Summary
Massive palindromes in the human Y chromosome harbor mirror-image gene pairs essential for spermatogenesis. During evolution, these gene pairs have been maintained by intrapalindrome, arm-to-arm recombination. The mechanism of intrapalindrome recombination and risk of harmful effects are unknown. We report 51 patients with isodicentric Y (idicY) chromosomes formed by homologous crossing-over between opposing arms of palindromes on sister chromatids. These ectopic recombination events occur at nearly all Y-linked palindromes. Based on our findings, we propose that intrapalindrome sequence identity is maintained via noncrossover pathways of homologous recombination. DNA double-strand breaks that initiate these pathways can be alternatively resolved by crossing over between sister chromatids to form idicY chromosomes, with clinical consequences ranging from spermatogenic failure to sex reversal and Turner syndrome. Our observations imply that crossover as well as noncrossover pathways are active in nearly all Y-linked palindromes, exposing an Achilles' heel in the mechanism that preserves palindrome-borne genes.
Introduction
Since emergence of the mammalian X-Y sex determination system hundreds of millions of years ago, functional specialization of the evolving Y chromosome (chrY) has driven the stepwise suppression of sexual recombination with the X chromosome (chrX), resulting in differentiation of what was once an ordinary pair of recombining autosomes (Lahn and Page, 1999). Such increasingly limited meiotic exchange has also fostered decay of chrY (Charlesworth and Charlesworth, 2000). Whereas the pseudoautosomal regions of the modern human sex chromosomes continue to pair and recombine, the remaining 95% of chrY, the male-specific region of the Y (MSY; Figure 1A), does not ordinarily recombine with chrX and has retained only a small minority of its ancestral genes (Skaletsky et al., 2003).
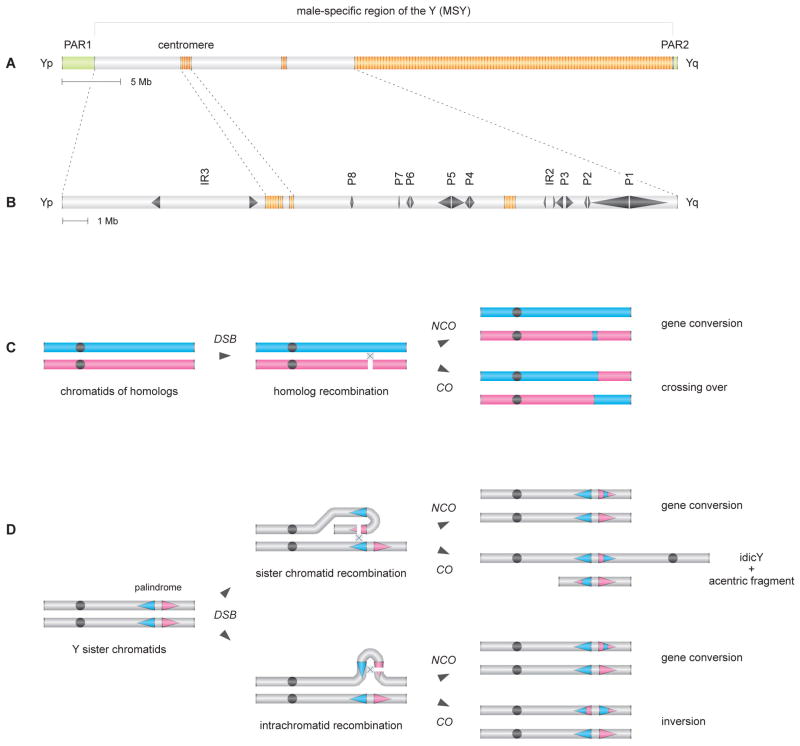
Figure 1. Hypothesized mechanism of idicY formation by homologous recombination in MSY palindrome.
(A) Schematic representation of chrY. MSY is flanked by pseudoautosomal regions PAR1 and PAR2 (green) and contains heterochromatic blocks (orange). Yp, short arm. Yq, long arm.
(B) Expanded view of MSY euchromatin, with palindromes P1 through P8 and inverted repeats IR2 and IR3.
(C) Conventional recombination between homologous chromosomes: DSB resolution by NCO and CO pathways yielding, respectively, gene conversion and crossing over. Second chromatid not shown for each of the two homologs.
(D) Model of homologous recombination in MSY palindromes: DSB resolution by NCO pathways yields gene conversion. Resolution by crossing over between sister chromatids can produce an idicY (and an acentric fragment), while crossing over within a chromatid yields an inversion.
Counterpoint to this decay in recombination's absence is provided by massive MSY palindromes that have been maintained by gene conversion. These palindromes (Figure 1B and Figure S1 in Supplemental Data) harbor mirror-image gene pairs that play critical roles in spermatogenesis. Each palindrome is composed of two large arms separated by a small “spacer” sequence at the center. Ranging in wingspan from 30 kilobases (kb) to 2.9 megabases (Mb), the palindromes comprise 25% of MSY euchromatin (Skaletsky et al., 2003). The palindromes predate divergence of the human and chimpanzee lineages, yet they exhibit arm-to-arm sequence identities of >99.9%. Evidence in modern human lineages of sequence homogenization in MSY palindromes indicates that gene conversion there remains a frequent process (Rozen et al., 2003).
As the mechanistic basis of the gene conversion that maintains MSY palindromes has yet to be elucidated, we considered possible parallels to a better understood process. Meiotic (or mitotic) recombination between homologous chromosomes frequently results in nonreciprocal exchange, or gene conversion, with no crossing over. Such gene conversion is the result of DNA double-strand break (DSB) formation followed by noncrossover (NCO) resolution of those breaks (Figure 1C) (Börner et al., 2004; Guillon et al., 2005). Similarly, we hypothesized that arm-to-arm sequence identity within palindromes might be maintained by DSB formation and NCO resolution, either between sister chromatids or within one chromatid (Figure 1D). Since DSB formation can also lead to crossing over in the case of homologous chromosomes, we further hypothesized that analogous options exist in the case of MSY palindrome recombination. A testable prediction of this hypothesis is that interchromatid crossover (CO) resolution of DSBs formed in MSY palindromes might give rise to isodicentric Y (idicY) chromosomes, mirror-imaged chromosomes with an axis of symmetry through the center of the involved palindrome (Figure 1D).
Indeed, idicY chromosomes had been identified through light microscopic studies (Jacobs and Ross, 1966; Hsu, 1994), but their molecular basis and mode of origin remained unknown. Moreover, we suspected that idicY chromosomes might sometimes go undetected or be misidentified in cytogenetic studies due to chrY's small size and paucity of microscopic landmarks. We thus conducted a broad screen for potential idicY chromosomes by molecular testing, using Y-DNA markers, to capture a large sample and obtain insight into mechanisms of origin. We identified 51 unrelated individuals with idicY chromosomes that evidently formed by homology-mediated crossing over between palindromes on sister chromatids. These idicY chromosomes are associated with a wide range of sex-linked reproductive disorders including spermatogenic failure, sex reversal, and Turner syndrome.
Results
We began by reanalyzing DNA samples from 2,380 patients studied in our laboratory during the past 25 years (Vergnaud et al., 1986; Vollrath et al., 1992; Reijo et al., 1995; Kuroda-Kawaguchi et al., 2001; Repping et al., 2002; our unpublished results). These individuals had been ascertained by one of two means. Fifteen-hundred and fifty patients were men with spermatogenic failure (< 5 million spermatozoa per ml semen; normal > 20 million spermatozoa per ml semen), a phenotype often associated with chrY anomalies. In the remaining 830 patients, light microscopy had revealed a structurally anomalous chrY, or a discrepancy between sex chromosome constitution and sex phenotype (“sex reversal”).
Employing precisely mapped, Y-specific sequence-tagged sites (STSs) as markers (Vollrath et al., 1992; Lange et al., 2008), we screened genomic DNAs from all 2,380 patients for evidence of idicY chromosomes. Most MSY palindromes are located on the long arm (Yq; Figure 1B), where recombination between opposing palindrome arms on sister chromatids should generate idicYp chromosomes, bearing two copies of the short arm (Yp) and centromere but lacking distal Yq (Figure 1D). Accordingly, we searched specifically for idicYp chromosomes.
49 Patients with Possible IdicYp Chromosomes Identified by Mapping of Breakpoints to Yq Palindromes
Our initial screen utilized three STS markers: sY14 derives from the sex-determining gene SRY and marks the most distal male-specific portion of Yp; sY78 derives from centromeric alpha satellite (DYZ3) sequences; and sY1273 marks the most distal male-specific portion of Yq (Figure 2A). Individuals bearing idicYp chromosomes should carry the Yp and centromeric markers, but lack the distal Yq marker. Among 2,380 patients tested, we identified 100 cases whose STS signatures were consistent with an idicYp: sY14 and sY78 present, but sY1273 absent. Of these 100 patients, 15 had been shown previously to carry either a Y;autosome translocation, a Y;X translocation, or a ring Y and therefore were not considered further. This left 85 individuals for further scrutiny.
