Abstract
Phospholipases A2 are represented in snake venoms by several types and possess diverse biological activities including neurotoxicity. Previously, we isolated and characterized two neurotoxic phospholipases A2 (HDP-1 and HDP-2) from the venom of Nikolski's viper (Vipera nikolskii), which were heterodimers composed of two non-covalently bound subunits. Each heterodimer consisted of an enzymatically active basic subunit and an inactive acidic subunit. In this work, we studied the in vivo biological activity of HDP-2 in mice. The acute toxicity (LD50 = 0.38 μg/gm) and maximal tolerated dose (0.1 μg/gm) were determined. In the hot plate test, HDP-2 at the maximal tolerated dose, reliably prolonged the time of the mouse staying on the plate. However, taking into account the neurotoxicity of HDP-2, we believe that this effect may be explained by a general intoxication rather than specific decrease of pain sensitivity. In this respect HDP-2 differs from other heterodimeric phospholipases A2 like crotoxin, which possess analgesic activity. This difference can be explained by the dissimilarity in the structure of the acidic subunits, suggesting an important role of this subunit in analgesic activity.
Keywords: Phospholipase A2, toxicity, venom, snake, nociception, α-neurotoxin
INTRODUCTION
One of the most prominent early symptoms of snakebite envenoming is disturbed pain sensitivity. Snakebites often induce severe pain at the site of the bite or in other parts of the body (Frangides et al, 2006; Alkaabi et al, 2011; Walker and Morrison, 2011). This is especially pronounced in bites by snakes of the family Viperidae. However, another set of data indicates anti-nociceptive properties of some snake venoms (Picolo et al, 1998) and their components. Thus, analgesic activity has been shown for so-called three-fingered α-neurotoxins. For example, α-cobratoxin from the Thai monocellate cobra Naja kaouthia (Chen et al, 2006) produced antinociceptive effects. Recently, it has been shown that a new class of three-finger peptides from the black mamba (Dendroaspis polylepis) venom is able to abolish pain through inhibition of acid sensing ion channels expressed either in central or peripheral neurons (Diochot et al, 2012). Antinociceptive activity was also reported for crotoxin, a phospholipase A2 (PLA2) from South American rattlesnake (Crotalus durissus terrificus) venom (Zhang et al, 2006). The crotoxin molecule is composed of two non-covalently bound subunits: a weakly toxic basic phospholipase A2 and an acidic non-toxic and non-enzymatic polypeptide named crotapotin. Similar to some other oligomeric PLA2, it possesses presynaptic neurotoxicity (Sampaio et al, 2010). In our studies of Nikolski's viper (Vipera nikolskii) venom, we isolated two hetrodimeric PLA2 (HDP-1 and HDP-2) also manifesting presynaptic neurotoxicity (Ramazanova et al, 2008). Crotoxin and V. nikolskii PLA2s are homologous proteins; the main structural difference between them is in their acidic subunits. The acidic crotapotin consists of three disulfide-linked polypeptide chains (α, β, γ) which result from proteolytic cleavage of a unique precursor (pro-CA) that has been identified from its cDNA (Bouchier et al, 1991). In HDP-1 and HDP-2 the acidic subunit consists of a single polypeptide chain that is homologous to the basic subunit, but lacks a histidine residue at the active site (Ramazanova et al, 2008). Such dissimilarity may result in different biological activities of proteins. The present work was undertaken to investigate whether neurotoxic HDP-2 has analgesic properties. We found that HDP-2 increases the time before the first hind paw licking and the first jump of CD-1 mice in the hot plate test. This might suggest decreased pain sensitivity. However, as intoxication by HDP-2 strongly inhibits locomotor activity in mice, this effect may be explained by a general neurotoxic effect of the toxin.
MATERIALS AND METHODS
The phospholipase HDP-2 was isolated from V. nikolskii venom as described (Ramazanova et al, 2008). For injection in mice, the protein was dissolved in saline. Adult male mice of the CD-1 strain (8-9 weeks old, 30-35 gm body weight) were used in this study. The animals were kept in a 12 hr light:dark cycle (18-26°C, 30-70% humidity) with food and water ad libitum in accordance with the World Health Organization's International Guiding Principles for Animal Research (WHO Chronicle, 1985). All animals were subjected to experimental operations only once and were not used for other tests. Intravenous injection of a single toxin dose was used for toxicity assays. The injection volume was 1 ml per 1000 gm of animal body weight. Animals were observed for 72 hr after injection. The quantitative toxicity parameters were calculated by the method of Litchfild and Wilkoxon (1949).
