Abstract
Epithelial ovarian cancer is a heterogeneous disease that is subdivided into five major histotypes but the mechanisms driving their differentiation are not clear. Mutations in adenomatous polyposis coli (APC) and β-catenin are commonly observed in the human ovarian endometrioid adenocarcinoma (OEA) patients. However, the mechanisms subsequent to APC deletion in ovarian tumorigenesis have not been well characterized. We have conditionally deleted APC in the murine ovarian surface epithelium (OSE) and showed that its loss leads to development of epithelial inclusion cysts. High-grade OEAs with tightly packed villoglandular histology were observed in older APC-deleted mice. Phosphatase and tensin homolog (PTEN) expression was elevated in the early lesions but lost after progression to the more advanced tumors. Knockdown of APC or expression of a gain-of-function β-catenin similarly induced human OSE cells to develop tumors with endometrioid histology in xenografts. Expression of HOXA10 was induced in both the advanced APC-deleted murine tumors and in the tumor xenografts of human OSE cells with knocked-down APC. These results show that reduced APC activity is sufficient to induce formation of epithelial inclusion cysts and support OEA development and suggest that induced HOXA10 expression and loss of PTEN are key mechanisms driving endometrioid histotype differentiation and progression.
Introduction
Human ovarian endometrioid adenocarcinoma (OEA) is one of the major histotypes of epithelial ovarian cancer, which is the most lethal gynecological cancer in the USA (1,2), but the etiology of the disease and the molecular mechanisms driving histotype differentiation are not clear. Activating mutations in exon 3 of the β-catenin gene (CTNNB1), which encodes the phosphorylation sites required for its degradation by the adenomatous polyposis coli (APC) complex (3), is observed in 16–38% patients of human OEAs (1), suggesting that dysregulated β-catenin activity might be a contributing factor in these patients. In support of this hypothesis, we have shown previously that conditional deletion of exon 3 of the β-catenin gene (Ctnnb1) in the ovarian surface epithelium (OSE) induces OEA development in mice (4). Additionally, most familial adenomatous polyposis patients with mutations in the APC gene usually succumb to colon cancer (5), but they are also at increased risk of developing tumors in other tissues, including the ovary (6–8).
Concomitant mutations in the tumor suppressor PTEN gene are also frequently observed in human OEA patients with defective WNT/β-catenin signaling but do not appear to play a significant role in histotype differentiation because loss of PTEN is found in serous ovarian carcinomas as well (9,10). Combined loss of PTEN, which inhibits AKT activation in the PI3K signaling pathway, and exon 3 of β-catenin increased tumor penetrance and aggressiveness in mice, but, as with human OEAs, PTEN deletion had no effect on the histopathology of these mouse tumors (11). In contrast, neither conditional loss of a flox allele of Apc after adenoviral delivery of Cre recombinase into the intrabursal space of murine ovaries (10) nor Apc haploinsufficiency (12) resulted in ovarian tumorigenesis. However, homozygous deletion of both Apc and Pten causes formation of aggressive OEAs within 6 weeks with 100% penetrance (10). These Apc/Pten-deleted ovarian tumors showed distinct epithelial glandular morphology with significant areas of cytokeratin-negative mesenchymal cells, indicative of epithelial–mesenchymal transition (10). Similar to Apc deletion, Pten loss or a gain-of-function mutation of the PI3K catalytic subunit alone in OSE is unable to cause tumor formation in mouse ovaries (4,10,13,14). Currently, it is unknown why alterations in these two specific pathways predominantly occur in human OEA patients and the individual contributions of APC and PTEN to the initiation, progression and differentiation of ovarian tumorigenesis is unclear.
The goal of this study was to determine whether conditional deletion of Apc in the OSE by using Amhr2-driven Cre expression is sufficient to induce ovarian tumorigenesis and whether loss of PTEN is involved in either tumorigenesis or disease progression. We show that conditional loss of APC alone leads to formation of ovarian epithelial inclusion cysts, a suspected precursor stage for human epithelial ovarian cancer and later to the development of OEAs. During the early stages of the disease, PTEN expression is prominently observed in the mutant cells, but in advanced stages, PTEN expression is lost. Because of observed differences between mouse and human OSE cells (15), we developed two xenotransplant models of OEA with either overexpression of an activating allele of β-catenin or with knockdown of APC expression in human OSE. Both models phenocopied each other suggesting that dysregulated nuclear β-catenin activity is an important driver of OEA differentiation. Lastly, we show that dysregulated β-catenin activates the expression of HOXA10, a homeobox protein linked to endometrioid histotype differentiation (16,17).
Materials and methods
Mouse genetics and husbandry
All animal experimentation is performed by following protocols approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Mice used in this study were maintained on a mixed genetic background (C57BL/6;129/SvEv) and housed under standard animal housing conditions. Amhr2tm3(cre)Bhr/Amhr2-cre [a gift from Dr Richard Behringer (18)] mice were crossed with Apcflox/flox (19) (National Cancer Institute) to generate conditional knockout Amhr2tm3(cre)Bhr; Apcflox/flox (Apccko), and control littermates Amhr2tm3(cre)Bhr; Apcflox/+ (Apcdel/+) and Apcflox/flox. The DNA from tail biopsies was used for genotyping using standard PCR protocols (20). Gross photography was performed with either a Nikon SMZ1500 and Spot digital camera (Diagnostic Instruments, Sterling Heights, MI) or a Nikon D60 digital camera.
