2E and S2F).
Our previous work demonstrated that RIG-I CARD phosphorylation at S8 and T170 suppresses the TRIM25-mediated K63-linked ubiquitination of RIG-I, thereby preventing RIG-I downstream signaling (Gack et al., 2010; Maharaj et al., 2012; Nistal-Villan et al., 2010). Our study also indicated that stimulus-induced dephosphorylation of the RIG-I CARDs is essential for RIG-I to initiate downstream signaling. We thus postulated that PP1α and PP1γ may be common activators for both MDA5 and RIG-I by inducing dephosphorylation of their N-terminal CARDs. To test this, we investigated the effect of silencing PP1α or PP1γ on downstream signaling triggered by the 2CARD of RIG-I, or by Sendai virus (SeV), known to activate RIG-I. Silencing of IRF3 served as control (Figures 3F and 3G). This showed that siRNA-mediated depletion of endogenous PP1α or PP1γ, but not of PP1β, strongly decreased the RIG-I(2CARD)- and SeV-induced IFN-β promoter activation (Figures 3F and 3G). In summary, these results suggest that the phosphatases PP1α and PP1γ are important regulators of the CARD-mediated signal transduction of both MDA5 and RIG-I.
PP1α and PP1γ interact with MDA5 and RIG-I upon virus infection, leading to their dephosphorylation
We next addressed whether PP1α and PP1γ specifically dephosphorylate S88 in MDA5, and the S8 and T170 residues in RIG-I. Exogenous PP1α or PP1γ substantially decreased the S88 phosphorylation of Flag-MDA5, but neither PP1β nor any of the other candidate phosphatases found in our siRNA screen, had any effect (Figures 4A and S3A). Crucially, specific gene-silencing of PP1α and PP1γ strongly enhanced the S88 phosphorylation of MDA5(2CARD) (Figure 4B). The effect of PP1α and PP1γ depletion on the S88 phosphorylation of MDA5 was specific, as PP1 depletion did not have any effect on MDA5 S104 phosphorylation levels (Figure S3B). Accordingly, ectopically expressed PP1α and PP1γ, but neither PP1β nor any of the other candidate phosphatases from our siRNA screen, led to a strong decrease in RIG-I CARD phosphorylation (Figures S3C and S3D). Inversely, depletion of PP1α and PP1γ profoundly enhanced the S8 and T170 phosphorylation of GST-RIG-I(2CARD) compared to transfection of non-targeting control siRNA (Figure 4C).
Figure 4. PP1α and PP1γ interact with RIG-I and MDA5, leading to their CARD dephosphorylation.
(A) WCLs of HEK293T cells, transfected with Flag-tagged MDA5 and HA-tagged phosphatase constructs, were used for IP with anti-Flag, followed by IB with anti-pS88-MDA5 or anti-Flag. (B and C) HEK293T cells were transfected with GST-MDA5(2CARD) (B) or GST-RIG-I(2CARD) (C), together with control siRNA (si.C) or siRNA specific for PP1α and PP1γ. WCLs were subjected to GST-PD, followed by IB with the indicated antibodies. (D) HEK293T cells were transfected with vector or Flag-MDA5. At 45 h posttransfection, cells were mock-treated or infected with EMCV (MOI 0.5) for 3 h. WCLs were subjected to IP with anti-Flag. (E and F) HEK293T (E) or NHLF (F) cells were either mock-treated, infected with SeV (50 HA units/ml) for 18 h (E), or infected with ΔNS1 PR8 influenza virus (MOI 2) for the indicated time points (F). WCLs were subjected to IP with anti-RIG-I antibody. n.s., non-specific band. (G and H) HEK293T (G) or NHLF (H) cells were either mock-treated, transfected with poly(I:C) for 36 h (G), or infected with EMCV (MOI 0.5) for 3 h (H). WCLs were subjected to IP with anti-MDA5 antibody. See also Figures S3 and S4.
We next tested the interaction between PP1α or PP1γ and MDA5. Consistent with their abilities to induce antiviral signaling independent of viral stimulation, exogenously expressed Flag-MDA5 and GST-MDA5(2CARD) efficiently interacted with PP1α and PP1γ in mock-infected cells (Figures 4D and S4A). Furthermore, EMCV infection further enhanced the binding of PP1α and PP1γ to Flag-MDA5 (Figure 4D). In contrast, PP1β did not bind Flag-MDA5 or GST-MDA5(2CARD) under these conditions (Figures 4D and S4A). Moreover, RIG-I(2CARD) and MDA5(2CARD), but not a RIG-I or MDA5 mutant in which the 2CARD had been deleted (RIG-I or MDA5 Δ2CARD), readily interacted with endogenous PP1α and PP1γ (Figure S4B).
