Abstract
Trichloroethylene (TCE) has been associated with a variety of immunotoxic effects and may be associated with an increased risk of non-Hodgkin lymphoma (NHL). Altered serum immunoglobulin (Ig) levels have been reported in NHL patients and in animals exposed to TCE. Recently, we reported that occupational exposure to TCE is associated with immunosuppressive effects and immune dysfunction, including suppression of B-cell counts and activation, even at relatively low levels. We hypothesized that TCE exposure would also affect Ig levels in humans. We measured serum levels of IgG, IgM and IgE, by enzyme-linked immunosorbent assay, in TCE-exposed workers (n = 80) and unexposed controls (n = 45), matched by age and gender, in a cross-sectional, molecular epidemiology study of occupational exposure to TCE in Guangdong, China. Exposed workers had about a 17.5% decline in serum levels of IgG compared with unexposed controls (P = 0.0002). Similarly, serum levels of IgM were reduced by about 38% in workers exposed to TCE compared with unexposed controls (P < 0.0001). Serum levels of both IgG and IgM were significantly decreased in workers exposed to TCE levels below 12 p.p.m., the median exposure level. Adjustment for B-cell counts had minimal impact on our findings. IgE levels were not significantly different between exposed and control subjects. These results provide further evidence that TCE is immunotoxic at relatively low exposure levels and provide additional biologic plausibility for the reported association of TCE with NHL.
Introduction
Increasing epidemiological and experimental evidence has suggested that exposure to trichloroethylene (TCE), a common groundwater contaminant and occupational solvent used primarily for metal degreasing, is associated with immunotoxic effects at relatively low exposure levels (1,2). Previously, in a cross-sectional study of occupational TCE exposure, we reported that exposed workers had significant declines in peripheral blood cell counts, total lymphocytes and some lymphocyte subsets, including B cells and CD4+ T cells, as well as in plasma markers of B-cell activation compared with unexposed factory workers (1,2). Moreover, both epidemiological and animal studies have indicated that TCE exposure alters blood levels of cytokines, including the anti-inflammatory IL-4 and type 1 cytokine IFN-γ, as well as other inflammatory markers (3–5). Collectively, these findings provide evidence that exposure to TCE is associated with immunosuppressive effects and immune dysfunction and provide biological plausibility for the elevated risk of some autoimmune and hypersensitivity disorders, as well as non-Hodgkin lymphoma (NHL), that have been observed in some epidemiological studies of TCE exposure (5,6).
Immunoglobulins (Igs) are produced by B cells and contribute to the immune response via antigen binding and/or by mediating specific effector functions. Structurally, Igs consist of four polypeptide chains including two light and heavy chains. The type of heavy chain produced determines the Ig isotype, of which five are produced in humans (IgA, IgD, IgG, IgE and IgM), each with its own specific composition and effector functions related to the immune response (7). Both IgG, the most abundant Ig in the body, and IgM, the initial Ig expressed in response to an acute infection, play critical roles in neutralizing toxins and other immunogens, whereas increased production of IgE specifically is closely associated with hypersensitivity and allergic responses (7).
Some evidence from animal studies indicates alterations in serum IgG or IgM levels following exposure to various levels of TCE (8,9). We hypothesized that TCE exposure would affect Ig levels in humans in a manner similar to its effect on other immune markers. To our knowledge, no previous epidemiological study has examined serum levels of Igs in healthy workers occupationally exposed to TCE. In order to test our hypothesis, we measured serum levels of IgG, IgM and IgE in exposed workers (n = 80) and unexposed controls (n = 45) in a cross-sectional study of occupational TCE exposure in Guangdong, China.
Materials and methods
Study population and exposure assessment
The design and exposure assessment protocol of this cross-sectional molecular epidemiology study of factory workers in Guangdong, China, has been described previously (2). Briefly, subjects were selected from six factories that used TCE in the manufacturing process and from four control factories in the same geographic region, which did not use TCE. Exposed and unexposed workers were frequency matched on age and sex. Workers with a history of cancer, chemotherapy, radiotherapy or a previous occupation with notable exposure to benzene, butadiene, styrene and/or ionizing radiation were excluded from the study. Informed consent was obtained from all subjects and the study was approved by the Institutional Review Boards at the U.S. National Cancer Institute and the Guangdong Poison Control Center in China.