Histology, immunofluorescence and immunohistochemistry
For histological analyses, ovaries were fixed in 4% paraformaldehyde overnight at 4°C. Hematoxylin and eosin staining was performed using standard protocols. The procedures for immunofluorescence (IF) and immunohistochemistry (IHC) are described in a previous study (21). The primary and secondary antibodies used in this study are β-catenin (BD Transduction Laboratories, San Jose, CA), lymphoid enhancer factor 1 (LEF1), T-cell factor 1 (TCF1), PTEN, non-phospho (active) β-catenin Ser33/37/Thr41 (Cell Signaling Technology, Danvers, MA), cytokeratin 8 (CK8; Developmental Studies Hybridoma Bank, Iowa City, IA), anti-Müllerian hormone (Santa Cruz Biotechnology, Santa Cruz, CA), cyclin d1 (Neomarkers, Fremont, CA), inhibinα (Biogenex, San Ramon, CA), phospho-histone 3 (Millipore, Billerica, MA), AlexaFluor secondary antibodies (Invitrogen, Carlsbad, CA) and biotinylated donkey secondary F(ab)2 fragments (Jackson ImmunoResearch, West Grove, PA). Photos were taken with a Nikon TE2000 microscope with epifluorescence and a Spot digital camera (Diagnostic Instruments).
Human ovarian endometrioid carcinoma sample analyses
Paraffin-embedded tissue blocks for human OEAs (n = 5) and normal/benign ovarian samples (n = 3) were obtained from the Department of Pathology, MGH, using Institutional Review Board-approved protocols. IF for LEF1, TCF1 and cyclin d1 was performed on 5 µm thick tissue sections.
Human ovarian surface epithelial in vitro and in vivo
Telomerase reverse transcriptase-immortalized human ovarian surface epithelial (HOSE) cells (22) were cultured in Medium 199 (Invitrogen):MCDB 105 medium (Sigma, St Louis, MO). For viral transduction, 0.3 million HOSE cells were plated in 6-well plates and incubated with constitutively activated β-catenin (CA-β-catenin) (23) (Addgene, Cambridge, MA), APC shRNA (Dana Farber/Harvard Cancer Center DNA Resource Core) and empty retroviral vectors. Green fluorescent protein (GFP) expression was used to monitor transduction efficiency for both the CA-β-catenin and empty vectors and for purification by cell sorting. Puromycin selection was performed after APC shRNA transduction. Tumor development was performed by injecting 106 HOSE cells from the three different groups in nude mice, both intraperitoneal and subcutaneous. Comparative histological analyses were done with endometrial cancer tumors made with 106 AN3CA cells (American Type Culture Collection, Manassas, VA).
Western blot analyses
Ovaries were collected from Apccko and control mice (n = 3 each) and analyzed by western blot as described previously (20). Antibodies used are GSK3-β, pGSK3-β, LEF1, TCF1 (Cell Signaling Technology), cyclin d1 (Neomarkers) and β-catenin (BD Transduction Laboratories).
Results
To study the function of APC in ovarian somatic cells, we generated mice with conditional deletion of exon 14 of the Apc gene by mating anti-Müllerian hormone receptor 2-Cre (Amhr2-Cre, also known as Misr2-Cre, Müllerian inhibiting substance receptor 2-Cre) mice with mice carrying flox alleles of Apc (20). Amhr2-Cre is expressed in OSE, granulosa cells of developing follicles as well as in the stroma of Müllerian duct mesenchyme-derived structures (20,24). Complete knockout of exon 14 of the Apc gene induces embryonic lethality and phenocopies other Apc gene complete knockouts, suggesting that deletion of exon 14 leads to loss or abnormal functions of APC (19). Confirmation that Amhr2-Cre causes faithful recombination of the Apc flox allele in the ovary, oviduct and uterus of Apccko mice was shown in a previous study (20). Since the APC complex controls the cellular level of β-catenin, we hypothesized that β-catenin nuclear accumulation would occur in cells with conditional deletion of Apc. Examination of total and active (non-phosphorylated) β-catenin expression in control ovaries (n = 3 each) shows mostly membranous expression of β-catenin in surface epithelial and granulosa cells (Supplementary Figure 1A, C and E, available at Carcinogenesis Online). However, prominent patches of cytoplasmic and nuclear accumulation of β-catenin were observed in the OSE but not in granulosa cells of the mutant ovaries (Supplementary Figure 1B, D and F, available at Carcinogenesis Online). Increased protein expression of TCF1 and LEF1 was also observed in mutant ovaries compared with controls (Supplementary Figure 1G, available at Carcinogenesis Online). Western blot analyses of APC downstream targets (β-catenin, TCF1, LEF1 and cyclin d1) with whole ovarian protein extracts showed increased expression of these proteins in the older mutant ovaries compared with controls (Supplementary Figure 1H, available at Carcinogenesis Online), confirming increased but varying levels of tumor burden with age. Increased phosphorylation of GSK3β was also observed in mutant ovaries (Supplementary Figure 1H, available at Carcinogenesis Online), which is consistent with destabilization of the APC complex (3). No difference was observed in total GSK3β protein between control and mutant ovaries. Gross examination of 6-week-old homozygous Apccko, heterozygous Apcdel/+ and unfloxed control ovaries revealed no obvious differences between these three groups at this age (n = 3 each) (Supplementary Figure 1I, available at Carcinogenesis Online). Examination of older ovaries (≥20 weeks) revealed that the size of nearly half the mutant ovaries was increased (n = 7/18) compared with control and Apcdel/+ ovaries (Supplementary Figure 1I, available at Carcinogenesis Online).