The hot plate test was performed on a Hot Plate Analgesia Meter (Columbus Instruments, Columbus, OH, USA). The animals were injected with HDP-2 (0.1 mg/kg) and placed on the thermostat surface at 55°C 15 min after injection. Latency to paw-lick response and latency to jumps was registered. Animals were taken off the hot surface after the first jump. Sodium chloride solution (0.9%) was injected into a control group of animals.
RESULTS AND DISCUSSION
Previously, we isolated two heterodimeric phospholipases A2 (HDP-1 and HDP-2) from V. nikolskii venom and showed their neurotoxic effects in vitro (Ramazanova et al, 2008). In particular, the nerve impulse transmission in the frog nerve-muscle preparation was affected. HDP-1 and HDP-2 are structurally and functionally very similar. However HDP-2 possesses higher biological activity and its content in the venom is also higher, that is why HDP-2 was chosen for this study. It was isolated from the V. nikolskii venom by ion-exchange chromatography as described (Ramazanova et al, 2008). To further study the biological activity in vivo, the mice were injected intravenously with increasing doses of HDP-2. The symptoms of HDP-2 intoxication were similar irrespective of the dose used. One to 3 min after injection, the animals showed severe depression, a strong decrease of locomotor activity, decreased breath rate and crooked posture followed at high doses by coma within the next 15-30 min. Their death was registered within the first 2 hrs. The general conditions of surviving animals were normal. The results of postmortem investigations showed that death was caused by asphyxia as manifested by wide eyes, a protruding tongue as well as cyanosis of the lips and the extremities. There were no obvious changes of internal organs. The data of the toxicity assays are given in Table 1. The calculated LD50 of HDP-2 is 0.38 μg/gm. This value is close to that of a heterodimeric PLA2 from Vipera aspis venom (0.288 μg/gm; Komori et al, 1990) and about two times higher than the value determined for a heterodimeric PLA2 from Taiwanese Daboia siamensis (Wang et al, 1992). The LD50 for crotoxin is 0.06-0.09 μg/gm (Okamotoet al, 1993; Rangel-Santos et al, 2004), therefore the toxicity of HDP-2 was substantially lower than that of crotoxin. The maximal tolerated dose of HDP-2 was 0.1 μg/gm. This value was used for the further study of HDP-2 influence on pain sensitivity.
Table 1.
The lethality of HDP-2 after single intravenous injection in mice.
| Number of mice injected | Dose (μg/gm) | Number of animals | |
|---|---|---|---|
| Dead | Survived | ||
| 3 | 1 | 3 | 0 |
| 5 | 0.5 | 4 | 1 |
| 6 | 0.35 | 2 | 4 |
| 6 | 0.25 | 1 | 5 |
| 12 | 0.1 | 0 | 12 |
To investigate the antinociceptive activity of HDP-2, the “hot plate” test was used. In this test, the time before the first heat induced jump and the first paw licking were registered. The results are summarized in Table 2. The data obtained indicate that the time spent on the hot plate before the first forepaw licking did not differ significantly between the control and experimental groups. However, the time before the first hind paw licking and the first jump increased significantly for the experimental animals as compared to the control indicating decreased pain sensitivity in the experimental mice. It should be mentioned that forepaw licking is a common grooming response or a response to warmth rather than noxious heat. Therefore, hind paw licking is a more reliable measure of discomfort. (Mogil et al, 2001). Earlier, an analgesic action had been shown for crotoxin by Zhang et al (2006). These authors observed dose dependent analgesia at doses ranging from 0.0295-0.0665 μg/gm. This last value is in the range of the LD50 reported for crotoxin (Rangel-Santos et al, 2004), however, no animal death was reported (Zhang et al, 2006). Moreover, intact locomotor activity was observed in injected animals (Zhang et al, 2008). We found a noticeable decrease in locomotor activity even after the injection of low doses (0.1-0.2 μg/gm) of HDP-2. Taking this fact into account we suggest that the delayed reaction of mice to nociceptive stimulus after HDP-2 injection may result from general intoxication slowing down all the reflexes in treated animals. In this respect, HDP-2 differs from crotoxin, which produces analgesia.