Next, we sought to explore the molecular details of how virus infection leads to RIG-I and MDA5 dephosphorylation by PP1, thereby inducing antiviral signal transduction. We first assessed the endogenous protein levels of PP1α, PP1γ, and RIG-I under normal conditions, or in cells either infected with SeV or treated with IFN-β by WB analysis (Figure S4C). Consistent with previous reports, endogenous RIG-I levels were low under normal conditions but significantly increased upon SeV infection or IFN-β stimulation. In contrast, the protein expression of endogenous PP1α and PP1γ was readily detectable in mock-treated cells and did not change upon IFN-β treatment or virus infection (Figure S4C). To investigate whether virus infection regulates the specific recruitment of PP1α and PP1γ to RIG-I and MDA5, we examined PP1α and PP1γ binding to endogenous RIG-I in HEK293T cells prior to and after SeV infection by Co-Immunoprecipitation (Figure 4E). PP1α and PP1γ specifically bound to RIG-I in cells infected with SeV, whereas no interaction was detected in uninfected cells (Figure 4E). Consistently, endogenous PP1α and PP1γ exclusively interacted with RIG-I in primary normal human lung fibroblasts (NHLF) that had been infected with ΔNS1 influenza virus, but not in mock-treated cells (Figure 4F). Accordingly, PP1α and PP1γ were detected in complex with endogenous MDA5 exclusively in poly(I:C)-transfected or EMCV-infected cells, but not in mock-treated cells (Figures 4G and 4H). Crucially, endogenous RIG-I and MDA5 that interacted with PP1 exhibited markedly decreased phosphorylation levels at S8 and S88, respectively (Figures 4F and 4H). To provide further evidence for the specific recruitment of PP1 to RIG-I and MDA5 upon virus infection or dsRNA stimulation, we employed confocal microscopy. To this end, HeLa cells were mock-treated, infected with SeV to activate RIG-I, or transfected with poly(I:C) to stimulate MDA5, followed by determining the localization of endogenous RIG-I or MDA5 as well as PP1α and PP1γ (Figures S4D and S4E). Whereas cytoplasmic RIG-I and MDA5 protein expressions were low in uninfected cells and increased upon SeV infection or poly(I:C) transfection, robust PP1α and PP1γ expression was detected in mock-treated and infected/stimulated cells both in the nucleus and cytoplasm. Furthermore, PP1α and PP1γ extensively co-localized with endogenous RIG-I in the cytoplasm specifically in SeV-infected cells, but not in uninfected cells (Figure S4D). Similar to RIG-I, extensive co-localization of PP1 with endogenous MDA5 was observed exclusively upon poly(I:C)-stimulation (Figure S4E). Collectively, these results indicate that PP1α and PP1γ are recruited to RIG-I and MDA5 upon virus infection or viral RNA binding, leading to their dephosphorylation and hence activation.