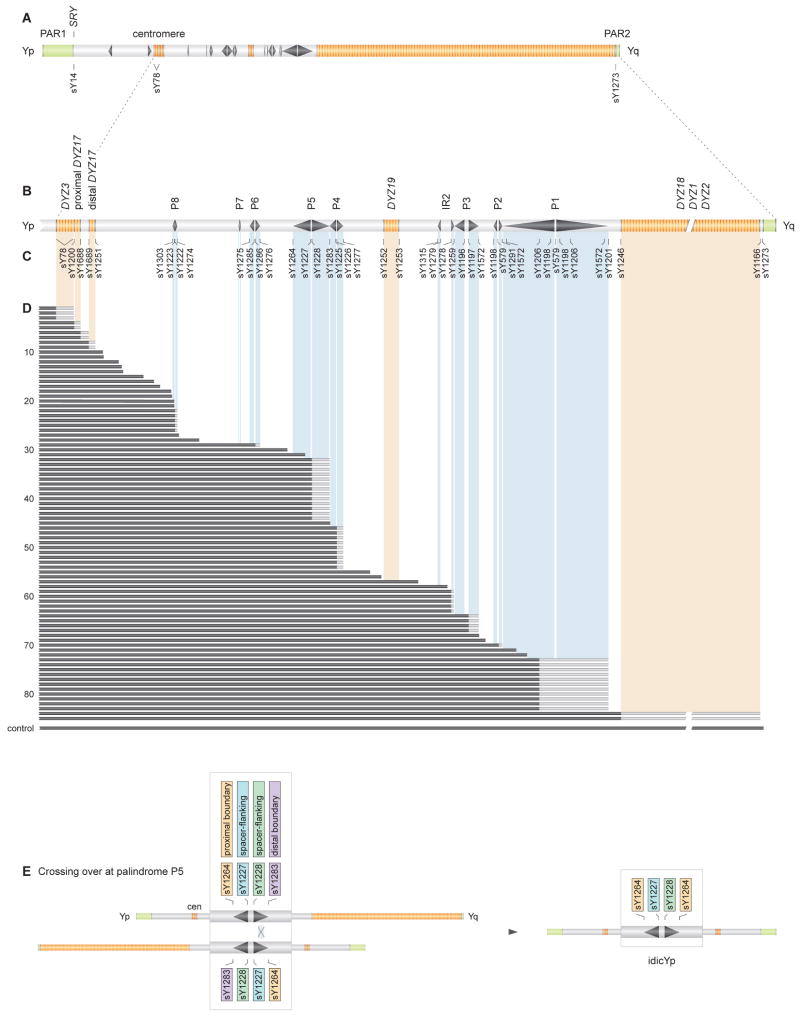
Figure 2. STS content of structurally anomalous Y chromosomes in 85 patients with deletion breakpoints in Yq or centromere.
(A) Locations of three STSs assayed in initial screen for Yq or centromeric breakpoints.
(B) Expanded view of centromere and Yq, with palindromes P1 - P8, inverted repeat IR2, and heterochromatic blocks (orange).
(C) STSs employed in fine mapping breakpoints. These include boundary and spacer-flanking markers for each palindrome and inverted repeat, and markers around each heterochromatic block.
(D) Results of testing DNAs from 85 patients for presence or absence of STSs. Solid black bars encompass STSs found to be present. Gray bars indicate breakpoint intervals that could not be further narrowed due to cross-amplification at other loci. See Figure S2 for identifiers of patients tested.
(E) Predicted molecular signature of idicYp formed by homologous recombination between sister chromatids at palindrome P5. Thirteen individuals with this molecular signature are shown in Figure 2D.
In each of the 85 cases, chrY evidently was broken between centromeric marker sY78 and Yq marker sY1273. To confirm this inference, and to fine-map breakpoints, we assayed additional Yq STSs, including STSs bordering each palindrome (Figures 2B and 2C). In each case, we localized a single Yq breakpoint; all examined STSs proximal to the breakpoint were present, while all those distal to the breakpoint were absent (Figure 2D). In 33 cases, we previously had mapped Yq breakpoints using more limited sets of Y-DNA markers, and in all cases the present localizations reinforced or refined previous reports (Table S1).
If any of the 85 patients carries an idicYp generated by crossing over between opposing palindrome arms on sister chromatids, then their breakpoint should lie within a Yq palindrome. In fact, in 56 cases, the breakpoint mapped within a palindrome or other inverted repeat. That is, in each case, an STS straddling the palindrome's proximal boundary was present while an STS straddling the palindrome's distal boundary was absent (breakpoints in blue regions in Figure 2D).
Our model further predicts that the resulting idicYp should retain the central spacer of the targeted palindrome (Figure 2E). We therefore tested spacer-flanking STSs in each of the 56 cases with palindrome breakpoints. In 49 cases, STSs flanking the spacer of the targeted palindrome were present, demonstrating the spacer's integrity (Figure 2D; Table S1A). The spacer was absent in six patients (Table S1B). One patient displayed a breakpoint within the spacer of IR2 (Table S1C).
In sum, STS screening identified 49 unrelated cases satisfying two predictions of our model of idicY formation: 1) absence of all chrY sequences distal to a particular palindrome and 2) retention of that palindrome's spacer (Figure 2E). Of nine palindromes and inverted repeats in Yq, all but the smallest (palindrome P7) was targeted in at least one case (Figure 2D, Table S2).
IdicYp Chromosomes Demonstrated by Metaphase Fluorescence In Situ Hybridization
Our model of idicYp formation by crossing over between opposing palindrome arms on sister chromatids predicts not only loss of some chrY DNA, but also mirror-image duplication of the retained portion of chrY (Figure 1D and 2E). In each of 49 patients identified in our STS screen, the sets of chrY loci retained and deleted were consistent with an idicYp formed by this model. However, neither the copy number of retained loci nor their arrangement could be surmised from these PCR assays. To explore these questions, we turned to fluorescence in situ hybridization (FISH) on metaphase spreads.
An idicYp generated by our model should bear a mirror-image duplication of Yp, the centromere, and the segment of Yq proximal to the targeted palindrome. The more distal the targeted palindrome, the larger the Yq segment retained. For example, the distance from the centromere to palindrome P8 is 2.2 Mb, while the distance from the centromere to palindrome P1 is 11.5 Mb (Figure 3A). Accordingly, the distance between the two centromeres in resulting idicYp chromosomes should increase as more distal palindromes are targeted (see schematics in Figure 3B). We tested these predictions using FISH probes that hybridized to the most distal male-specific portion of Yp (SRY) and to centromeric alpha satellite (DYZ3) sequences (Figure 3A). We thereby determined the copy number and spatial relationship of the distal Yp locus and centromere. We selected 27 cases for study, including at least one representative for each of the eight targeted palindromes and inverted repeats.
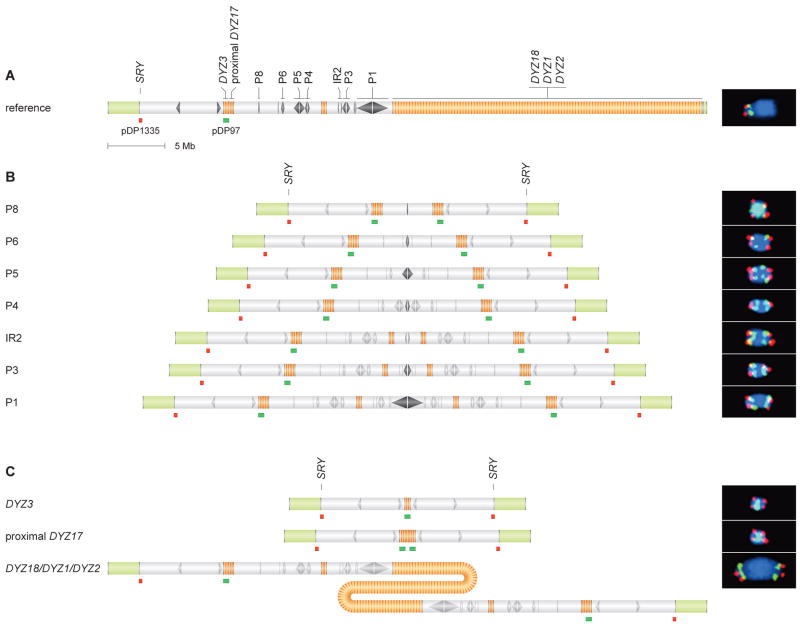
Figure 3. IdicYp and isoYp chromosomes confirmed by metaphase FISH.
(A) Probe hybridization sites shown below chrY schematic. At right: Hybridization to chrY of normal male control produced expected pattern of red and green signals. Chromosome counterstained with DAPI (blue).
(B) Hybridizations to presumed idicYp chromosomes with breakpoints in Yq palindromes P8, P6, P5, P4, P3 and P1, or in IR2 inverted repeat. Hybridization sites shown below schematic of each predicted idicYp; targeted palindrome or inverted repeat lies at center of each schematic. At right: FISH produced predicted red-green-green-red patterns.
(C) Hybridization to presumed isoYp or idicYp chromosomes with breakpoints in heterochromatin.
All FISH micrographs at same magnification.