Full-shift personal air exposure measurements using 3M organic vapor monitoring badges were made before the blood sample was collected as described previously (2). All samples were analyzed for TCE and a subset was analyzed for a panel of other organic hydrocarbons, including benzene, methylene chloride, perchloroethylene and epichlorohydrin. All subjects were interviewed using a questionnaire that assessed demographic and lifestyle characteristics, as well as occupational history.
Analysis of serum IgG, IgM and IgE by enzyme-linked immunosorbent assay
All subjects provided blood, buccal, and postshift and overnight urine samples and underwent a physical examination. Blood samples were delivered to the processing laboratory within 6h of collection and the complete blood count and differential were analyzed on the same day of collection. Serum concentrations of IgG, IgM and IgE from 80 exposed workers and 45 controls were measured using an enzyme-linked immunosorbent assay (ELISA). Briefly, each well of four MaxiSorp (F) 96-well plates (Nalge Nunc International, Rochester, NY) was coated with 100 µl of capture antibody for IgG, IgM and IgE (Bethyl Laboratories, Montgomery, TX; catalog numbers A80-104A, A80-100A and A80-108A, respectively) diluted 1:100 in freshly prepared coating buffer (0.05M carbonate–bicarbonate, pH 9.6, Sigma–Aldrich, St Louis, MO). The plates were incubated at room temperature for 60min after which the coating buffer was removed by aspiration and the wells were washed three times with wash buffer (50mM Tris pH 8.0, 0.14M NaCl, 0.05% Tween-20). At the end of this and all subsequent wash sets, excess wash buffer was removed by tapping the plate gently upside down on a wet paper towel.
Blocking buffer (50mM Tris pH 8.0, 0.14M NaCl, 1.0% bovine serum albumin; 200 µl) was added to each well, and the plates were sealed and incubated for 30min at room temperature. The blocking buffer was removed, the wells were washed three times and the plates were sealed, kept at 4°C, and loaded within 1h. An eight-point serial dilution (using Sample/Conjugate diluent 50mM Tris pH 8.0, 0.14M NaCl, 1.0% bovine serum albumin, with 0.05% Tween-20) of the calibrator sample (Bethyl Laboratories; IgG, RS10-110-3; IgM, RS10-110-3; IgE, RC80-108-5) was prepared for use as a standard curve. Samples were diluted 1:200 000 (IgG), 1:10 000 (IgM) and 1:1 (IgE). Standard curve dilutions and samples (100 µl) were loaded in duplicate onto the freshly prepared ELISA plate. Samples were randomized across the four assay plates and laboratory personnel were blinded to the exposure status of the samples. The plates were sealed, incubated at room temperature for 5min on a shaker, then for 55min without shaking, after which the supernatants were aspirated and the wells washed five times.
The horseradish peroxidase-conjugated secondary antibody (IgG, A80-104P; IgM, A80-100P and IgE, A80-108P) was serially diluted (1:100 000; 1:50 000; 1:100 000) with conjugate dilution buffer to the working concentration and 100 µl was added to each well of the reaction plate. The plates were sealed, incubated at room temperature for 60min, after which the wells were washed five times. Freshly prepared enzyme substrate (Bethyl Laboratories; 100 µl) was added to each well. The plates were incubated, uncovered at room temperature for 12min, after which the reactions were stopped by the addition of 100 µl of 2M H2SO4. The absorbance of the wells was immediately read at 450nM in a PowerWave XS plate reader (BioTek, Winooski, VT).
To measure intra- and interassay variation, a master internal control sample, prepared from a single human volunteer serum sample, was diluted as described for the samples above, aliquoted and stored at −80°C. The internal control was measured twice on each of the four plates for each assay. From these readings, it was determined that both the inter- and intraplate coefficients of variation values (averaged across all four plates) for each assay were less than 10%, a value that is considered good for a sandwich ELISA.
Statistical analysis
Unadjusted summary measures were calculated for IgG, IgM and IgE in exposed workers and controls. Linear regression using the natural logarithm of each endpoint was conducted in order to assess differences in the serum concentration of Igs in exposed cases and controls, as well as to test for trend across TCE exposure categories using a three-level ordinal variable based on the median exposure level in the overall study population (unexposed controls, <12 p.p.m., ≥12 p.p.m.). All models were adjusted for the matching factors, age and sex. Other potential confounders including current smoking (yes/no), alcohol consumption (yes/no), body mass index and recent infection (yes/no) were included in the final model if the regression coefficient of the TCE exposure variable was altered by ±10%. All statistical analyses were conducted using SAS v. 9.1 (Cary, NC).