Enhanced phosphorylation and abolished signaling activity of PP1-binding deficient mutants of MDA5 and RIG-I
Several known PP1-binding motifs have been identified in PP1 substrates or PP1-binding proteins, including the canonical R/K-x(0,1)-V/I-x-F/W motif (commonly termed RVxF motif), and the more recently discovered F-x-x-R/K-x-R/K motif (Garcia et al., 2004; Roy and Cyert, 2009). Indeed, RIG-I and MDA5 harbor an F-x-x-R/K-x-R/K consensus motif in their N-terminal CARDs (referred to as motif I [MI]), and an RVxF motif in their helicase domain (referred to as motif II [MII]) (Figure 5A, upper panel). To corroborate the role of PP1 for RIG-I and MDA5 dephosphorylation-mediated activation, hydrophobic and basic residues in the PP1-binding motif I or II of MDA5 and RIG-I were replaced with alanine; these mutants were then tested for their signal-transducing abilities in an IFN-β promoter assay (Fig. 5A, lower panel and Figure S5A). Strikingly, mutating PP1-binding motif MI or MII in MDA5, and motif MI in RIG-I caused a near-complete loss of signal-transducing activity, while mutation of motif MII in RIG-I did not affect the RIG-I signaling ability (Figure 5A, lower panel). Furthermore, MDA5 mutants MI and MII as well as RIG-I mutant MI exhibited an abolished PP1 binding ability, and consistent with this, strongly enhanced S88 or S8 phosphorylation levels compared to WT MDA5 or RIG-I (Figures 5B and 5C). In contrast, the RIG-I mutant MII exhibited a comparable PP1 binding activity and S8 phosphorylation levels to WT RIG-I. Notably, when tested for their RNA-binding ability the PP1-binding mutants MI and MII of RIG-I and MDA5 bound as efficiently as WT RIG-I or MDA5 to in vitro-transcribed 5’triphosphate rabies virus leader RNA or poly(I:C), respectively (Figure S5B). We next complemented Mda5-deficient (Ifih1−/−) and Rig-I-deficient (Ddx58−/−) MEFs with the PP1-binding mutant MI or MII of MDA5 and RIG-I, respectively (Figure 5D). MEFs complemented with WT MDA5 or WT RIG-I served as control. Poly(I:C)-induced IFN-β production was higher in Ifih1−/− MEFs reconstituted with MDA5 WT than in Ifih1−/− MEFs expressing vector, or the MDA5 mutants MI or MII (Figure 5D, left panel). Moreover, SeV infection triggered a robust IFN-β production in Ddx58−/− MEFs complemented with Flag-tagged RIG-I WT or RIG-I mutant MII, while no IFN-β was detected in SeV-infected cells complemented with the PP1-binding deficient mutant MI of RIG-I (Figure 5D, right panel). Since our previous work indicated that RIG-I S8 and T170 phosphorylation inhibits the RIG-I CARD ubiquitination (Gack et al., 2010; Nistal-Villan et al., 2010), we tested the phosphorylation and ubiquitination levels, as well as signal-transducing abilities of GST-RIG-I(2CARD) WT and PP1-binding deficient mutant MI (Figures 5E and 5F). GST-RIG-I(2CARD) MI exhibited strongly enhanced S8 phosphorylation levels compared to WT RIG-I(2CARD) and, correlated with this, an abolished CARD ubiquitination (Figure 5E). Furthermore, while GST-RIG-I(2CARD) WT robustly activated the IFN-β promoter, RIG-I(2CARD) MI did not have any signaling activity (Figure 5F). These results indicate that PP1 binding and PP1-dependent dephosphorylation of RIG-I and MDA5 are critical for their IFN-inducing activities in response to virus infection. In the case of RIG-I, dephosphorylation by PP1 is critical for RIG-I CARD ubiquitination and thus signal transducing ability.
Figure 5. PP1-binding deficient mutants of RIG-I and MDA5 exhibit enhanced CARD phosphorylation levels and abolished downstream signaling abilities.
(A) (upper) Identified PP1-binding motifs in MDA5 and RIG-I. (lower) Flag-tagged MDA5 WT or mutants (left), or RIG-I WT or mutants (right) together with IFN-β luciferase and pGK-β-gal were transfected into HEK293T cells. 48 h later, luciferase and β-gal activities were measured. The results are expressed as means +/− s.d. (n=3). (B) HEK293T cells were transfected with vector, Flag-MDA5, Flag-RIG-I or the indicated mutants together with HA-tagged PP1α or PP1γ, followed by infection with EMCV (MOI 0.5) (left panel) or SeV (50 HA units/ml) (right panel) for 3 h. WCLs were used for IP with anti-Flag. (C) WCLs of HEK293T cells transfected with Flag-MDA5, Flag-RIG-I or the indicated mutants were used for IP with anti-Flag. (D) Ifih1−/− and Ddx58−/− MEFs were complemented with MDA5 WT or mutants and RIG-I WT or mutants, respectively. IFN-β production upon mock-treatment, poly(I:C) (0.1 µg/ml) transfection (left), or SeV infection (50 HAU/ml) (right) was determined by ELISA. The results are expressed as means +/− s.d. (n=3). ND, non-detectable. (E) WCLs of HEK293T cells, transfected with GST-RIG-I(2CARD) WT or MI, were subjected to GST-PD. Arrows, ubiquitinated bands. (F) HEK293T cells were transfected with GST, GST-RIG-I(2CARD) WT or MI mutant together with IFN-β luciferase and pGK-β-gal. 48 h after transfection, luciferase and β-gal activities were determined. The results are expressed as means +/− s.d. (n=3). See also Figure S5.