Early phenotypic changes in the ovaries of mutant mice
Histological examination of ovaries at different time points was performed to examine microscopic changes after loss of APC. No abnormal growth was observed in 5-week-old Apcflox/flox, and Apcdel/+ control ovaries (n = 6) (Supplementary Figure 2A–D, available at Carcinogenesis Online) but darkly stained areas that resemble the CK8-positive areas in Figure 1D and F were observed in Apccko ovaries (Supplementary Figure 2E and F, available at Carcinogenesis Online). Normal looking follicles and corpora lutea were present in all three group of ovaries examined in this study (Supplementary Figure 2A–F, available at Carcinogenesis Online). Oocytes were collected from control and mutant ovaries to analyze ovarian function. Fewer oocytes (MII) were retrieved from superovulated mutant ovaries compared with controls (Supplementary Table I, available at Carcinogenesis Online), which was likely due to the presence of shortened or nearly absent oviducts in the APCcko mice (4). However, fertilization efficiency and embryo quality were equivalent in oocytes collected from the control and mutant ovaries (Supplementary Table II, available at Carcinogenesis Online), suggesting that follicular functions were normal in both groups of mice. Histological assessment of 4-month-old ovaries from control (n = 7) and mutant ovaries (n = 6/8) showed hyperplasia of the OSE cells in mutant ovaries (Supplementary Figure 2G–J, available at Carcinogenesis Online), cells that were often shed and observed in the intrabursal space (Supplementary Figure 2H and J, available at Carcinogenesis Online). Granulosa cells continued to appear normal at this age.
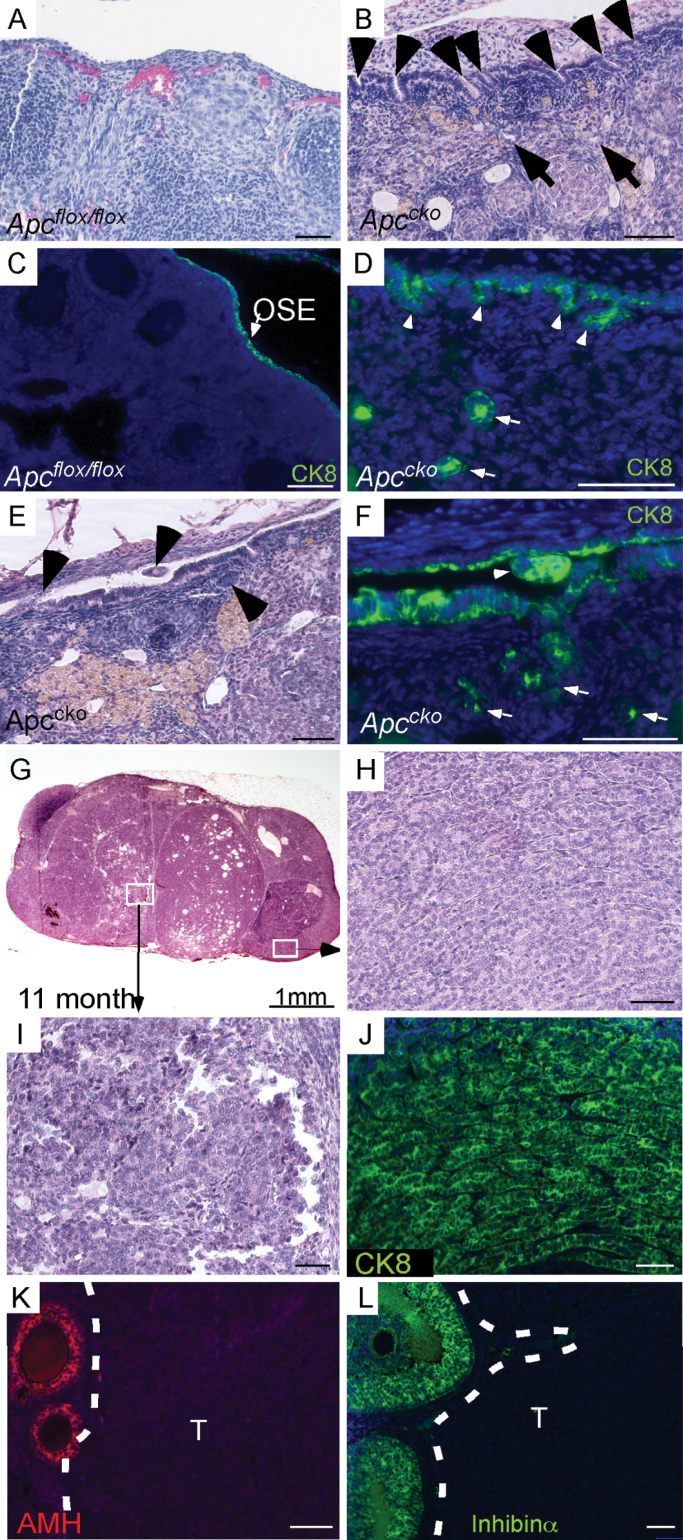
Fig. 1.
Histology of Apccko ovaries. (A) Normal histology of the OSE in older (>4 month) control ovaries by hematoxylin and eosin. (B) Invasion of stromal cells by hyperplastic OSE cells (arrowheads) in Apccko ovaries at 7–9 months. Epithelial gland-like structures (arrows) were observed in ovarian stroma. (C and D) CK8-specific staining showed normal OSE cells in control ovaries (arrow), and hyperplastic OSE cells (arrowheads) and glandular (arrows) epithelial cells in stroma of mutant ovaries at 7–9 months. (E and F) CK8-positive epithelial cells were observed in the space between the OSE and the ovarian bursa (arrowhead). Mutant OSE cells forming epithelial glandular structures (arrows) in mutant ovaries at 7–9 months. (G–I) OEAs formed in mutant ovaries. IF of the Apccko tumors with CK8 (J), anti-Müllerian hormone (K) and inhibinα (L). T = tumor area in K and L at 7–9 months. In panels C, D, F, J, K and L, nuclei are shown stained with 4′,6-diamidino-2-phenylindole (DAPI). Bars: 50 µm.