In 25 of 27 cases examined, we detected hybridization of both SRY and DYZ3 on metaphase spreads. In each of these 25 cases, we observed duplication of both Yp and the centromere, and the predicted pattern of two centromeres flanked by two Yp arms (Figure 3B, Table S2). Comparison of FISH results across all 25 cases demonstrated an increase in the distance between centromeric signals as more distal palindromes were targeted (Figure 3B). In the remaining two of 27 cases, we observed no FISH signal for either SRY or DYZ3, despite both loci having tested positive by PCR. This discordance may be due to high-grade mosaicism for a 45,X (“XO”) cell line. In sum, we microscopically observed an idicYp in all 25 scorable cases.
Proposed Mechanism of IdicYp Formation Validated by Interphase FISH
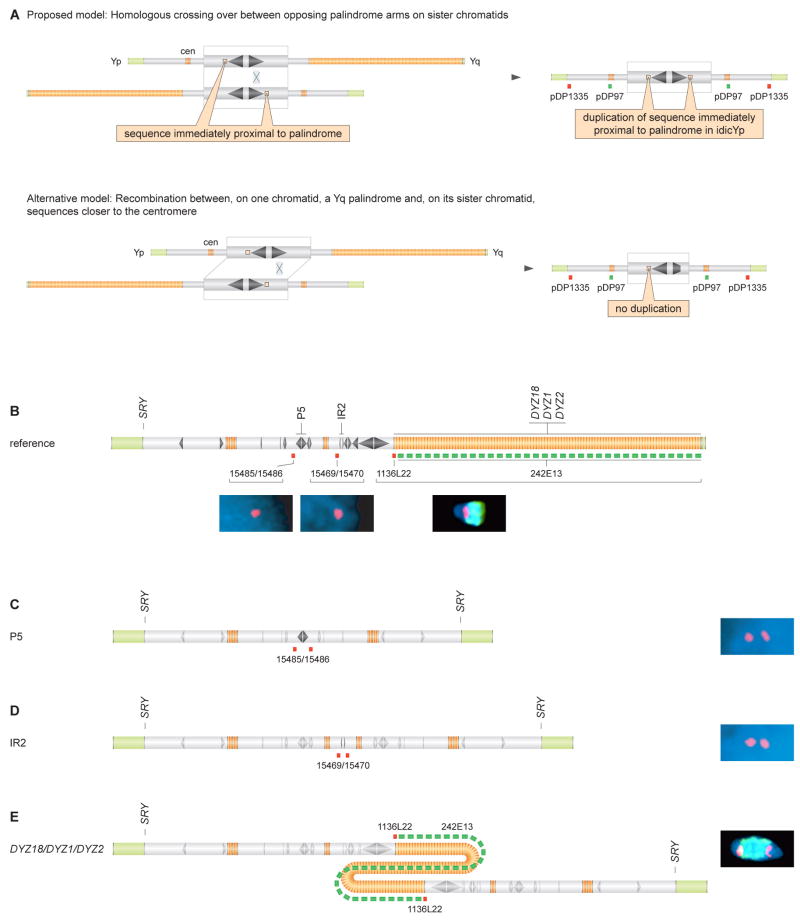
Our results to this point were consistent with idicYp formation via homology-mediated crossing over between opposing palindrome arms on sister chromatids. However, our results could also be explained by recombination between, on one chromatid, a Yq palindrome and, on its sister chromatid, sequences closer to the centromere. These two models yield different predictions regarding the copy number of DNA sequences located immediately proximal to the targeted palindrome (Figure 4A). Whereas our proposed model predicts duplication of these sequences, the alternative explanation predicts that these sequences would not be duplicated.
Figure 4. Duplication in idicYp chromosomes of sequences immediately proximal to targeted palindromes.
(A) Two models of idicYp formation can be distinguished by determining copy numbers of sequences immediately proximal to targeted palindrome.
(B) Probe hybridization sites shown below chrY schematic. Hybridizations to chrY of normal male produced expected signals; 15485/15486 and 15469/15470 on interphase spreads, and co-hybridization of 1136L22 and 242E13 on metaphase spreads.
(C) Interphase FISH demonstrates duplication of 15485/15486 on idicYp with breakpoint in palindrome P5.
(D) Interphase FISH demonstrates duplication of 15469/15470 on idicYp with breakpoint in inverted repeat IR2.
(E) Metaphase FISH demonstrates duplication of 1136L22 on idicYp with breakpoint in DYZ18/DYZ1/DYZ2 heterochromatin. Co-hybridization of 1136L22, in red, with 242E13, in green, produced predicted red-green-red pattern.
All micrographs at same magnification. In each interphase FISH experiment, ≥100 nuclei were scored (Figures S3, S4, and S5).
We designed FISH assays to examine this question. For each targeted palindrome, we isolated a genomic DNA clone or prepared a long-range PCR product that would hybridize specifically to sequences just proximal to that palindrome. Anticipating that metaphase FISH would offer insufficient resolution, we performed FISH on interphase spreads. We tested 23 of 25 cases in which we had confirmed an idicYp on metaphase spreads, including at least one representative for each of seven targeted palindromes. (In the other two cases, metaphase FISH had revealed a very low percentage of cells with an idicYp, likely reflecting mosaicism for a 45,X cell line, and making statistical analysis of interphase nuclei difficult.) To determine the number of probe hybridization sites and thereby differentiate the two models, we scored at least 100 nuclei in each case. In 22 of 23 cases, we found that sequences just proximal to the targeted palindrome were duplicated (Figures 4C, 4D, S3, and S4; Table S2), as predicted by our model.
These findings eliminated the alternative model in all but one case, WHT3189. Further analysis of this seemingly exceptional case confirmed our model. WHT3189 displays a breakpoint in palindrome P1, large blocks of which are repeated in palindromes P2, P3, and P5 (Figure S4). We postulated that WHT3189's idicYp was due to homologous crossing over between sister chromatids, with one recombination point in palindrome P1 and the other in P2, P3, or P5. Additional interphase studies indicated that this idicYp likely arose by homologous recombination between, on one chromatid, palindrome P1, and on the sister chromatid, a 1.1-Mb block of 99.9% sequence identity encompassing palindrome P2 (Figure S4). In sum, in all 23 cases examined, interphase FISH studies validated our model of idicYp formation (Table S2).
IsoYp and IdicYp Chromosomes Arising from Recombination in Heterochromatin
As described above, of 85 patients with Yq breakpoints potentially relevant to this study, 56 proved to have breakpoints within palindromes. We turn now to the remaining 29 cases, with Yq breakpoints outside palindromes. In 20 of these cases, we mapped breakpoints to pericentromeric euchromatin or to non-palindromic Yq sequences (Figure 2D, Table S1D).
In each of the nine remaining cases, we mapped a breakpoint to one of four large blocks of centromeric or Yq heterochromatin (breakpoints in orange regions in Figure 2D; Table S1E). These blocks contain tandem arrays of short (5- to 171-basepair) sequence units, and they range in size from about 200 kb (DYZ17 arrays) to >30 Mb (distal Yq heterochromatin; DYZ18/DYZ1/DYZ2) (Skaletsky et al., 2003; Kirsch et al., 2005). In theory, inversion of some repeats within these heterochromatic regions could generate targets for isoYp (monocentric) or idicYp formation by our proposed mechanism. Thus we explored the possibility that the nine cases with breakpoints in centromeric or Yq heterochromatin carry isoYp or idicYp chromosomes.
We selected three cases with centromeric breakpoints and two cases with distal Yq breakpoints for further study by FISH. Hybridization of SRY (distal Yp) and DYZ3 (centromere) probes to metaphase spreads of these five cases yielded the predicted patterns (Figure 3C, Table S2). In the cases with centromeric breakpoints, we observed two Yp signals flanking one centromeric signal. In the cases with distal Yq breakpoints, we observed two Yp signals flanking two distinct centromeric signals. Thus, isoYp or idicYp chromosomes are present in all five scrutinized cases with centromeric or Yq heterochromatic breakpoints.
Our model predicts the duplication of all retained sequences apart from the targeted heterochromatic regions. We designed additional FISH assays to explore this matter in all five cases. In the two cases with breakpoints in distal Yq heterochromatin, the proximal boundary of this heterochromatin was duplicated, as predicted (Figure 4E, Table S2). Similarly, in the three cases with centromeric breakpoints, a Yp sequence located 280 kb from the centromere was duplicated (Figure S5, Table S2). In sum, these FISH studies are consistent with our proposed mechanism. Future studies may identify inverted repeats within heterochromatic regions that would facilitate isoYp or idicYp formation by this mechanism. Alternatively, isochromosome formation via centromere misdivision (Darlington, 1939) could account for the cases with centromeric breakpoints.