PP1α and PP1γ are critical for the IFN-mediated antiviral activity of RIG-I and MDA5 in response to VSV and EMCV
To determine the physiological role of PP1 in regulating type-I IFN production induced by RIG-I and MDA5, we silenced endogenous PP1α and PP1γ in primary NHLF cells and examined the production of IFN-β upon transfection of poly(I:C) or EMCV-RNA, both of which stimulate MDA5, or upon infection with SeV or ΔNS1 influenza A virus, both of which are sensed by RIG-I (Figure 6A). Depletion of PP1α and PP1γ significantly decreased the IFN-β production induced by viral RNA or by viral infection (Figure 6A). To determine the individual contribution of PP1α and PP1γ to MDA5 and RIG-I dephosphorylation and antiviral signaling, we examined the effect of silencing either PP1α or PP1γ, or both on IFN-β production and MDA5 or RIG-I CARD phosphorylation (Figure S6). While PP1α or PP1γ single depletion markedly decreased the poly(I:C)- and SeV-induced IFN-β production, silencing of both PP1α and PP1γ reduced the IFN-β levels even further (Figures S6A and S6B). Consistently, depletion of either PP1α or PP1γ detectably enhanced the MDA5 S88 and RIG-I S8 phosphorylation, and these phosphorylations were further increased in PP1α and PP1γ doubly depleted cells (Figures S6C and S6D). These results suggest that both PP1α and PP1γ, which share ~90% identity at the amino acid level, contribute to RIG-I and MDA5 dephosphorylation-dependent activation in these cells. We next addressed whether PP1α and PP1γ specifically regulate the signaling activity of RIG-I-like receptors (RLR). To this end, we tested the effect of silencing PP1α and PP1γ on the signal transduction induced by Toll-like receptor 3 (TLR3) (Figure S7A). This showed that PP1α and PP1γ depletion did not have any effect on TLR3-induced NF-κB promoter activation upon poly(I:C) stimulation, suggesting that PP1 specifically regulates RLR signaling but not TLR3 signal transduction.
Figure 6. Depletion of PP1α and PP1γ decreases antiviral IFN production and enhances viral replication.
(A) NHLF cells were transfected with the indicated siRNAs. 48 h later, cells were either mock-transfected or transfected with poly(I:C) or Vero-EMCV-RNA, or infected with ΔNS1 A/PR/8/34 influenza virus or SeV. 24 h later, IFN-β production was measured by ELISA. PP1 knockdown was confirmed by IB. The results are expressed as means +/− s.d. (n=3). *p < 0.05; **p < 0.005. ND, non-detectable. (B and C) NHLF cells were transfected with the indicated siRNAs. 48 h later, cells were infected with VSV-eGFP (MOI 0.05). 24 h after infection, eGFP expression was determined by microscopy and WB, and virus titers were determined by plaque assay. PP1 knockdown was confirmed by IB (C). Pfu, plaque forming unit. (D and E) THP-1 cells were transfected with control siRNA (si.C), or with PP1α and PP1γ specific siRNAs, followed by mock-infection or infection with EMCV (MOI 1) for 5 h. Total RNA was isolated and subjected to RT-PCR using gene-specific primers for ISG15, ISG54 and ISG56 (D). PP1 knockdown was confirmed by IB (E). See also Figure S6.
To examine the functional role of PP1α and PP1γ in regulating the antiviral activity of RIG-I and MDA5, we examined virus replication or the induction of IFN-stimulated genes (ISGs) by viruses known to activate RIG-I (vesicular stomatitis virus [VSV]) or MDA5 (EMCV), in cells in which PP1α and PP1γ have been silenced (Figures 6B–E). Depletion of endogenous PP1α and PP1γ in primary NHLF cells substantially enhanced the replication of VSV-eGFP compared to transfection of non-targeting control siRNA as determined by fluorescence microscopy, plaque assay, and anti-eGFP immunoblotting (Figures 6B and 6C). Efficient depletion of PP1α and PP1γ was confirmed by immunoblot (Figure 6C). Furthermore, PP1α and PP1γ doubly depleted THP-1 macrophages had markedly reduced ISG transcript amounts upon EMCV infection compared to cells transfected with non-targeting control siRNA (Figures 6D and 6E).