Progressive development of OEAs in Apccko mice
Disrupted APC expression initially affects the crypt architecture and later induces changes in epithelial cellular proliferation in colon cancer mouse models (25). Additionally, overexpression of full-length APC in a colon cancer cell line decreases cell migration and increases cell adhesion (26), suggesting that normal APC plays an important role in cell migration and adhesion. In this study, we observed that OSE cells of mutant ovaries invade the ovarian stroma (n = 5/20), which were similar to the inclusion cysts that have been implicated in the etiology of epithelial ovarian cancers (15), and appeared to form glandular structures (Figure 1B and D–F). Unlike controls (Figure 1A and C), epithelial cells were observed in the intrabursal space of mutant ovaries (n = 4/20) (Figure 1E and F). IF for CK8, an epithelial cell-specific marker, confirmed the presence of epithelial cells in the ovarian stroma of Apccko ovaries (Figure 1D and F). APCcko mice progressively develop ovarian tumors through 11 months of age (n = 7/20) (Figure 1G–J). However, no tumor formation was observed in control mouse ovaries (n = 14). These tumors were often admixed with cysts and limited to the ovaries. Histological examination of the tumors showed that they were largely composed of tightly packed epithelial cells similar to advanced Type I endometrial cancer (Figure 1G–J). CK8-specific staining confirmed the epithelial nature of these tumors (Figure 1J). Expression analyses of anti-Müllerian hormone or Müllerian inhibiting substance and inhibinα, two well-known markers of ovarian stromal tumors (4), showed that tumors were negative for both markers (Figure 1K and L), which again supports the epithelial nature of these tumors. As expected, nuclear accumulation of β-catenin was observed in the fully developed tumors (Supplementary Figure 3A and B, available at Carcinogenesis Online).
Activating mutations in exon 3 of the β-catenin gene accompanied by nuclear accumulation of β-catenin protein have been observed in human ovarian microcystic stromal tumors (27). Because Amhr2-Cre is also expressed in stromal cells of the mouse ovary (4,24), we examined mutant mice for the stromal abnormalities/tumors and observed cysts in some (n = 4/20) of the Apccko ovaries (Supplementary Figure 3C, available at Carcinogenesis Online). However, further examination of these cysts revealed that they were positive for CK8 staining (Supplementary Figure 3D, available at Carcinogenesis Online), suggesting an epithelial origin. Stromal granulosa cell tumors were not observed in any of the mutant ovaries (n = 28) examined, indicating a limited role for mutated APC in the development of stromal tumors in mice, which is consistent with the lack of nuclear β-catenin in either human adult or juvenile granulosa cell tumors (28).
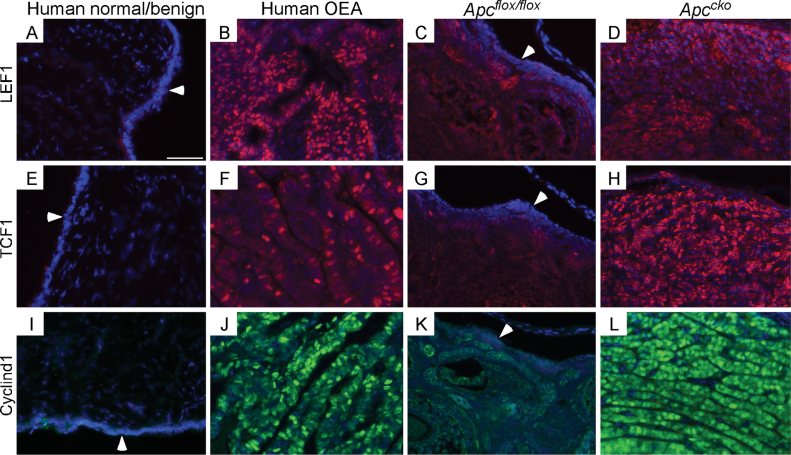
Increased nuclear or total β-catenin ultimately leads to the activation of its target genes, including LEF1, TCF1 and cyclin d1 (29). Dominant-stable activation of β-catenin causes formation of intestinal polyps and cancer similar to the mice with mutated Apc (30), and expression of dominant-negative TCFs or deletion of cyclin d1 suppressed APC-mutated intestinal cancer cell proliferation (3). Additionally, previous studies have illustrated that activation of Wnt signaling occurs in human OEAs (1,4). The expression of LEF1, TCF1 and cyclin d1 in human and Apccko mice OEAs was elevated in both human (n = 5) and murine (n = 3) OEAs compared with controls (Figure 2), suggesting that dysregulated nuclear β-catenin activity might be contributing to progression of the disease in both settings.
Fig. 2.
Induction of β-catenin-responsive genes in human and Apccko OEAs. Sections from human and Apccko OEAs were analyzed by IF for expression of LEF1 (B and D), TCF1 (F and H) and cyclin d1 (J and L) for comparison with normal human (A, E and I) and control murine (C, G and K) ovaries. Murine OEAs and controls are from 11-month-old mice. Arrowheads in panels A, C, E, G, I and K are pointing toward the normal OSE. Nuclei are shown by DAPI staining. Bar: 50 µm.