Results
The demographic characteristics of the exposed and control workers are shown in Table I. Age and body mass index were similar in exposed workers and controls, whereas the percentages of current smokers and those reporting recent infection were slightly higher in exposed workers. Exposed subjects were designated as low exposed (<12 p.p.m.) and high exposed (≥12 p.p.m.), based on the median TCE exposure level during the month prior to phlebotomy. The mean TCE exposure level was 22.2 p.p.m. (±35.9 p.p.m.) in exposed workers, 5.2 p.p.m. (±3.5 p.p.m.) in the low exposure group and 38.4 p.p.m. (±44.6 p.p.m.) in the high exposure group.
Table I.
Demographic characteristics of the study population from a cross-sectional molecular epidemiology study of TCE in China
| Characteristic | Control | Exposed | Low exposed, <12 p.p.m.a | High exposed, ≥12 p.p.m.a |
|---|---|---|---|---|
| Number of subjects | 45 | 80 | 39 | 41 |
| Sex (n, %) | ||||
| Female | 16 (36) | 23 (29) | 15 (38) | 8 (20) |
| Male | 29 (64) | 57 (71) | 24 (62) | 33 (80) |
| Mean age (SD) | 25 (6) | 25 (7) | 24 (5) | 27 (8) |
| BMI (SD) | 22 (3) | 21 (3) | 21 (2) | 22 (3) |
| Smoking (n, %) | ||||
| No | 30 (67) | 46 (58) | 22 (56) | 24 (59) |
| Yes | 15 (33) | 34 (42) | 17 (44) | 17 (41) |
| Alcohol use (n, %) | ||||
| No | 26 (58) | 54 (68) | 26 (67) | 28 (68) |
| Yes | 19 (42) | 26 (32) | 13 (33) | 13 (32) |
| Recent infection (n, %) | ||||
| No | 39 (87) | 65 (81) | 31 (79) | 34 (83) |
| Yes | 6 (13) | 15 (19) | 8 (21) | 7 (17) |
| TCE air exposure mean p.p.m. (SD) | <0.03 | 22.2 (35.9) | 5.2 (3.5) | 38.4 (44.6) |
BMI, body mass index; SD, standard deviation.
aBased on median TCE exposure level in overall study population (80 exposed, 45 control).
The associations of serum IgG, IgM and IgE levels with TCE exposure are shown in Figure 1A–C, respectively. Workers exposed to TCE had a 17.5% decrease in serum levels of IgG compared with unexposed controls (P = 0.0002). The decline was similar for workers exposed to low (16%; P = 0.006) and high (19%; P = 0.0002) levels of TCE exposure, after adjusting for age and gender (Figure 1A).
Fig. 1.
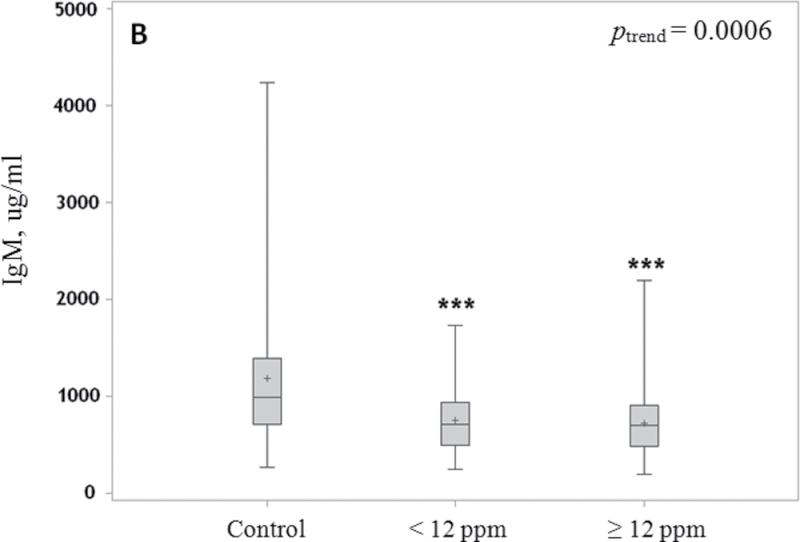
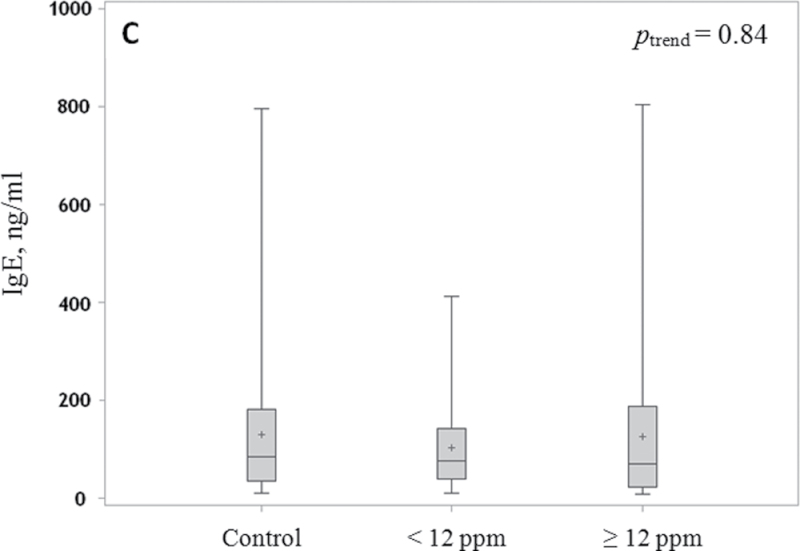
Box and whisker plot of serum levels of IgG, IgM and IgE. Median (line), mean (+) and interquartile range (box) and whiskers to the lowest and highest values in relation to TCE exposure level are shown for IgG (A), IgM (B) and IgE (C) in control subjects and workers exposed to <12 p.p.m. and ≥12 p.p.m. TCE. P-values are indicated as **P < 0.01 and ***P < 0.001. Levels of IgG and IgM were adjusted for age and sex, and levels of IgE were adjusted for age, sex, alcohol and recent infection.