IdicYq Chromosomes Generated by Recombination at an Inverted Repeat on Yp
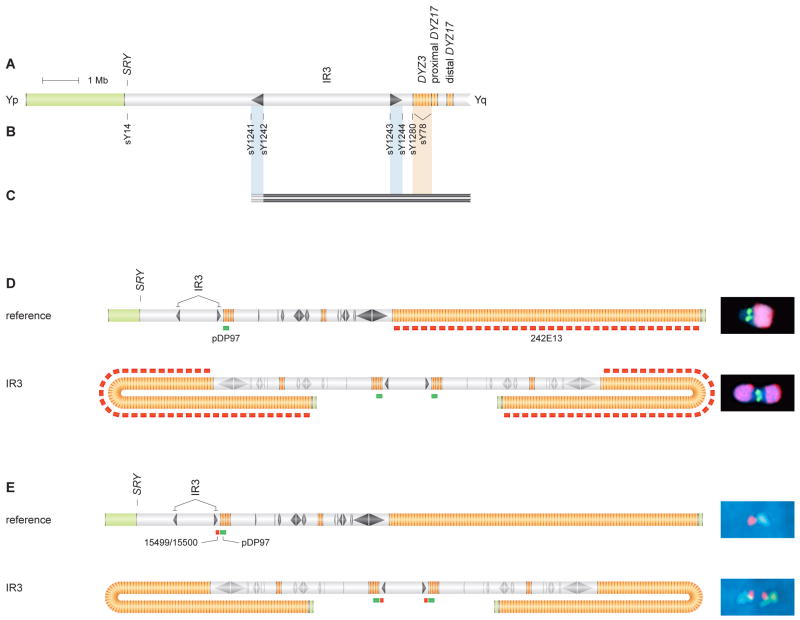
While most MSY palindromes and inverted repeats are located on Yq, the 298-kb arms of inverted repeat IR3 are located on Yp (Figures 1B and 5A). The 3.6-Mb spacer bounded by IR3's arms has been inverted repeatedly during human history (Jobling et al., 1998; Tilford et al., 2001; Skaletsky et al., 2003; Repping et al., 2006). Such inversion might be due to DSB formation in one IR3 arm and intrachromatid resolution of that DSB by crossing over with the other IR3 arm (see Figure 1D). Further, we postulated that interchromatid resolution of such a DSB could generate an idicYq bearing two copies of the centromere and Yq but lacking the portion of Yp distal to IR3.
Figure 5. IdicYq chromosomes formed by crossing over between sister chromatids at Yp inverted repeat: STS content and FISH analyses.
(A) Schematic representation of Yp and centromeric region.
(B) STSs employed in mapping deletion breakpoints in two individuals in whom sY14 (SRY) was absent, and sY78 and sY1273 (Figure 2) were present. These STSs include boundary and spacer-flanking markers for IR3 inverted repeat and a marker in Yp pericentromeric euchromatin.
(C) Results of testing DNAs from two patients for presence or absence of STSs. Solid black bars encompass STSs found to be present. Gray bars indicate breakpoint intervals within IR3's distal arm that could not be further narrowed due to cross-amplification of identical sequences in IR3's proximal arm. See Figure S6 for identifiers of patients tested.
(D) Hybridization of probes pDP97 (to centromeric DYZ3 repeats), in green, and 242E13 (to DYZ1 repeats in distal Yq heterochromatin), in red, to metaphase chrY of normal male (above) and to idicYq with breakpoint in IR3 (below). (Metaphase FISH precludes resolution of two centromeric signals in this idicYq.) All images at same magnification.
(E) Interphase FISH demonstrates duplication of sequences proximal to IR3 in idicYq. Hybridization of probes 15499/15500 (proximal to IR3), in red, and pDP97, in green, produced expected patterns of red-green on chrY of normal male (above) and green-red-red-green on idicYq with breakpoint in IR3 (below). All images at same magnification. Two hundred nuclei were scored (Figure S7).
We screened our collection of patients for such idicYq chromosomes using eight STSs, the first three of which had figured prominently in our idicYp screen: sY14, sY78, and sY1273, which mark distal Yp, centromere, and distal Yq, respectively (Figure 2A). IdicYq chromosomes should lack the distal Yp marker but retain the centromeric and distal Yq markers. The other five STSs were as follows: sY1241 and sY1244 straddle the distal and proximal boundaries of IR3, sY1242 and sY1243 flank its 3.6-Mb spacer, and sY1280 marks Yp pericentromeric euchromatin (Figure 5B). In an idicYq formed by crossing over between opposing IR3 arms on sister chromatids, the distal boundary STS should be deleted, but the proximal boundary STS, both spacer-flanking STSs, and the pericentromeric STS should be retained. Among 2,380 patients tested, we identified two individuals whose STS data were consistent with an idicYq formed by this model at IR3 (Figure 5C, Table S1F). (This screen also would have identified monocentric isoYq chromosomes with breakpoints in centromeric DYZ3 repeats, but we found none.)
An idicYq generated by this model should bear a mirror-image duplication of Yq, the centromere, and the segment of Yp immediately proximal to IR3. We used FISH assays to test these predictions in one of the two cases identified by STS screening. Co-hybridizing centromeric and distal Yq heterochromatin probes to metaphase chromosomes, we observed the predicted duplication of Yq (Figure 5D). By interphase FISH, we observed mirror-image duplication of the centromere and of sequences located 28 kb proximal to IR3, all as predicted by our model of idicYq formation at IR3 (Figures 5E and S7).
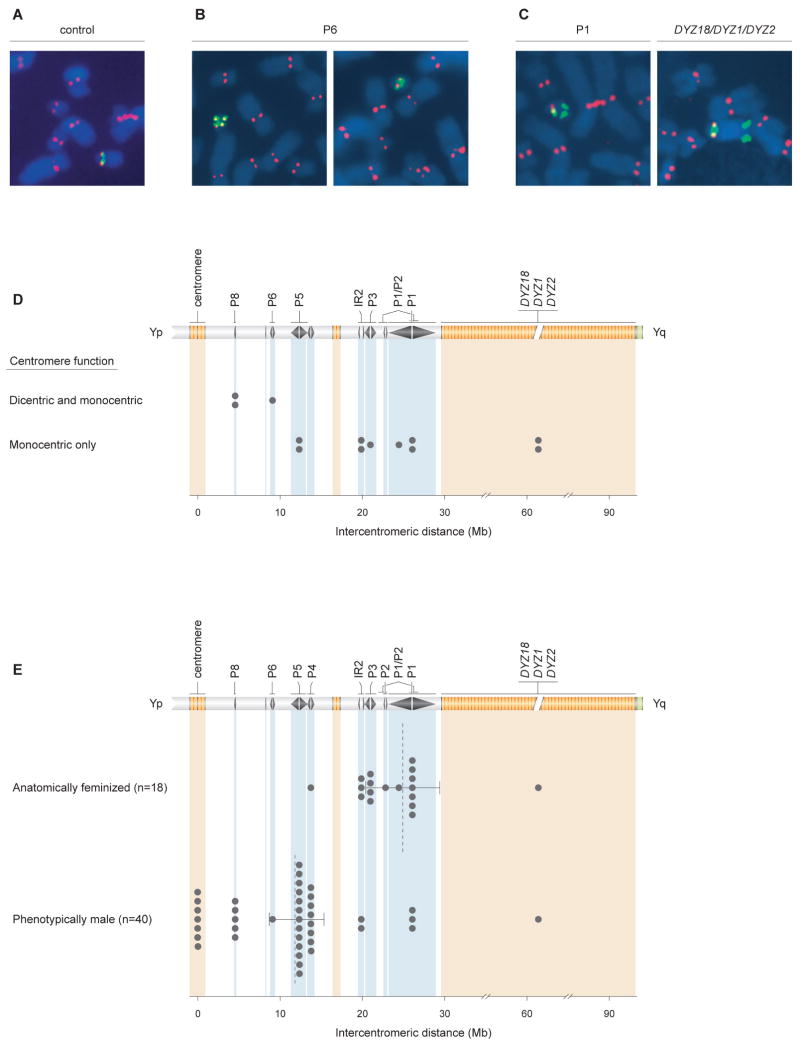
Many IdicYp Chromosomes have Only One Active Centromere
By definition, the idicYp chromosomes that we identified contain two copies of the centromeric region (Figures 3 and 5). If these two regions were to function independently as centromeres, then the idicYp might be vulnerable to damage or loss during mitosis. This raised the question of whether one of the two centromeric regions in the idicYp chromosomes is functionally inactive, as has been observed in many human dicentric chromosomes of X chromosome or autosomal origin (Therman et al., 1974; Hsu et al., 1975; Earnshaw and Migeon, 1985; Wandall, 1989; Page et al., 1995; Page and Shaffer, 1998; Sullivan and Willard, 1998). Specifically, we speculated that among the idicYp chromosomes studied here, those with longer intercentromeric distances would rely more heavily upon functional inactivation of one centromere to achieve mitotic stability, while those with shorter intercentromeric distances would be less dependent upon this mechanism. As other investigators have suggested, when intercentromeric distances are modest, mitotic stability may be achieved because two centromeres function as one, or because close proximity physically constrains them from attaching to opposite spindle poles during mitotic division (Hair, 1953; Niebuhr, 1972; John and Freeman, 1975; Daniel and Lam-Po-Tang, 1976; Camargo and Cervenka, 1984; Therman et al., 1986; Koshland et al., 1987; Page and Shaffer, 1998; Sullivan and Willard, 1998).