Upregulation of Pten expression in pretumoral lesions present in mutant ovaries
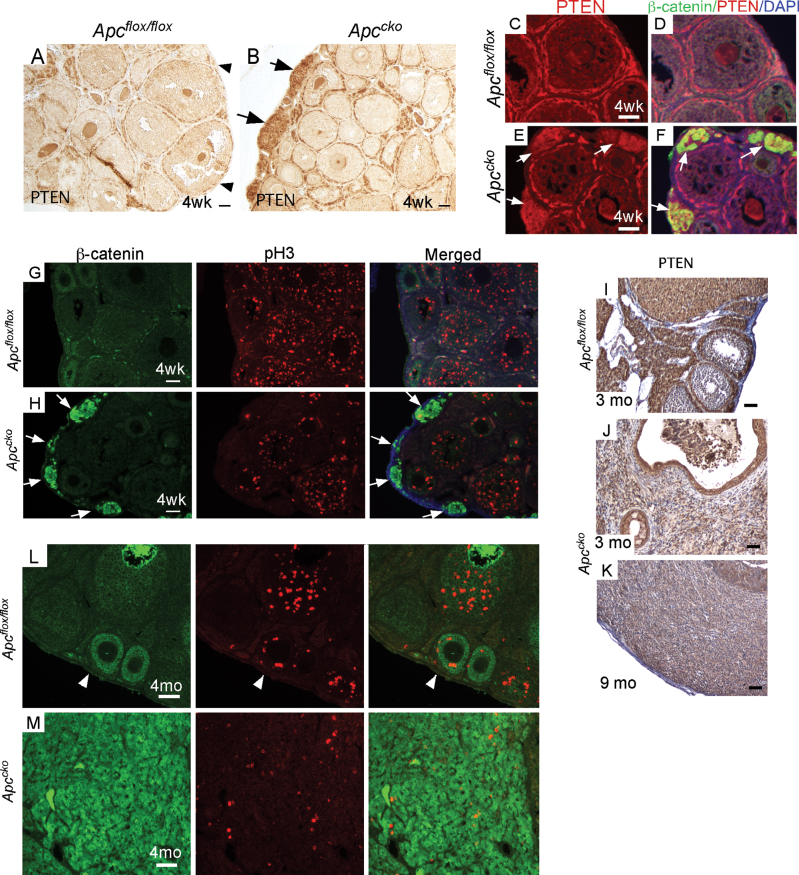
Deletion of PTEN and activation of PIK3CA alone are not sufficient to initiate ovarian tumorigenesis (10,13,24). However, loss of PTEN appears to potentiate tumorigenesis in ovaries with mutations of other genes (4,10,24) and the combined loss of PTEN and APC causes a 100-fold increase in the penetrance of OEAs (10). We observed increased expression of PTEN in the pretumoral lesions of Apccko ovaries (Figure 3A and B), suggesting that PTEN expression might be inhibited by normal APC activity or induced by nuclear β-catenin. Colocalization of β-catenin and PTEN confirms that upregulation of PTEN only occurred in lesions with nuclear β-catenin accumulation in the Apccko ovaries (Figure 3C–F). The expression of the cell cycle regulator, P27kip1, a downstream target of PTEN/AKT signaling, was also increased in pretumoral lesions (Supplementary Figure 4A and B, available at Carcinogenesis Online). However, FOXO1, a tumor suppressor and downstream target of AKT signaling (11), was not increased in the pretumoral lesions (Supplementary Figure 4C and D, available at Carcinogenesis Online).
Fig. 3.
PTEN expression is induced and proliferation is inhibited in early Apccko lesions. Expression of PTEN (representative of n = 3 in each group) in juvenile control (A) and mutant ovaries (B) by IHC. Colocalization of β-catenin and PTEN in juvenile control (C and D) and mutant ovaries (E and F) by IF. Coimmunostaining of β-catenin and pH3 in juvenile control (G) and mutant (H) ovaries. PTEN protein expression in adult control (I) and mutant (J and K) ovaries by IHC. IF for β-catenin and pH3 in adult control (L) and mutant (M) ovaries. Arrowheads indicate OSE in A and L; arrows in B, E, F and H indicate precancerous lesions. Bars: 50 µm.
Expression of the proliferation marker, phospho-histone H3 (pH3), was examined to determine whether abnormal proliferation was observed in cells with dysregulated β-catenin and showed that precancerous lesions with nuclear β-catenin accumulation had a very low proliferative index at 4 weeks compared with the surrounding tissue of mutant mice (Figure 3G and H). Increased PTEN and P27kip1 expression, combined with the lower proliferation rate of pretumoral lesions and the loss or suppression of PTEN expression accompanied by a higher proliferation rate in fully developed tumors (Figure 3L and M), suggests that β-catenin-induced expression of PTEN suppresses progression of tumor development in Apccko ovaries. Although precancerous lesions are present in 4–6-week-old Apccko ovaries, cancer development took up to 11 months, indicating that either another mechanism suppressing PTEN expression or PTEN mutation is required for progression.
β-Catenin activation or APC suppression in HOSE cells causes formation of tumors with OEA characteristics
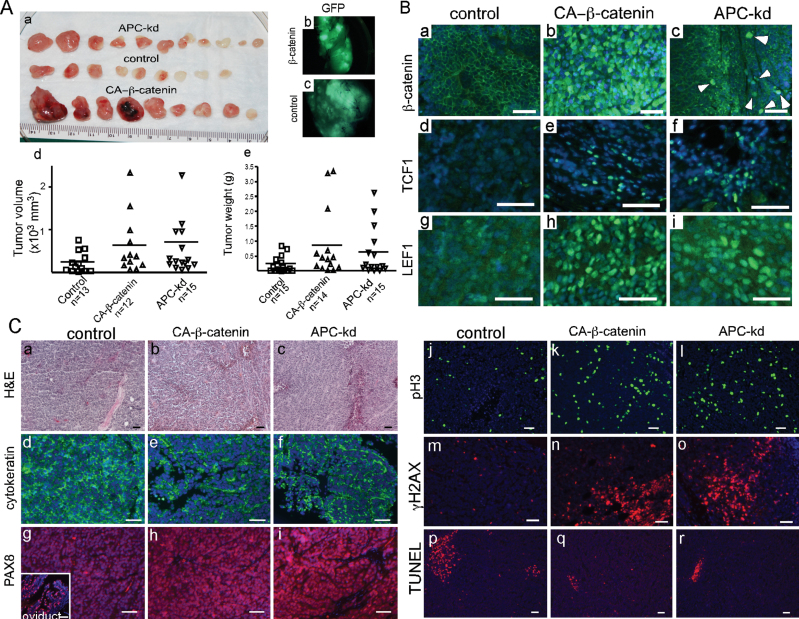
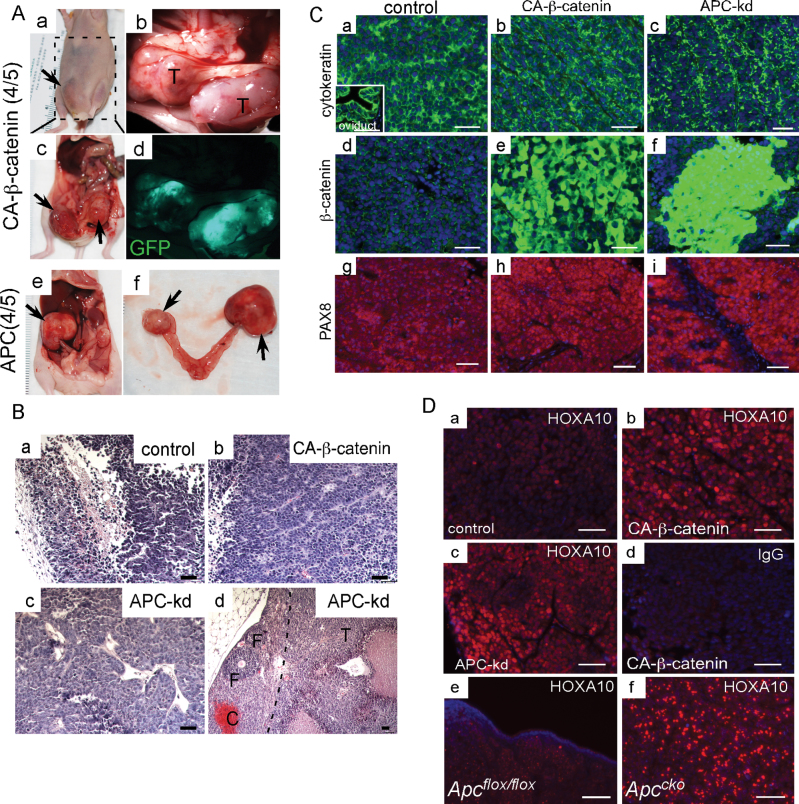