Similarly, serum levels of IgM were reduced by about 38% in workers exposed to TCE compared with unexposed controls (P < 0.0001), after adjusting for age and gender. The magnitudes of decline in levels of IgM were similar in workers exposed to lower (37%; P = 0.0002) and higher (39%; P = 0.0008) levels of TCE compared with the unexposed workers (Figure 1B). There was no evidence of a correlation between IgM and IgG levels in the exposed (rSp = −0.12, P = 0.31) or in control workers (rSp = +0.16, P = 0.30).
Serum IgE levels were not significantly different between TCE-exposed and -unexposed workers after adjustment for age, gender, alcohol consumption and recent infection (P = 0.64; Figure 1C).
Discussion
To the best of our knowledge, this is the first study to examine the effect of TCE on serum levels of Ig family components in healthy adult workers. We observed significant declines in serum levels of IgG and IgM, but not IgE, in workers exposed to TCE compared with unexposed controls. Notably, these significant alterations persisted after adjustment for infection status and other demographic variables. Results were essentially unchanged by further adjustment for smoking and alcohol and the magnitude of the declines was similar in workers exposed to low and high levels of TCE. As most workers were exposed to TCE levels below the current permissible exposure limit of 100 p.p.m. (8h time-weighted average) of Occupational Safety and Health Administration (http://www.osha.gov/dts/chemicalsampling/data/CH_273000.html), our findings provide further evidence that TCE is capable of altering the immunologic milieu even at relatively low exposure levels (median = 12 p.p.m., mean = 22.2 p.p.m.).
Evidence of altered Ig levels in TCE-exposed animals supports the plausibility of our findings. Specifically, a study conducted in mice evaluating immune status and immune marker concentrations following exposure to TCE noted declines in levels of IgG and IgM at relatively high TCE exposure levels, ranging from 500 to 2000 p.p.m., but effects at lower concentrations were not considered (8). Further, higher levels of antinuclear antibodies in mice have been associated with ingestion of drinking water contaminated with TCE and related metabolites (5,10). Also consistent with our findings in exposed workers is the fact that a clear association between TCE exposure and serum IgE levels has not been demonstrated in animals, with studies either reporting no effect or conflicting findings based on the length of the exposure period (11,12).