We explored these ideas by determining the number of active Y centromeres in cells from 13 patients whose idicYp chromosomes spanned a wide range of intercentromeric distances. We counted active Y centromeres by simultaneously visualizing 1) active centromeres on all nuclear chromosomes and 2) centromeric DNA sequences in idicYp chromosomes. Specifically, we stained metaphase spreads from lymphoblastoid cell lines using antibodies to CENP-E, a protein that localizes exclusively to kinetochores at active centromeres (Sullivan and Schwartz, 1995), followed by FISH to Y centromeric (DYZ3) DNA (Figure 6A). We studied 13 cases in which we had confirmed the presence of an idicYp on metaphase spreads and the duplication of sequences immediately proximal to the targeted palindrome or heterochromatic repeat. These cases encompass the range of observed intercentromeric distances: from 4.5 Mb to roughly 60 Mb (Figure 6A-6D).
Figure 6. Correlates of intercentromeric distance in idicYp chromosomes: centromere inactivation and sex reversal.
(A) Active chrY centromeres identified by immunofluorescence followed by FISH (IF-FISH). Anti-CENP-E antibody staining, in red, identifies all active centromeres. FISH probe pDP97 (to DYZ3 alpha satellite repeats), in green, identifies chrY centromeric DNA. IF-FISH to chrY of normal male control produced expected signals at single centromere.
(B) IF-FISH to idicYp case with breakpoint in palindrome P6 demonstrates cell-to-cell mosaicism for functionally dicentric (left) or functionally monocentric (right) chromosomes.
(C) IF-FISH to idicYp cases with breakpoints in palindrome P1 (left) or in DYZ18/DYZ1/DYZ2 heterochromatin (right) demonstrates functional inactivation of one Y centromere.
(D) Plots of recombination sites in 13 idicYp cases assayed by IF-FISH and displaying either cell-to-cell mosaicism for functionally dicentric and monocentric idicYp chromosomes (three cases, above) or exclusively functionally monocentric idicYp chromosomes (ten cases, below). For each cell line, ≥20 idicYp-bearing spreads of good morphology were scored. Among the three cases with cell-to-cell variation, fraction of cells displaying functionally dicentric idicYp chromosomes ranged from 41% to 77%. See Figure S8 for additional data.
(E) Plots of intercentromeric distances in SRY-positive idicYp or isoYp chromosomes in 18 feminized individuals (above) and 40 males (below). Dotted lines indicate mean intercentromeric distances for the two groups; 95% confidence intervals calculated by bootstrapping method.
In three of 13 cases examined, we detected cell-to-cell variation with respect to centromere activity, with some cells displaying one active centromere on the idicYp and other cells displaying two active centromeres there (Figures 6B, 6D, and S8). These three mosaics for centromere activity had the shortest intercentromeric distances, ranging from 4.5 Mb (recombination at palindrome P8) to 9.1 Mb (recombination at palindrome P6), among the 13 cases tested. All examined cells from each of the ten remaining cases displayed one active centromere on the idicYp (Figures 6C, 6D, and S8). These cases had intercentromeric distances ranging from 12.3 Mb (recombination at palindrome P5) to roughly 60 Mb (recombination at DYZ18/DYZ1/DYZ2 heterochromatin). Thus, all ten tested cases with intercentromeric distances of 12.3 Mb or greater displayed one active centromere on the idicYp, while all three tested cases with intercentromeric distances of 9.1 Mb or less were mosaic for one versus two active centromeres. We concluded that functional inactivation of one centromere is commonplace in our series of idicYp chromosomes and is especially manifest among cases with greater intercentromeric distances.
Sex Phenotype Correlates with Intercentromeric Distance in SRY-bearing Chromosomes
Given chrY's role in masculinizing the fetus, we then sought to correlate the idicYp and isoYp chromosomes that we had characterized with the sexual phenotypes of the individuals carrying them. In humans and mice, the zygote's sexual fate is determined by the presence or absence of a single gene, SRY, on chrY (Sinclair et al., 1990; Berta et al., 1990; Jäger et al., 1990; Koopman et al., 1991). Moreover, in mice, studies of XX ← → XY chimeras have demonstrated that Sry (mouse ortholog of human SRY) acts in one and only one cell lineage – the supporting cells of the fetal gonad – to drive testis development and anatomic masculinization in utero (Burgoyne et al., 1988; Palmer and Burgoyne, 1991). In effect, the sexual phenotype at birth provides a retrospective report on the presence or absence of SRY in the supporting cell lineage.
Our screen had identified 60 individuals whose STS content was consistent with the presence of an idicY or isoY: 58 have idicYp or isoYp chromosomes with recombination points in palindromes and heterochromatic regions on Yq and in the centromere (Figure 2D), and two have idicYq chromosomes with recombination points in an inverted repeat, IR3, on Yp (Figure 5C). The idicYq chromosomes lack the SRY gene, which is located on distal Yp (Figure 5). As expected, the individuals carrying idicYq chromosomes were phenotypic females.
By contrast, the idicYp and isoYp chromosomes each carry two copies of SRY (Figure 3). Despite this, 18 of the 58 individuals with such SRY-bearing chromosomes 1) were raised as females based on the appearance of their external genitalia at birth and/or 2) were identified in childhood as having one degenerate ovary (“streak gonad”) and one testis. To exclude SRY point mutations as the cause of this sex reversal, we sequenced the gene's coding region and found it to be intact in all 18 anatomically feminized individuals.
What might account for sex reversal in individuals with SRY-bearing chromosomes? We speculated that this reflected mitotic instability of an idicYp – and resultant mosaicism for 45,X (“XO”) cells that had lost the idicYp – during embryonic development in these 18 feminized individuals. Further, since mitotic instability might be expected to increase with intercentromeric distance (Hair, 1953; Koshland et al., 1987; Sullivan and Willard, 1998), we hypothesized that, among the 58 individuals with SRY-bearing idicYp or isoYp chromosomes, the likelihood or frequency of female anatomic development would rise as intercentromeric distance increased.
To test this prediction, we compared the distributions of intercentromeric distances in the 18 anatomically feminized individuals and 40 phenotypic males with idicYp or isoYp chromosomes (Figure 6E). Whereas recombination points in 16 of 18 feminized individuals were located in the more distal Yq palindromes – palindrome P1, P2, P3, or inverted repeat IR2 – recombination points in 34 of 40 males were located in more proximal palindromes – P4, P5, P6 or P8 – or in the centromeric region. Indeed, all seven individuals carrying isoYp chromosomes, with centromeric breakpoints, were phenotypic males (Figure 6E). Aggregating the data for all 58 individuals with idicYp or isoYp chromosomes, we observed that the average intercentromeric distance in the feminized individuals (24.9 Mb; 95% CI: 20.4–29.4 Mb, bootstrapping method) was twice that in the males (12.0 Mb; 95% CI: 8.7–15.3 Mb), and that this difference was statistically significant (p < 10-6, Wilcoxon two-sample test). The likelihood of female anatomic development rises sharply with increasing intercentromeric distance in idicYp chromosomes.
XO Mosaicism in Individuals with IdicYp Chromosomes
This correlation supports the hypothesis that mitotic instability and resultant XO mosaicism cause sex reversal (anatomic feminization) in many individuals with idicYp chromosomes. Two observations provide additional support for this XO mosaicism hypothesis. First, five of the females with idicYp chromosomes exhibited somatic, anatomic features of Turner syndrome, which is classically associated with a 45,X (“XO”) karyotype. This suggests that XO mosaicism is not restricted to the gonadal supporting lineage but extends, in these five individuals, to other cell lineages that contribute to phenotypic components of Turner syndrome. Second, mosaicism for a 45,X (“XO”) cell line had been discovered microscopically, prior to any studies in our laboratory, in 35 of 49 individuals in whom we identified idicYp chromosomes. Taken together, these findings indicate that individuals with 46,X,idicYp cells are frequently mosaic for 45,X cells, and that this mosaicism can give rise to sex reversal or to phenotypic features of Turner syndrome.
Spermatogenic Failure in Phenotypic Males with IsoYp Chromosomes
Our findings indicate that idicYp or isoYp chromosomes are among the more common genetic causes of severe spermatogenic failure. Eighteen patients in whom we identified such chromosomes were otherwise healthy men with greatly diminished or no sperm production. Moreover, the set of 2,380 individuals that we screened included systematically ascertained series of men with various degrees of spermatogenic failure: 293 men with nonobstructive azoospermia (the clinical diagnosis arising from complete failure of sperm production), 207 men with severe oligozoospermia (> 0 but < 5 million spermatozoa per ml semen), and 81 men with moderate oligozoospermia (5 – 20 million spermatozoa per ml semen). We found evidence of an idicYp or isoYp in eight (2.7%) of 293 men with nonobstructive azoospermia, but in none of 288 men with severe or moderate oligozoospermia (p < 0.01, Fisher's exact test). Given that the population incidence of nonobstructive azoospermia (complete spermatogenic failure) is much lower than that of oligozoospermia (Hull et al., 1985), these data imply that, in the large majority of cases, idicYp or isoYp formation precludes sperm production. In this respect, the consequences of idicYp or isoYp formation appear to be more severe than those of AZFc deletions, which are found in men with either severe oligozoospermia or nonobstructive azoospermia, and which constitute the most frequently recurrent interstitial deletions in chrY (Reijo et al., 1995; Vogt et al., 1996; Kuroda-Kawaguchi et al., 2001). In our series of 293 men with nonobstructive azoospermia, idicYp or isoYp chromosomes were about one third as frequent as AZFc deletions (22 cases, 7.5%). In no case did we observe transmission of an idicYp or isoYp from one generation to the next – at least in the absence of assisted reproduction. In one case, a 46,X,idicYp man with a palindrome P1 breakpoint produced small numbers of sperm sufficient to father a child by intracytoplasmic sperm injection.