Because dysregulated Wnt signaling is associated with development of human OEAs (4,10), we investigated the role of Wnt signaling in HOSE cells, an immortalized human cell line used as a surrogate for primary ovarian surface epithelial cells (22). The HOSE cells were transduced with viral vectors either to express CA-β-catenin (23) or to suppress APC expression (Dana Farber/Harvard Cancer Center DNA Resource Core) (Supplementary Figure 5A, a–g, available at Carcinogenesis Online). HOSE cells appeared more epithelial with a cobblestone morphology 4 days after transduction with the CA-β-catenin and APC vectors (Supplementary Figure 5A, d–g, available at Carcinogenesis Online) but not with control vector (Supplementary Figure 5A, a–c, available at Carcinogenesis Online). To analyze the effects of CA-β-catenin expression and APC knockdown on the tumor-forming ability of these cells, 106 cells were combined with Matrigel and injected into the dorsal flanks of nude mice (2 site/mouse and minimum 15 sites each group; n = 8 mice/group). Four weeks after injection, tumors were harvested from the animals (Figure 4A, a). As expected, GFP was expressed in tumors derived from CA-β-catenin and control vector expressing cells (Figure 4A, b and c); the APC knockdown viruses do not express GFP and thus could not be similarly analyzed. The mean tumor volume and weight was higher in CA-β-catenin and APC tumors compared with control group (Figure 4A, d and e). One injection site each from control and CA-β-catenin group did not form grossly visible tumors (Figure 4A, d). All animals injected with HOSE cells transduced with APC knockdown shRNA developed tumors (Figure 4A).
Fig. 4.
Xenotransplants of HOSE cells with either CA-β-catenin or APC knockdown. HOSE cells were transduced with control virus or, virus to express CA-β-catenin or to knockdown (kd) expression of APC. Gross tumors harvested 4 weeks after subcutaneous injection of the transduced HOSE cells into the dorsal flanks of nude mice (A, a). Direct GFP fluorescence in control and CA-β-catenin tumors (A, b and c). Tumor volume and weight in three different groups of tumors (A, d and e). Expression of β-catenin, TCF1 and LEF1 was detected by IF in tumors as indicated (B). Histological and IF (cytokeratin, PAX8, pH3, γ-H2AX and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) examination of tumors (C). Inset in panel C, g, is a positive control for PAX8 staining in mouse oviduct. DAPI staining was used to indicate nuclei. Bars: 50 µm.
Next, we examined the status of WNT signaling in these tumors by analyzing the expression of β-catenin and its downstream targets (TCF1 and LEF1) (Figure 4B). As predicted by the observed increase in nuclear β-catenin accumulation, which is indicative of activated WNT signaling, increased expression of TCF1 and LEF1 was observed in CA-β-catenin and APC tumors but not in controls (Figure 4B). Histological examination revealed that tumors formed from HOSE cells with either CA-β-catenin or APC knockdown showed features similar to human OEAs and also shared histological characteristics with tumors formed after subcutaneous injections of AN3CA, a human endometrial cancer cell line (Figure 4C, a–c and Supplementary Figure 5B, available at Carcinogenesis Online). Similar to human OEAs, these tumors were positive for cytokeratin and PAX8 (markers for human ovarian carcinomas (31); Figure 4C, d–i). Additionally, increased proliferation, DNA damage and decreased cell death examined by pH3 staining, γ-H2AX staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling, respectively, were observed in CA-β-catenin and APC knockdown tumors compared with controls (Figure 4C, j–r). None of the tumors derived from control vector-transduced HOSE cells showed typical OEA histology (Figure 4C, a).