Our findings that TCE exposure alters serum Ig levels in humans adds to the accumulating evidence that TCE is associated with a wide range of immunotoxic effects, including reductions in immune cells and in soluble immune markers, as well as in serum levels of cytokines that could potentially result in the disruption of the Th1/Th2-mediated immune response (1–3). IgG and IgM are involved in a variety of host immunological functions, as increased levels of IgM are produced by B cells following antigenic stimulation and function in the primary immune response during an acute infection, after which IgG antibodies are produced to mediate the secondary immune response (7). IgG specifically mediates several crucial functions associated with an effective immune response, including clearance of immune complexes and phagocytosis (13). Further, IgM and specific subclasses of IgG antibodies are involved in the activation of the classical pathway of the complement system, which is a critical component of the innate immune response and serves as a first line of defense against infection (14).
Therefore, it is plausible that lower levels of these Igs may be indicative of a reduced immune capacity and greater susceptibility to infection following exposure to TCE, as has been reported in studies of animals exposed to TCE by inhalation. Mice exposed to 10 p.p.m. of TCE showed significantly increased mortality after Klebsiella pneumoniae infection and significantly decreased pulmonary bactericidal activity (15). In another study, significantly increased mortality from Streptococcus zooepidermicus infection and reduced clearance of the bacteria were found in mice exposed to levels of 50 and 100 p.p.m., respectively (16). Dogs exposed to TCE had significantly reduced numbers of lymphocytes and neutrophils 30min after exposure to 200 p.p.m. TCE (17). Although the mechanisms underpinning these associations need further elucidation, we considered whether the reduced Ig levels were related to the suppression of B-cell numbers that we reported previously in exposed workers (2). However, B-cell counts were uncorrelated with IgG (r = −0.01, P = 0.91) and IgM (r = 0.07, P = 0.51) levels and adjustment for B-cell counts and other peripheral blood cell counts had minimal impact on our findings of a reduction in IgM and IgG levels attributed to TCE exposure. This suggests that TCE inhibits Ig production independently of its effect on B-cell numbers. It is possible that TCE impairs B-cell stimulation and consequently the generation of Igs. Evidence supporting this mechanism comes from the reduced levels of soluble CD27 and CD30 that we observed previously in TCE-exposed workers (2). Another possible mechanism is the impaired differentiation of B cells; this could be assessed by measuring the relative proportions of B cells at various stages of maturity, in exposed and control subjects.
Our findings are further notable given that reductions in serum Ig levels have previously been associated with disorders associated with immune deficiency. Reduced levels of IgM or IgG, either overall or specific subclasses, in patients with immune deficiency, have been demonstrated to have greater susceptibility to infections, with the greatest risk associated with very low and prolonged deficiencies (13,18,19). Further, several studies have reported lower levels of either IgM or IgG as well as other Igs in patients with lymphoma compared with controls and in association with more advanced stages of disease (20–22). Our finding of reduced Ig levels in TCE-exposed workers provides biologic plausibility for the suggestive epidemiologic evidence that TCE is a lymphomagen, which is associated, in particular, with NHL (23). At the same time, prospective studies measuring serum Ig levels before the diagnosis of NHL (and other diseases associated with altered Ig levels) would be particularly informative in evaluating the association of Ig levels and subsequent disease development.
In summary, we found that serum concentrations of IgG and IgM, but not IgE, were significantly reduced in workers exposed to TCE compared with controls. These findings provide further evidence that TCE is immunotoxic at relatively low exposure levels and provide additional biologic plausibility for the reported associations of TCE with NHL. Future studies with a larger number of exposed workers are needed to more extensively evaluate potential dose–response relationships between serum Ig levels and TCE exposure levels, as well as to conduct a more detailed evaluation of potential confounding factors such as smoking and alcohol use that have previously been demonstrated to alter Ig levels (24,25).
Funding
Intramural funds from National Institutes of Health and National Cancer Institute; National Institute of Environmental Health Sciences (P42ES04705 and P30ES01896 to M.T.S.); Northern California Center for Occupational and Environmental Health and Department of Science and Technology of Guangdong Province, China (2007A050100004 to X.T.).
Acknowledgements
We thank Jackie King, M.D. (BioReliance Corp., Rockville, MD), for logistical support; the study participants and members of the Guangdong field team.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ELISA
enzyme-linked immunosorbent assay
- Ig
immunoglobulin
- NHL
non-Hodgkin lymphoma
- TCE
trichloroethylene.