PP1α and PP1γ are critical for IFN-mediated inhibition of Dengue virus replication
As our results indicate that PP1α and PP1γ are common activators of both RIG-I and MDA5, we next explored the effect of PP1α or PP1γ overexpression as well as their gene silencing on dengue virus type 2 (DenV) replication, a virus detected by both RIG-I and MDA5 (Figures 7A and 7B). This showed that expression of increasing amounts of PP1α or PP1γ strongly suppressed DenV replication in a dose-dependent manner compared to vector-transfection (Figure 7A). To complement these gain-of-function assays, we depleted PP1α and PP1γ in HeLa cells, followed by infection with DenV (Figure 7B). Silencing of PP1α and PP1γ enhanced DenV replication by more than 7-fold compared to cells transfected with non-targeting siRNA (Figure 7B). Furthermore, PP1α and PP1γ readily interacted with endogenous MDA5 and RIG-I in DenV-infected but not uninfected cells, and this correlated with enhanced IRF3 phosphorylation at S396, a marker of activation of the RLR signaling pathway (Figures 7C and 7D). Consistent with our data showing that PP1 is critical for RIG-I- and MDA5-mediated IFN-β induction, the enhanced DenV replication in PP1α and PP1γ doubly depleted cells directly correlated with markedly reduced ISG protein expressions following DenV infection compared to cells transfected with control siRNA (Figure 7E). Taken together, these results indicate that the dephosphorylation of RIG-I and MDA5 by PP1α and PP1γ is essential for these sensors to elicit immune signaling for the control of RNA virus infections.
Figure 7. PP1α and PP1γ are critical for ISG induction and host antiviral activity to Dengue virus.
(A) At 30 h posttransfection with vector, PP1α, or PP1γ, HEK293T cells were infected with DenV (MOI 0.5). 42 h later, cells were stained for viral envelope protein (red). Nuclei were stained with DAPI (blue). Quantification of infection is presented as relative infection compared to control. The results are expressed as means +/− s.d. (n=3). *p < 0.05; **p < 0.005. (B) At 48 h posttransfection with the indicated siRNAs, HeLa cells were infected with DenV (MOI 0.01). 42 h later, cells were stained for viral envelope protein (red). Nuclei were stained with DAPI (blue). Quantification of infection is presented as relative infection compared to control. The results are expressed as means +/− s.d. (n=3). *p < 0.05; **p < 0.005. (C and D) NHLF cells were either mock-treated or infected with DenV (MOI 0.1) for the indicated time points. WCLs were used for IP with anti-MDA5 (C) or anti-RIG-I (D) antibody. (E) NHLF cells were transfected with the indicated siRNAs. 48 h later, cells were either mock-infected or infected with DenV (MOI 0.25) for 24 h. WCLs were subjected to IB with the indicated antibodies. See also Figure S7.
DISCUSSION
The role of kinases, such as IKK-ε, TBK1, and RIP1 in activating immune responses to viral infection has long been established (Lee and Kim, 2007); in contrast, the impact of phosphatases in antiviral signaling pathways is largely undetermined. Here we have provided evidence demonstrating that the phosphatases PP1α and PP1γ are essential for an effective antiviral innate immune response mediated by the cytosolic pattern-recognition receptors RIG-I and MDA5.
Using mass spectrometry and phosphorylation state-specific antibodies, we found that the CARDs of MDA5 are robustly phosphorylated in uninfected cells. Mutational analyses further indicated that phosphorylation of MDA5 at S88 keeps it in an inactive state. Combined with our previous studies on RIG-I (Gack et al., 2010; Maharaj et al., 2012; Nistal-Villan et al., 2010), this corroborates that constitutive Ser-Thr phosphorylation is a common mechanism for suppressing the signal-transducing activity of RIG-I and MDA5, thereby preventing premature IFN production prior to virus infection. Moreover, our study shows that upon virus infection or stimulation by dsRNA, the MDA5 CARDs, as seen with RIG-I, are rapidly dephosphorylated, which is necessary for the initiation of downstream signaling, thereby triggering antiviral innate immune responses. Our study thus establishes a novel concept of regulation for the cytosolic sensors RIG-I and MDA5, that is characterized by a dynamic balance between constitutive phosphorylation for their inactivation and stimulus-dependent dephosphorylation, which triggers RLR activation upon virus infection.