Given these severe reproductive deficits, we would expect that each of the idicYp and isoYp chromosomes studied here arose de novo, and thus would not be found in the father of the affected individual. Indeed, in 17 of the cases studied here, the fathers' Y chromosomes were examined by DNA or light microscopic analysis, and in all 17 cases the fathers' Y chromosomes were found to be intact (data not shown).
Discussion
We explored the hypothesis that homologous recombination between opposing arms of MSY palindromes could generate idicY chromosomes. Screening of patients with spermatogenic failure, or with microscopically detected anomalies of chrY, resulted in our identification and characterization of idicY or isoY chromosomes in 60 unrelated individuals. Of these 60 cases, 51 evidently arose via the hypothesized palindrome mechanism (yielding an idicYp in 49 cases and an idicYq in two cases), while the remaining nine arose via recombination in heterochromatic sequences (yielding an idicYp in two cases and an isoYp in seven cases)(Table S2).
Here we will discuss two topics. First, we will consider the mechanisms of idicY formation, and implications for a comprehensive model of MSY recombination. Second, we will consider the biological and medical consequences of idicY and isoY formation.
A Model of MSY Recombination
Our findings, combined with previous results, reveal fundamental, mechanistic parallels between recombination within the MSY and meiotic (or mitotic) recombination between homologous chromosomes as studied in yeast and other experimental systems. The resulting expanded model of MSY recombination provides a broad framework for understanding much of the biology of chrY in health and disease.
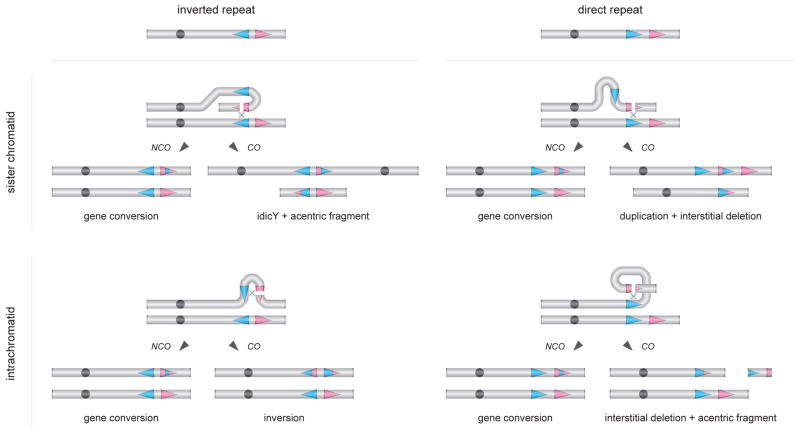
According to our model, idicY formation and MSY palindrome maintenance are alternative products of a common mechanism reminiscent of conventional recombination between homologous chromosomes (Figure 1C). That common mechanism involves 1) generation of a DSB within one arm of an MSY palindrome and 2) homologous repair of that DSB using the opposing arm of the same palindrome as template. As in conventional meiotic or mitotic recombination between homologs, MSY recombination intermediates generated during DSB repair can be resolved with or without crossing over. Resolution with crossing over (CO) can yield an idicY (when recombination occurs between sister chromatids) or an inversion within the palindrome (when recombination occurs within a chromatid). Alternatively, NCO resolution results in gene conversion and palindrome maintenance. This model provides an economical explanation for all of the following: 1) our present evidence of idicY formation by crossing-over between opposing arms of palindromes, 2) previous evidence of gene conversion at these same palindromes (Rozen et al., 2003), and 3) previous evidence of repeated inversion, during human history, of the 3.6-Mb spacer of the IR3 inverted repeats on Yp (Jobling et al., 1998; Tilford et al., 2001; Skaletsky et al., 2003; Repping et al., 2006). As reported here, idicY chromosomes appear to have arisen via this mechanism at nine of the 10 large palindromes and inverted repeats identified in the MSY, suggesting the generality of the model.
An even more inclusive model (Figure 7) stems from the realization that many palindromic sequences are repeated elsewhere in the MSY in direct (as opposed to inverted) orientation (Kuroda-Kawaguchi et al., 2001; Skaletsky et al., 2003). What if a DSB arising within one arm of a palindrome were homologously repaired using the more geographically distant, direct repeat as template? CO resolution would then yield a tandem duplication, or an interstitially deleted chrY lacking the region between the direct repeats – as observed in many men with spermatogenic failure (Sun et al., 2000; Kamp et al., 2000; Blanco et al., 2000; Kuroda-Kawaguchi et al., 2001; Repping et al., 2002; Repping et al., 2003). Alternatively, NCO resolution would result in gene conversion between the direct repeats (Hurles et al., 2004). Such NCO events could explain the high sequence identity observed between widely separated direct repeats in the MSY (Skaletsky et al., 2003). In sum, many aspects of MSY structure, behavior, and evolution are economically explained by DSBs arising within palindrome arms or other lengthy repeats. Homologous repair of these DSBs yields diverse classes of outcomes, these being determined by three binary variables in the process of repair: 1) inverted versus direct-repeat templates, 2) sister chromatid versus intrachromatid templates, and 3) NCO versus CO resolution (Figure 7).
Figure 7. Model of DSB resolution in MSY direct and inverted repeats (palindromes) by NCO and CO pathways.
Sequence identity between palindrome arms or inverted repeats, and between direct repeats, is maintained by NCO resolution of DSBs; such gene conversion could occur between sister chromatids or within a single chromatid. In the case of palindromes or inverted repeats, CO resolution between sister chromatids can generate an idicY and an acentric fragment, while CO resolution within a chromatid results in inversion. In the case of direct repeats, CO resolution between sister chromatids can generate a duplication and an interstitial deletion, while CO resolution within a chromatid yields an interstitial deletion and an acentric fragment.
The Medical Consequences of IdicYp and IsoYp Formation
In considering the medical significance of our findings, we will focus on idicYp and isoYp chromosomes, as these represent 58 of the 60 isodicentric and isochromosomes identified in this study. We will consider how these chromosomes come to be associated with a wide range of reproductive disorders, ranging from spermatogenic failure in men to sex reversal and Turner syndrome in girls and women.
Spermatogenic Failure
We find that idicYp and isoYp chromosomes are among the more common genetic causes of severe spermatogenic failure in otherwise healthy men. IdicYp or isoYp formation likely interferes with sperm production via several distinct mechanisms. First, many idicYp and all isoYp chromosomes lack distal Yq genes that play critical roles in spermatogenesis (Table S3) (Reijo et al., 1995; Vogt et al., 1996; Sun et al., 2000; Kamp et al., 2000; Blanco et al., 2000; Kuroda-Kawaguchi et al., 2001; Repping et al., 2002; Skaletsky et al., 2003; Repping et al., 2003). Second, idicYp or isoYp formation results in duplication of the Yp pseudoautosomal region and deletion of the Yq pseudoautosomal region – outcomes that may disrupt meiotic pairing of chrX and chrY and thereby preclude progression through meiosis (Mohandas et al., 1992). This may help explain why some men with idicYp chromosomes formed at palindrome P1 do not produce sperm despite carrying a full complement of MSY genes (Table S3). Finally, mitotic instability and resultant XO mosaicism in the germ line may also contribute to the spermatogenic defects observed in men with idicYp chromosomes formed at palindrome P1.
Sex Reversal and Turner Syndrome: Sequellae of Mitotic Instability
While spermatogenic failure may often be a direct consequence of idicYp or isoYp formation, we postulate that sex reversal and Turner syndrome arise indirectly – as consequences of mitotic instability of idicYp chromosomes and the resultant emergence of cells with an “XO” sex chromosome constitution during embryonic and fetal development.
In yeast, and in plant and animal cells, the mitotic instability of dicentric chromosomes becomes more pronounced as the distance between the two centromeres increases (Hair, 1953; Koshland et al., 1987; Sullivan and Willard, 1998). In humans, a positive correlation between intercentromeric distance and mitotic instability had been demonstrated previously in cultured cells (Camargo and Cervenka, 1984; Sullivan and Willard, 1998) but had not been examined in vivo. In the patient series that we report, a readily scored genetic marker – the male-determining gene SRY – was located on dicentric chromosomes of varying intercentromeric distance, providing an unusual opportunity to examine these phenomena in vivo. We observed that, in individuals with an SRY-bearing dicentric chromosome, the probability of female differentiation increases with intercentromeric distance and the size of the idicYp.