References
- 1. Hosgood H.D, 3rd, et al. (2011). Decreased numbers of CD4(+) naive and effector memory T cells, and CD8(+) naïve T cells, are associated with trichloroethylene exposure. Front. Oncol., 1, 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lan Q, et al. (2010). Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis, 31, 1592–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iavicoli I, et al. (2005). Effects of occupational trichloroethylene exposure on cytokine levels in workers. J. Occup. Environ. Med., 47, 453–457 [DOI] [PubMed] [Google Scholar]
- 4. Lehmann I, et al. (2002). The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environ. Toxicol., 17, 203–210 [DOI] [PubMed] [Google Scholar]
- 5. Cooper G.S, et al. (2009). Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Perspect., 117, 696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. United States Environmental Protection Agency (2011). Toxicological Review of Trichloroethylene http://www.epa.gov/iris/toxreviews/0199tr/0199tr.pdf (1 November 2012, date last accessed)
- 7. Schroeder H.W, et al. (2010). Structure and function of immunoglobulins. J. Allergy Clin. Immunol., 125, S41–S52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaneko T, et al. (2000). Immunotoxicity of trichloroethylene: a study with MRL-lpr/lpr mice. J. Appl. Toxicol., 20, 471–475 [DOI] [PubMed] [Google Scholar]
- 9. Griffin J.M, et al. (2000). Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology, 46, 123–137 [DOI] [PubMed] [Google Scholar]
- 10. Blossom S.J, et al. (2004). Activation and attenuation of apoptosis of CD4+ T cells following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid. J. Autoimmun., 23, 211–220 [DOI] [PubMed] [Google Scholar]
- 11. White K.L, et al. (2000). Assessment of autoimmunity-inducing potential using the brown Norway rat challenge model. Toxicol. Lett., 112–113, 443–451 [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi R, et al. (2010). Enhancement of immediate allergic reactions by trichloroethylene ingestion via drinking water in mice. J. Toxicol. Sci., 35, 699–707 [DOI] [PubMed] [Google Scholar]
- 13. Furst D.E. (2009). Serum immunoglobulins and risk of infection: how low can you go? Semin. Arthritis Rheum., 39, 18–29 [DOI] [PubMed] [Google Scholar]
- 14. Daha N.A, et al. (2011). Complement activation by (auto-) antibodies. Mol. Immunol., 48, 1656–1665 [DOI] [PubMed] [Google Scholar]
- 15. Aranyi C, et al. (1986). The effects of inhalation of organic chemical air contaminants on murine lung host defenses. Fundam. Appl. Toxicol., 6, 713–720 [DOI] [PubMed] [Google Scholar]
- 16. Selgrade M.K, et al. (2010). Suppression of pulmonary host defenses and enhanced susceptibility to respiratory bacterial infection in mice following inhalation exposure to trichloroethylene and chloroform. J. Immunotoxicol., 7, 350–356 [DOI] [PubMed] [Google Scholar]
- 17. Hobara T, et al. (1984). Acute effects of 1,1,1-trichloroethane, trichloroethylene, and toluene on the hematologic parameters in dogs. Arch. Environ. Contam. Toxicol., 13, 589–593 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein M.F, et al. (2006). Selective IgM immunodeficiency: retrospective analysis of 36 adult patients with review of the literature. Ann. Allergy Asthma Immunol., 97, 717–730 [DOI] [PubMed] [Google Scholar]
- 19. Yel L, et al. (2009). Clinical and immunological features in IgM deficiency. Int. Arch. Allergy Immunol., 150, 291–298 [DOI] [PubMed] [Google Scholar]
- 20. Grulich A.E, et al. (2007). Re: atopy and risk of non-Hodgkin lymphoma. J. Natl Cancer Inst., 99, 1417 [DOI] [PubMed] [Google Scholar]
- 21. Ellison-Loschmann L, et al. (2007). Immunoglobulin E levels and risk of lymphoma in a case-control study in Spain. Cancer Epidemiol. Biomarkers Prev., 16, 1492–1498 [DOI] [PubMed] [Google Scholar]
- 22. Biggar R.J, et al. (2009). Immunoglobulin subclass levels in patients with non-Hodgkin lymphoma. Int. J. Cancer, 124, 2616–2620 [DOI] [PubMed] [Google Scholar]
- 23. Scott C.S, et al. (2011). Trichloroethylene and cancer: systematic and quantitative review of epidemiologic evidence for identifying hazards. Int. J. Environ. Res. Public Health, 8, 4238–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMillan S.A, et al. (1997). Effect of low to moderate levels of smoking and alcohol consumption on serum immunoglobulin concentrations. J. Clin. Pathol., 50, 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonzalez-Quintela A, et al. (2008). Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol., 151, 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]