Table 2.
Results of the hot plate test for HDP-2 (0.1μg/gm) in CD-1 mice.
| Animal group | Time (seconds) spent on the hot plate before: | ||
|---|---|---|---|
| First forepaw licking | First hindpaw licking | First jump | |
| HDP-2 (n=12) | 6.7±0.7 | 21.3±0.7* | 41.6±1.8* |
| Control (n=15) | 7.7±0.4 | 14.6±1 | 31.5±1.7 |
*Significant difference (P < 0.05 by Student’s t-test) relative to control.
As mentioned in the introduction both crotoxin and HDP-2 are heterodimers consisiting of two non-covalently bound subunits: a basic PLA2 and an acidic enzymatically inactive protein homologous to the basic subunit. The basic subunits of crotoxin and HDP-2 are homologous proteins sharing about 60% identical residues (Figure 1). In contrast, a big difference is found when the structures of the acidic subunits are compared (Figure 1). The acidic subunit of crotoxin, crotapotin, is shorter and consists of three polypeptide fragments produced from a single-chain precursor during posttranslational processing. Such dissimilarity may result in distinct biological activities of crotoxin and HDP-2. Taking these considerations into account, one may suggest an important role of crotapotin in the analgesic activity of crotoxin.
Figure 1.
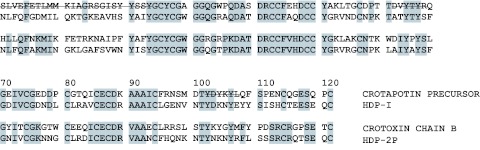
Amino acid sequences of crotoxin and HDP-2. HDP-I and HDP-2P are acidic and basic subunits of HDP-2, respectively (Ramazanova et al, 2008). Crotoxin chain B - basic subunit of crotoxin (Bouchier et al, 1991). Identical amino acid residues are shaded in grey. Crossed residues indicate fragments removed from the precursor during processing.
Although there are no perspectives of using HDP-2 or its modified forms as potential analgesics in view of the presented results, our data indicate an important role of the acidic subunits in the analgesic activity of heterodimeric PLA2.
CONCLUSIONS
The toxicity of a hetrodimeric PLA2 from V. nikolskii venom for mice was determined. Its LD50 (0.38 μg/gm) is close to those obtained for other heterodimeric PLA2s.
In the hot plate test HDP-2 increased the time before the first hind paw licking and the first jump at the maximal tolerated dose of 0.1 μg/gm. However, this effect may be explained by a decrease in locomotor activity rather than analgesic activity of HDP-2.
Comparison of the analgesic effect produced by crotoxin with that of HDP-2 indicates an important role of acidic subunits in the analgesic activity of heterodimeric PLA2.
ACKNOWLEDGEMENTS
This work was supported by the Russian Foundation for Basic Research (Grant No 12-04-01523); the Russian Ministry of Education and Science (Contract No 02.740.11.0865); and the EC FP7 programme Neurocypres (Grant No 202033).
LIST OF ABBREVIATIONS
- PLA2
phospholipase A2
COMPETING INTERESTS
None declared.