As with most human molecular genetic and cytogenetic studies, our molecular analyses were conducted on cells of the hematopoietic lineage. It follows that each patient whom we identified as possessing an idicYp retained it in the hematopoietic lineage during the course of his or her development. Inclusion in our study did not require retention of the idicYp in other cell lineages during embryonic, fetal, and postnatal development – as illustrated by the sex reversal observed in 18 of our study subjects.
Consider then the consequences of an alternative scenario in which mitotic instability of an idicYp leads to its loss not only in the gonadal supporting cell lineage but also in the hematopoietic lineage, the standard target of cytogenetic studies. The result would be a phenotypic female with a 45,X (“XO”) blood karyotype who would likely receive a diagnosis of Turner syndrome. This reasoning leads us to conjecture that many 46,X,idicYp zygotes give rise to 45,X Turner females. Conversely, we speculate that many 45,X Turner girls and women developed from 46,X,idicYp zygotes.
Closer examination of our findings, together with published information on the genetic origins of Turner syndrome, lends support to these hypotheses. Unlike trisomy 21 and trisomy X, which are most often the result of nondisjunction in maternal meiosis, and whose incidence increases dramatically with maternal age, the incidence of 45,X Turner syndrome does not increase with maternal age, suggesting that maternal nondisjunction is not its principal cause (Warburton et al., 1980; Hassold et al., 1980; Hassold and Chiu, 1985; Hassold et al., 1988; Hassold and Hunt, 2001). Indeed, in about 75% of 45,X girls and women, the single X chromosome is of maternal origin (Jacobs et al., 1997; Uematsu et al., 2002); what is missing or absent in these 45,Xmaternal individuals is a paternally derived sex chromosome, which could be either a chrX or a chrY, or a derivative thereof. We speculate that, in many 45,Xmaternal girls and women, the missing paternal sex chromosome was an idicYp generated in the father's germ line and present in the zygote, but lost during subsequent development. Such cases would have been systematically excluded from the present study if the idicYp chromosomes had been lost in the hematopoietic lineage.
A surprising aspect of our data could be explained by such losses. Spanning 2.9 Mb of Yq, palindrome P1 is by far the largest known palindrome in the human genome, and it is by far the most frequent site of interstitial Yq deletions arising via homologous recombination (AZFc and related deletions) (Kuroda-Kawaguchi et al., 2001; Repping et al., 2002; Repping et al., 2003). Palindrome P1 represents 52% of the theoretical target for idicYp formation by the palindrome recombination mechanism proposed here. Nonetheless, in the present study, P1 accounts for only 11 (22%) of the 49 idicYp chromosomes arising via palindrome recombination. We suggest that this apparent “deficit” of P1-derived idicYp chromosomes reflects their loss to mitotic instability during embryonic, fetal, or postnatal development.
In conclusion, our findings underscore the recombinogenic nature of MSY palindromes, and they expand the gamut of associated risks. The prevalence of idicY chromosomes in patients with spermatogenic failure, sex reversal, and Turner syndrome reveals an Achilles' heel in the mechanism by which MSY palindromes and genes are maintained and preserved.
Experimental Procedures
PCR Mapping of MSY Breakpoints
Patient genomic DNAs were extracted from either peripheral blood leukocytes or cultured cells, which in most cases were Epstein-Barr-virus-transformed lymphoblastoid cell lines. DNAs were tested for the presence or absence of chrY STSs by PCR using primer pairs catalogued and indexed in MSY Breakpoint Mapper (http://breakpointmapper.wi.mit.edu/) (Lange et al., 2008). In addition, all PCR primer sequences and thermocycling conditions have been deposited in GenBank; see Table S4 for accession numbers.
Fluorescence In Situ Hybridization (FISH) Analysis of IdicY and IsoY Structure
Metaphase chromosomes for FISH were obtained from lymphoblastoid cells treated with colcemid. Interphase chromosomes were obtained from lymphoblastoid cells by starvation, with no colcemid treatment. FISH probes were labeled by nick translation with biotin-dUTP, digoxigenin-dUTP, Cy3-dUTP, or FITC-dUTP. Biotin-labeled probes were detected by incubation with Cy3-conjugated avidin. Digoxigenin-labeled probes were detected by incubation with mouse anti-digoxigenin antibody followed by incubation with FITC-labeled rabbit anti-mouse IgG antibody. Chromosomes were counterstained with DAPI or propidium iodide. In each interphase experiment that involved the counting of sites of hybridization, at least 100 nuclei were scored. See Table S5 for specific information on individual FISH probes and assays. See Supplemental Experimental Procedures for details.
Immunofluorescence (IF) – FISH Counting of Active Centromeres
Metaphase chromosomes for IF-FISH were obtained from lymphoblastoid cells treated with colcemid. For IF, anti-CENP-E antibody (Harrington et al., 1997) was detected with Cy3-conjugated donkey anti-rabbit IgG antibody. For FISH, probe pDP97 was labeled by nick translation with SpectrumGreen dUTP (Abbott Molecular). Chromosomes were counterstained with DAPI in mounting medium (Vector Laboratories). For each cell line, at least 20 idicYp-chromosome-bearing spreads of good morphology were scored. See Supplemental Experimental Procedures for details.
Sequencing of SRY Gene
The coding sequence of SRY was PCR amplified (Table S4E) from genomic DNAs of anatomically feminized patients carrying idicYp chromosomes. The resulting PCR products were sequenced on an ABI3730 automated sequencer using the BigDye Terminator protocol (Applied Biosystems).
Human Subjects
These studies were approved by the Massachusetts Institute of Technology's Committee on the Use of Humans as Experimental Subjects. Informed consent was obtained from all subjects.
Supplementary Material
Acknowledgments
We thank the following individuals for patient samples or cell lines: M. Alamares, G. Beverstock, H. Bode, J. Bodurtha, A. Brothman, B. Clark, J. Crawford, A. Daniel, A. Donnenfeld, P. Donohoue, S. Fallet, G. Feole, S. Hahm, K. Harper, D. Hoar, A. Hughes, J. Jacobs, L. Jenkins, D. Manchester, P. McDonough, B. McGillivray, R. McVie, W. Miller, A. Milunsky, C. Palmer, S. Patil, S. Peacock, M. Phelan, J. Priest, J.B. Ravnan, C. Rudlin, L. Shapiro, J. Shulkin, E. Simpson, D. Solomon, C. Stevens, P. Storto, R. Stratton, M. Summers, J. Tollis, J. Vigorita, N. Wang, L. Weiss, V. Wilson, P. Wyatt, T. Yang-Feng, J. Zenger Hain, and J. Zunich. We thank A. Amon, I. Cheeseman, G. Fink, A. Hochwagen, E. Keen, S. Keeney, N. Mohibullah, H. Willard and the Page lab for helpful discussions and comments. Supported by the National Institutes of Health, the Howard Hughes Medical Institute, the Netherlands Organization for Scientific Research, and the Academic Medical Center of the University of Amsterdam. J.L. received a Boehringer Ingelheim fellowship.