The central finding of this study – that the phosphatases PP1α and PP1γ are key regulators of RIG-I and MDA5 antiviral signaling – was established with a complementary set of overexpression and siRNA gene targeting studies, that measured IFN-β production, ISG protein and mRNA levels, and the replication of viruses belonging to five different families, paramyxoviruses (SeV), rhabdoviruses (VSV), orthomyxoviruses (influenza virus), picornaviruses (EMCV) and Flaviviruses (Dengue virus). In each of these assays, we found that PP1α and PP1γ are critical regulators for RIG-I- and MDA5-mediated antiviral IFN production, thereby suppressing viral replication. Furthermore, the identification of PP1-binding motifs in RIG-I and MDA5 as well as the functional characterization of PP1-binding deficient mutants of both sensors provided genetic evidence that PP1 plays a crucial role in RLR signaling.
PP1 is a major eukaryotic Ser-Thr phosphatase implicated in a large variety of dephosphorylation events catalyzed by one of its three catalytic subunits (PP1α, PP1β, and PP1γ). Our study demonstrates for the first time that PP1α and PP1γ are crucial for eliciting an antiviral innate immune response by inducing dephosphorylation of the CARDs of RIG-I and MDA5, which allows MAVS-dependent downstream signaling. Our study further revealed some of the details of how virus infection triggers RIG-I and MDA5 dephosphorylation by PP1. In contrast to RIG-I and MDA5, PP1α and PP1γ expression levels were stable and did not change upon virus infection or dsRNA stimulation. Moreover, our study showed that PP1α and PP1γ are in complex with RIG-I or MDA5 exclusively in cells that were virus-infected or stimulated by dsRNA, but not in uninfected cells, suggesting that the specific recruitment of PP1 to these sensors triggers the rapid dephosphorylation of their CARDs upon virus infection. In addition, it has been recently shown that Ser-Thr phosphorylation of the RIG-I CTD also suppresses RIG-I downstream signaling (Sun et al., 2011). It will thus be interesting to investigate whether PP1 also dephosphorylates the CTD, thereby leading to RIG-I activation. Furthermore, our data showed that while exogenous PP1γ strongly enhanced the IFN-β promoter activation induced by MDA5 WT, it also enhanced signaling induced by the MDA5 S88A mutant albeit to a lesser extent (~40% of the effect seen with WT MDA5) (data not shown), suggesting that there are additional phosphorylation sites located in the MDA5 C-terminal region that are dephosphorylated by PP1. Moreover, it is well established that the complex formation of PP1 catalytic subunit with different regulatory subunits can determine its substrate specificity or subcellular location (Cohen, 2002). Thus, the potential regulation of PP1’s enzymatic activity or its recruitment to RIG-I and MDA5 by specific regulatory subunits remains ground for future studies.
Our findings combined with previous studies and the recently solved crystal structure of RIG-I support the following model of RLR activation (Figure S7B) (Jiang et al., 2011; Kowalinski et al., 2011; Luo et al., 2011): Under normal conditions, RIG-I is kept inactive by a closed conformation, where the CARD2 interacts with the helicase and possibly CTD, and where the tandem CARD forms a head-to-tail conformation characterized by an interaction between the N-terminus of CARD2 and the C-terminus of CARD1. Our data showed that constitutive phosphorylation of the CARDs of both RIG-I and MDA5 prevents premature downstream signaling, possibly by keeping the tandem CARD in its inactive head-to-tail conformation, and we have recently shown that conventional PKC interacts with RIG-I, leading to its CARD phosphorylation (Maharaj et al., 2012). The first step of RIG-I and MDA5 activation is binding of 5’-triphosphate-containing dsRNA or long dsRNA, respectively, first to the CTD and subsequently to the helicase domain. For RIG-I, viral RNA-binding is believed to trigger a conformational change, leading to ATP hydrolysis and release of the CARDs. The data presented here indicate that the exposed CARDs then allow recruitment of PP1α and PP1γ, which then rapidly dephosphorylate S8 and T170 of RIG-I, and S88 of MDA5. CARD dephosphorylation is likely to lead to a structural change within the tandem CARD. In the case of RIG-I, dephosphorylation of S8 and T170 allows binding of TRIM25 to CARD1 (Gack et al., 2010; Nistal-Villan et al., 2010), which induces K63-linked ubiquitination or unanchored polyubiquitin binding of CARD2 (Gack et al., 2007; Zeng et al., 2010). Future studies are directed towards elucidating the molecular mechanisms by which dephosphorylation of S88 in MDA5 triggers MAVS binding, and whether MDA5 phosphorylation regulates other posttranslational modifications of MDA5, including free ubiquitin binding as recently reported (Jiang et al., 2012).