REFERENCES
- Alkaabi JM, Al Neyadi M, Al Darei F, et al. Terrestrial snakebites in the South East of the Arabian Peninsula: patient characteristics, clinical presentations, and management. PLoS One. 2011;6:e24637. doi: 10.1371/journal.pone.0024637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchier C, Boulain JC, Bon C, Menez A. Analysis of cDNAs encoding the two subunits of crotoxin, a phospholipase A2 neurotoxin from rattlesnake venom: the acidic non enzymatic subunit derives from a phospholipase A2-like precursor. Biochim Biophys Acta. 1991;1088:401–408. doi: 10.1016/0167-4781(91)90132-6. [DOI] [PubMed] [Google Scholar]
- Chen R, Robinson SE. Effect of cholinergic manipulations on the analgesic response to cobrotoxin in mice. Life Sci. 1990;47:1949–1954. doi: 10.1016/0024-3205(90)90407-i. [DOI] [PubMed] [Google Scholar]
- Chen ZX, Zhang HL, Gu ZL, et al. A long-form α-neurotoxin from cobra venom produces potent opioid-independent analgesia. Acta Pharmacol Sin. 2006;27:402–408. doi: 10.1111/j.1745-7254.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Diochot S, Baron A, Salinas M, et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- Frangides CY, Koulouras V, Kouni SN, et al. Snake venom poisoning in Greece. Experiences with 147 cases. Eur J Intern Med. 2006;17:24–27. doi: 10.1016/j.ejim.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Hawgood BJ, Smith JW. The mode of action at the mouse neuromuscular junction of the phospholipase A-crotapotin complex isolated from venom of the South American rattlesnake. Br J Pharmacol. 1977;61:597–606. doi: 10.1111/j.1476-5381.1977.tb07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendon RA, Fraenkel-Conrat H. Biological role of the two components of Cro. Proc Natl Acad Sci USA. 1971;68:1560–1563. doi: 10.1073/pnas.68.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Guiding Principles for Biomedical Research involving Animals The WHO Chronicle. 1985;39:51–56. [PubMed] [Google Scholar]
- Komori Y, Nikai T and Sugihara H. Comparative study of three phospholipase A2s from the venom of Vipera aspis. Comp Biochem Physiol B. 1990;97:507–514. doi: 10.1016/0305-0491(90)90151-i. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- Mogil JS, Wilson SG and Wan Y. Assessing Niciception in Murine Subjects. In: Kruger L, editor. Methods in Pain Research. CRC Press LLC; Boca Raton, Florida: 2001. pp. 11–39. [Google Scholar]
- Okamoto M, Viskatis LJ, de la Roza G, Vidal JC. Induction of tolerance to crotoxin in mice. J Pharmacol Exp Ther. 1993;265:41–46. [PubMed] [Google Scholar]
- Picolo G, Giorgi R, Bernardi MM and Cury Y. The antinociceptive effect of Crotalus durissus terrificus snake venom is mainly due to a supraspinally integrated response. Toxicon. 1998;36:223–227. doi: 10.1016/s0041-0101(97)00048-2. [DOI] [PubMed] [Google Scholar]
- Pu XC, Wong PT and Gopalakrishnakone P. A novel analgesic toxin (hannalgesin) from the venom of king cobra (Ophiophagus hannah) Toxicon. 1995;33:1425–1431. doi: 10.1016/0041-0101(95)00096-5. [DOI] [PubMed] [Google Scholar]
- Ramazanova AS, Zavada LL, Starkov VG, et al. Heterodimeric neurotoxic phospholipases A2--the first proteins from venom of recently established species Vipera nikolskii: implication of venom composition in viper systematics. Toxicon. 2008;51:524–537. doi: 10.1016/j.toxicon.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Rangel-Santos A, Dos-Santos EC, Lopes-Ferreira M, et al. A comparative study of biological activities of crotoxin and CB fraction of venoms from Crotalus durissus terrificus, Crotalus durissus cascavella and Crotalus durissus collilineatus. Toxicon. 2004;43:801–810. doi: 10.1016/j.toxicon.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Sampaio SC, Hyslop S, Fontes MR, et al. Crotoxin: novel activities for a classic beta-neurotoxin. Toxicon. 2010;55:1045–1060. doi: 10.1016/j.toxicon.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470–474. doi: 10.1016/j.jamcollsurg.2010.12.049. [DOI] [PubMed] [Google Scholar]
- Wang YM, Lu PJ, Ho CL, Tsai IH. Characterization and molecular cloning of neurotoxic phospholipases A2 from Taiwan viper (Vipera russelli formosensis) Eur J Biochem. 1992;209:635–641. doi: 10.1111/j.1432-1033.1992.tb17330.x. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Han R, Chen ZX, et al. Opiate and acetylcholine-independent analgesic actions of crotoxin isolated from Crotalus durissus terrificus venom. Toxicon. 2006;48:175–182. doi: 10.1016/j.toxicon.2006.04.008. [DOI] [PubMed] [Google Scholar]