In human patients, ovarian cancer is a peritoneal disease. To mimic conditions of orthotopic tumor progression of human ovarian cancer patients, we injected 3×106 transduced HOSE cells intraperitoneally per mouse (n = 5 mice/group). Visibly enlarged tumors were observed 5 weeks after injection in 80% (8/10) of mice injected with either CA-β-catenin or APC knockdown HOSE cells, often attached to the bowel (Figure 5A and Supplementary Figure 5C, available at Carcinogenesis Online). In comparison, only one small tumor was discovered in mice injected with control vector-transduced HOSE cells (data not shown). CA-β-catenin tumors were GFP positive (Figure 5A, c and d), indicating that they were derived from the transduced cells. Interestingly, 50% (2/4) of the tumors in mice injected with APC knockdown HOSE cells developed in ovaries of the nude mice (Figure 5A, e and f), suggesting that these cells preferentially target the ovary, which was not observed in either CA-β-catenin or control HOSE-injected mice. Histological examination of tumors showed that CA-β-catenin and APC knockdown HOSE tumors are similar to tumors developed after subcutaneous injections (Figure 4) and human OEAs (Figure 5B, b–d). The control HOSE tumor was largely cystic (Figure 5B, a). Cytokeratin staining confirmed the epithelial origin of these tumors (Figure 5C, a–c). Similar to subcutaneous tumors (Figure 4), nuclear accumulation of β-catenin was only observed in CA-β-catenin and APC knockdown HOSE tumors (Figure 5C, d–f), and like human OEAs, these tumors were also positive for PAX8 (Figure 5C, g–i).
Fig. 5.
Tumor development with intraperitoneal injection of transduced HOSE cells. (A) Gross analysis of tumors formed after intraperitoneal injections of CA-β-catenin (a–d) and APC knockdown (e–f) HOSE cells. CA-β-catenin tumors were GFP positive (d). Histology of control (B, a), CA-β-catenin (B, b) and APC knockdown (B, c and d) tumors. In panel B, d the black dotted line demarcates tumor area of cells that targeted the normal ovary of nude mice; follicles (F). Localization of cytokeratin, β-catenin and PAX8 in tumors from all three groups of animals by IF (C). Mouse oviduct was used as positive control for cytokeratin localization (inset in panel C, a). HOXA10 protein expression in control (D, a), CA-β-catenin (D, b) and APC knockdown (D, c) HOSE tumors. (D, e and f) HOXA10 expression in Apccko ovarian tumors and controls. (D, d) Negative control for HOXA10-specific staining with an equal amount of non-immune IgG. Nuclei are stained with DAPI. Bars: 50 µm.
During embryonic development, segmental expression of the HOXA homeobox gene cluster is thought to play a central role in differentiation of the embryonic Müllerian duct into the female reproductive tract. For example, HOXA10 expression appears to drive uterine differentiation and its deletion leads to homeotic transformation of the uterus to a more oviductal phenotype (32). WNT signaling is a known inducer of HOX genes and tightly regulated Wnt signaling is required for proper patterning of the female reproductive tract (32). Additionally, forced expression of HOXA10 in mouse OSE causes endometrioid-like differentiation (16). Since the OEA histotype closely resembles the uterine endometrium, we examined expression of HOXA10 in both orthotopic and ectopic tumors with either CA-β-catenin or APC knockdown (Figure 5D, a–d), as well as in Apccko tumors (Figure 5D, e and f), and observed increased expression of HOXA10 compared with controls, suggesting that dysregulated Wnt signaling contributes to the formation of OEAs partially by inducing aberrant expression of HOX genes.
Discussion
Ovarian cancer is broadly classified into five different categories on the basis of histological features: serous, endometrioid, mucinous, clear cell and undifferentiated, with the serous, endometrioid and mucinous subtypes so named because of their histological similarity to the oviductal, endometrial, and cervical epithelia, respectively (1). Mutated OSE, oviductal epithelial and endometrial epithelial cells have all been proposed as possible cells of origin of different ovarian cancer subtypes (15,33–35). Studies in various ovarian cancer mouse models have shown that specific genetic alterations in OSE cells lead to the development of ovarian carcinomas resembling serous, endometrioid and mucinous subtypes (4,10,14,16,24,36–38). For example, activation of KRAS and loss of PTEN in OSE cells lead to the formation of poorly differentiated ovarian serous papillary adenocarcinomas and endometrioid adenocarcinomas in two different mouse models using adenoviral- and Amhr2-driven Cre recombinase (24,36). Similarly, overexpression of HOXA9, 10 and 11 in ovarian epithelial tumors is associated with serous, endometrioid and mucinous characteristics, respectively (16). These studies collectively support that OSE cells are capable of forming ovarian carcinomas with Müllerian epithelial characteristics. Our studies reported here showing development of the inclusion cysts in the OSE also support the theory that OEAs are derived from the OSE. However, the other suspected origins cannot be ruled out, particularly since we have shown previously that older APCcko mice also develop endometrial hyperplasia that can sometimes progress to endometrial cancer (20). Therefore, we are unable to completely rule out an endometrial origin for OEAs in this model. In humans, double primary carcinoma of the ovary and endometrium is relatively rare, approximately 3% of each (39), a compelling rationale for further investigation of the APCcko mice to determine whether bilateral oophorectomy or hysterectomy can interfere with either endometrial or ovarian carcinogenesis, respectively.