In summary, our results provide insights into the complex regulatory network of the RIG-I and MDA5 signaling pathway, and identify PP1α and PP1γ as molecules to be exploited for therapeutic intervention of emerging virus-associated disorders.
EXPERIMENTAL PROCEDURES
Cell Culture
HEK293T, HeLa, Vero, NHLF, and MEF cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 1% penicillin-streptomycin (Gibco-BRL). HEK293 cells stably expressing TLR3 (Imgenex) were cultured in complete DMEM containing 10 µg/ml blasticidin (Invitrogen). THP-1 cells (ATCC) were cultured in RPMI supplemented with 10% FBS, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate and 1% penicillin-streptomycin.
WT and Mda5-deficient MEFs were isolated from wild-type mice and Mda5-deficient (Ifih1−/−) mice (kindly provided by M. Colonna [Washington University]) (Gitlin et al., 2010). Isolated MEFs were immortalized with LXSN-E6/E7 retroviral vector containing human papilloma virus 16 E6 and E7 oncogenes using a standard protocol of selection with 200 µg/ml of neomycin. Immortalized Rig-I-deficient (Ddx58−/−) MEFs were described previously (Gack et al., 2007).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants R01 AI087846, R21 AI097699, and RR00168 (M.U.G.) and by the German Research Foundation (E.W.). We greatly thank J. Jung, L. Gehrke, S. Whelan, M. Colonna, and A. García-Sastre for providing reagents, and R. Tomaino for mass spectrometry.
E. Wies and M. Wang performed the majority of the experiments. M. Gack performed experiments and analyzed data. N. Maharaj and K. Chen performed experiments and assisted in data collection. S. Zhou and R. Finberg provided Mda5-deficient MEFs. M. Gack organized this study and wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aebersold R, Goodlett DR. Mass spectrometry in proteomics. Chem Rev. 2001;101:269–295. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007;104:7500–7505. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe. 2009;5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Kirchhofer A, Shin YC, Inn KS, Liang C, Cui S, Myong S, Ha T, Hopfner KP, Jung JU. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci U S A. 2008;105:16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Nistal-Villan E, Inn KS, Garcia-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J Virol. 2010;84:3220–3229. doi: 10.1128/JVI.02241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Garcia A, Cayla X, Caudron B, Deveaud E, Roncal F, Rebollo A. New insights in protein phosphorylation: a signature for protein phosphatase 1 interacting proteins. Comptes rendus biologies. 2004;327:93–97. doi: 10.1016/j.crvi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gitlin L, Benoit L, Song C, Cella M, Gilfillan S, Holtzman MJ, Colonna M. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 2010;6:e1000734. doi: 10.1371/journal.ppat.1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Jiang F, Ramanathan A, Miller MT, Tang GQ, Gale M, Jr, Patel SS, Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj NP, Wies E, Stoll A, Gack MU. Conventional protein kinase C-alpha (PKC-alpha) and PKC-beta negatively regulate RIG-I antiviral signal transduction. J Virol. 2012;86:1358–1371. doi: 10.1128/JVI.06543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Nakhaei P, Genin P, Civas A, Hiscott J. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Nistal-Villan E, Gack MU, Martinez-Delgado G, Maharaj NP, Inn KS, Yang H, Wang R, Aggarwal AK, Jung JU, Garcia-Sastre A. Negative role of RIG-I serine 8 phosphorylation in the regulation of interferon-beta production. J Biol Chem. 2010;285:20252–20261. doi: 10.1074/jbc.M109.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Hatakeyama S, Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009;284:807–817. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- Roy J, Cyert MS. Cracking the phosphatase code: docking interactions determine substrate specificity. Sci Signal. 2009;2:re9. doi: 10.1126/scisignal.2100re9. [DOI] [PubMed] [Google Scholar]
- Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M., Jr Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Sumpter R, Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M., Jr Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Ren H, Liu Y, Teeling JL, and Gu., J Phosphorylation of RIG-I by casein kinase II inhibits its antiviral response. J Virol. 2011;85:1036–1047. doi: 10.1128/JVI.01734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.