Footnotes
Supplemental Data: Supplemental Data include nine figures and five tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Blanco P, Shlumukova M, Sargent CA, Jobling MA, Affara N, Hurles ME. Divergent outcomes of intrachromosomal recombination on the human Y chromosome: male infertility and recurrent polymorphism. J Med Genet. 2000;37:752–758. doi: 10.1136/jmg.37.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/s0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX← →XY chimaeric mouse testes. Development. 1988;102:443–450. doi: 10.1242/dev.102.2.443. [DOI] [PubMed] [Google Scholar]
- Camargo M, Cervenka J. DNA replication and inactivation patterns in structural abnormality of sex chromosomes. I.X-A translocations, rings, fragments, isochromosomes, and pseudo-isodicentrics. Hum Genet. 1984;67:37–47. doi: 10.1007/BF00270556. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. The degeneration of Y chromosomes. Philos Trans R Soc Lond B Biol Sci. 2000;355:1563–1572. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel A, Lam-Po-Tang PR. Structure and inheritance of some heterozygous Robertsonian translocation in man. J Med Genet. 1976;13:381–388. doi: 10.1136/jmg.13.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CD. Misdivision and the genetics of the centromere. J Genet. 1939;37:341–364. [Google Scholar]
- Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92:290–296. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Guillon H, Baudat F, Grey C, Liskay RM, de Massy B. Crossover and noncrossover pathways in mouse meiosis. Mol Cell. 2005;20:563–573. doi: 10.1016/j.molcel.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Hair JB. The origin of new chromosomes in Agropyron. Heredity. 1953;6(Suppl):215–233. [Google Scholar]
- Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet. 1997;15:345–355. doi: 10.1038/ng0497-345. [DOI] [PubMed] [Google Scholar]
- Hassold T, Jacobs P, Kline J, Stein Z, Warburton D. Effect of maternal age on autosomal trisomies. Ann Hum Genet. 1980;44:29–36. doi: 10.1111/j.1469-1809.1980.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- Hassold T, Benham F, Leppert M. Cytogenetic and molecular analysis of sex-chromosome monosomy. Am J Hum Genet. 1988;42:534–541. [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hsu LY. Phenotype/karyotype correlations of Y chromosome aneuploidy with emphasis on structural aberrations in postnatally diagnosed cases. Am J Med Genet. 1994;53:108–140. doi: 10.1002/ajmg.1320530204. [DOI] [PubMed] [Google Scholar]
- Hsu TC, Pathak S, Chen TR. The possibility of latent centromeres and a proposed nomenclature system for total chromosome and whole arm translocations. Cytogenet Cell Genet. 1975;15:41–49. doi: 10.1159/000130497. [DOI] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurles ME, Willey D, Matthews L, Hussain SS. Origins of chromosomal rearrangement hotspots in the human genome: evidence from the AZFa deletion hotspots. Genome Biol. 2004;5:R55. doi: 10.1186/gb-2004-5-8-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P, Dalton P, James R, Mosse K, Power M, Robinson D, Skuse D. Turner syndrome: a cytogenetic and molecular study. Ann Hum Genet. 1997;61:471–483. doi: 10.1046/j.1469-1809.1997.6160471.x. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Ross A. Structural abnormalities of the Y chromosome in man. Nature. 1966;210:352–354. doi: 10.1038/210352a0. [DOI] [PubMed] [Google Scholar]
- Jäger RJ, Anvret M, Hall K, Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990;348:452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Jobling MA, Williams GA, Schiebel GA, Pandya GA, McElreavey GA, Salas GA, Rappold GA, Affara NA, Tyler-Smith C. A selective difference between human Y-chromosomal DNA haplotypes. Curr Biol. 1998;8:1391–1394. doi: 10.1016/s0960-9822(98)00020-7. [DOI] [PubMed] [Google Scholar]
- John B, Freeman M. Causes and consequences of Robertsonian exchange. Chromosoma. 1975;52:123–136. doi: 10.1007/BF00326262. [DOI] [PubMed] [Google Scholar]
- Kamp C, Hirschmann P, Voss H, Huellen K, Vogt PH. Two long homologous retroviral sequence blocks in proximal Yq11 cause AZFa microdeletions as a result of intrachromosomal recombination events. Hum Mol Genet. 2000;9:2563–2572. doi: 10.1093/hmg/9.17.2563. [DOI] [PubMed] [Google Scholar]
- Kirsch S, Weiss B, Miner TL, Waterston RH, Clark RA, Eichler EE, Munch C, Schempp W, Rappold G. Interchromosomal segmental duplications of the pericentromeric region on the human Y chromosome. Genome Res. 2005;15:195–204. doi: 10.1101/gr.3302705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Koshland D, Rutledge L, Fitzgerald-Hayes M, Hartwell LH. A genetic analysis of dicentric minichromosomes in Saccharomyces cerevisiae. Cell. 1987;48:801–812. doi: 10.1016/0092-8674(87)90077-8. [DOI] [PubMed] [Google Scholar]
- Kuroda-Kawaguchi T, Skaletsky H, Brown LG, Minx PJ, Cordum HS, Waterston RH, Wilson RK, Silber S, Oates R, Rozen S, et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet. 2001;29:279–286. doi: 10.1038/ng757. [DOI] [PubMed] [Google Scholar]
- Lahn BT, Page DC. Four evolutionary strata on the human X chromosome. Science. 1999;286:964–967. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- Lange J, Skaletsky H, Bell GW, Page DC. MSY Breakpoint Mapper, a database of sequence-tagged sites useful in defining naturally occurring deletions in the human Y chromosome. Nucleic Acids Res. 2008;36:D809–814. doi: 10.1093/nar/gkm849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas TK, Speed RM, Passage MB, Yen PH, Chandley AC, Shapiro LJ. Role of the pseudoautosomal region in sex-chromosome pairing during male meiosis: meiotic studies in a man with a deletion of distal Xp. Am J Hum Genet. 1992;51:526–533. [PMC free article] [PubMed] [Google Scholar]
- Niebuhr E. Unusual findings by fluorescence microscopy of a t(13q14q) Humangenetik. 1972;15:96–98. doi: 10.1007/BF00273440. [DOI] [PubMed] [Google Scholar]
- Page SL, Earnshaw WC, Choo KH, Shaffer LG. Further evidence that CENP-C is a necessary component of active centromeres: studies of a dic(X; 15) with simultaneous immunofluorescence and FISH. Hum Mol Genet. 1995;4:289–294. doi: 10.1093/hmg/4.2.289. [DOI] [PubMed] [Google Scholar]
- Page SL, Shaffer LG. Chromosome stability is maintained by short intercentromeric distance in functionally dicentric human Robertsonian translocations. Chromosome Res. 1998;6:115–122. doi: 10.1023/a:1009286929145. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepuberal and adult XX← →XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–393. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- Repping S, Skaletsky H, Lange J, Silber S, Van Der Veen F, Oates RD, Page DC, Rozen S. Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet. 2002;71:906–922. doi: 10.1086/342928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repping S, Skaletsky H, Brown L, van Daalen SK, Korver CM, Pyntikova T, Kuroda-Kawaguchi T, de Vries JW, Oates RD, Silber S, et al. Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nat Genet. 2003;35:247–251. doi: 10.1038/ng1250. [DOI] [PubMed] [Google Scholar]
- Repping S, van Daalen SK, Brown LG, Korver CM, Lange J, Marszalek JD, Pyntikova T, van der Veen F, Skaletsky H, Page DC, et al. High mutation rates have driven extensive structural polymorphism among human Y chromosomes. Nat Genet. 2006;38:463–467. doi: 10.1038/ng1754. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H, Marszalek JD, Minx PJ, Cordum HS, Waterston RH, Wilson RK, Page DC. Abundant gene conversion between arms of palindromes in human and ape Y chromosomes. Nature. 2003;423:873–876. doi: 10.1038/nature01723. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Sullivan BA, Schwartz S. Identification of centromeric antigens in dicentric Robertsonian translocations: CENP-C and CENP-E are necessary components of functional centromeres. Hum Mol Genet. 1995;4:2189–2197. doi: 10.1093/hmg/4.12.2189. [DOI] [PubMed] [Google Scholar]
- Sullivan BA, Willard HF. Stable dicentric X chromosomes with two functional centromeres. Nat Genet. 1998;20:227–228. doi: 10.1038/3024. [DOI] [PubMed] [Google Scholar]
- Sun C, Skaletsky H, Rozen S, Gromoll J, Nieschlag E, Oates R, Page DC. Deletion of azoospermia factor a (AZFa) region of human Y chromosome caused by recombination between HERV15 proviruses. Hum Mol Genet. 2000;9:2291–2296. doi: 10.1093/oxfordjournals.hmg.a018920. [DOI] [PubMed] [Google Scholar]
- Therman E, Sarto GE, Patau K. Apparently isodicentric but functionally monocentric X chromosome in man. Am J Hum Genet. 1974;26:83–92. [PMC free article] [PubMed] [Google Scholar]
- Therman E, Trunca C, Kuhn EM, Sarto GE. Dicentric chromosomes and the inactivation of the centromere. Hum Genet. 1986;72:191–195. doi: 10.1007/BF00291876. [DOI] [PubMed] [Google Scholar]
- Tilford CA, Kuroda-Kawaguchi T, Skaletsky H, Rozen S, Brown LG, Rosenberg M, McPherson JD, Wylie K, Sekhon M, Kucaba TA, et al. A physical map of the human Y chromosome. Nature. 2001;409:943–945. doi: 10.1038/35057170. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Yorifuji T, Muroi J, Kawai M, Mamada M, Kaji M, Yamanaka C, Momoi T, Nakahata T. Parental origin of normal X chromosomes in Turner syndrome patients with various karyotypes: implications for the mechanism leading to generation of a 45,X karyotype. Am J Med Genet. 2002;111:134–139. doi: 10.1002/ajmg.10506. [DOI] [PubMed] [Google Scholar]
- Vergnaud G, Page DC, Simmler MC, Brown L, Rouyer F, Noel B, Botstein D, de la Chapelle A, Weissenbach J. A deletion map of the human Y chromosome based on DNA hybridization. Am J Hum Genet. 1986;38:109–124. [PMC free article] [PubMed] [Google Scholar]
- Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, Kohn FM, Schill WB, Farah S, Ramos C, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933–943. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Foote S, Hilton A, Brown LG, Beer-Romero P, Bogan JS, Page DC. The human Y chromosome: a 43-interval map based on naturally occurring deletions. Science. 1992;258:52–59. doi: 10.1126/science.1439769. [DOI] [PubMed] [Google Scholar]
- Wandall A. Kinetochore development in two dicentric chromosomes in man. A light and electron microscopic study. Hum Genet. 1989;82:137–141. doi: 10.1007/BF00284046. [DOI] [PubMed] [Google Scholar]
- Warburton D, Kline J, Stein Z, Susser M. Monosomy X: a chromosomal anomaly associated with young maternal age. Lancet. 1980;1:167–169. doi: 10.1016/s0140-6736(80)90658-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.