Unlike other ovarian cancer histotypes, defective WNT signaling is commonly observed in human OEAs (1), suggesting that WNT signaling plays an important role in initiation and/or progression of this type of cancer. In a previous study, only compound loss of APC and PTEN generated OEAs (10). However, no tumor formation was observed in that study by deletion of either APC or PTEN alone, and the contribution of the individual mechanisms disrupted by mutation of each of these genes to development of OEAs is unknown (10). The activation of WNT signaling by deleting exon 3 of β-catenin with PTEN loss in mouse ovary also caused formation of tumors with mesenchymal characteristics (11), which further confounded the role of this pathway in OEAs. In Figure 1, we show that deletion of APC alone can lead to the development of OEAs in older mutant mice. The precancerous lesions in younger mutant ovaries (4–6 week old) overexpress PTEN (Figure 3) and are composed of very few proliferating cells. We hypothesize that the increased level of PTEN in the early lesions, which we show is associated with elevated nuclear β-catenin activity, might be suppressing progression of the tumors to a more cancerous phenotype. This would explain the formation of aggressive OEAs with 100% penetrance in combined APC/PTEN-deleted mice (10). The discrepancy between those results and those reported here could be because in the previous study, ovaries of the mutants were only examined from 30 to 42 weeks postnatal after Cre delivery and we report tumor development at 11 months, even though precancerous lesions were observed in younger ovaries. Alternatively, APC conditional deletion in the previous study was performed using adenoviral Cre injection into the ovarian intrabursal space of postpubertal mice. In our study, Amhr2-Cre is used, which is continuously expressed in the OSE from the embryonic period (40) through prepubertal ovaries and into adult ovaries (24), suggesting that both studies targeted OSE cells at different stages of development.
Although the major emphasis of this study is the early events in OEA tumorigenesis, it is important to note that classification of the later, high-grade tumors derived in the Apccko mice and in the xenotransplants, as well as those in the previously reported CA-β-catenin mice (4), as OEAs is not without its caveats. These tumors tend to be poorly differentiated and difficult to discern from high-grade serous cystadenocarcinomas (41). We have relied on the histology of the early or low-grade tumors to guide their classification as OEAs but we cannot rule out the possibility that these high-grade tumors might be serous cystadenocarcinomas. Additionally, whereas the preponderance of evidence from human serous cystadenocarcinomas and mouse studies suggests that neither dysregulated WNT/β-catenin signaling nor APC inactivation plays significant roles in serous ovarian cancer biology, they have been linked to a majority of OEAs (10,42,43). The lack of proper differentiation of the human cell lines after subcutaneous or intraperitoneal transplantation in immunocompromised mice could also be attributed to the lack of mesenchymal or fibroblast cells. A recent study showed that transplantation of a pure population of endometrial epithelial cells is unable to form endometrial epithelial glands (44). However, transplantation of equal numbers of epithelial and stromal cells leads to the proper differentiation of the epithelial cells and resulting tumors were similar to the endometrial adenocarcinoma. Since the stromal microenvironment is well known to play a role in cancer development, we avoided adding stromal cells in our model system with the HOSE cell line, fearing that it might complicate interpretation of our results. The contribution of the stromal cells to carcinogenesis in gynecological tissues is an active focus of our laboratory and we plan to explore the mechanisms involved in that process in future studies.
The interaction of WNT and PI3K signaling pathways has been shown to play an important role in carcinogenesis (4,10). For example, activation of β-catenin is observed in PTEN-null prostate cancer cell lines and restoration of wild-type expression of PTEN leads to increased phosphorylation and degradation of β-catenin (45). Overexpression of PTEN suppresses proliferation, reduces cellular β-catenin levels and decreases tumor formation in a WNT1-induced mammary cancer mouse model (46), suggesting that PTEN antagonizes WNT-induced tumor formation. In a previous study, we showed that sustained activation of β-catenin induces PTEN expression and contributes to a cellular senescence phenotype in OSE cells (4). Loss of PTEN combined with activation of β-catenin caused very aggressive ovarian tumor development with 100% penetrance (4). Lastly, mutations in WNT and PI3K signaling members have been prominently observed in human OEAs (9,43,47,48). However, the individual contributions of these two signaling pathways in ovarian carcinogenesis were not known. In this study, we show that APC loss alone can initiate development of OEAs, which is suppressed by activation of PTEN signaling (Figures 1 and 3). Although the loss of PTEN alone is unable to initiate cancer formation, it appears essential for progression of the disease.
We (49) and others (32, 50–52) have shown that WNT/β-catenin signaling is important for normal endometrial differentiation and can induce OEA when dysregulated (4,10,42,43,53); however, the mechanisms involved are not well understood. Induction of HOXA10 expression has also been shown to be important for normal endometrial differentiation (32,51,54) and OEA differentiation (16). Here, we have linked induced β-catenin expression with induced HOXA10 expression during OEA differentiation (Figure 5). We attempted to determine whether that link was direct or indirect by assaying the expression of CDX2 and CDX4, both of which have been shown to induce HOXA10 and are themselves induced by β-catenin (55,56). No changes in either CDX2 or CDX4 were observed in the tumors by IHC in the transduced HOSE cells by western analyses (data not shown). In our future studies, we will investigate whether other possible indirect mechanisms are involved or whether β-catenin directly induces HOXA10 at the transcriptional level.
In summary, we have shown that deletion of the APC gene is sufficient to initiate the formation of OEAs. The increase in PTEN protein levels and lower proliferation were observed in precancerous lesions present in younger (4–6 week old) ovaries, suggesting that PTEN suppresses early tumor progression in Apccko ovaries. Because of previously reported functional and physiological differences in rodent and human OSE, we have also developed ectopic and orthotopic xenograft models to study human OEAs by altering WNT signaling in HOSE cells (Figures 4 and 5), the first such report of OEAs developing from human OSE cells. We also showed that dysregulated WNT/β-catenin signaling is associated with induced HOXA10 expression, a likely mechanism driving OEA histotype differentiation. We are planning to use these model systems to determine the molecular details of those mechanisms leading to OEA development and progression with the hope that we can identify novel therapeutic targets for better management of human OEA patients.
Supplementary material
Supplementary Tables I and II and Figures S1–S5 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (HD052701) and Vincent Center Memorial Funds to J.M.T.
Supplementary Material
Acknowledgements
We would like to acknowledge the generosity of Drs Richard Behringer and Ronny Drapkin for supplying the Amhr2-Cre mice and the immortalized HOSE cells, respectively.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- APC
adenomatous polyposis coli
- CA-β-catenin
constitutively activated β-catenin
- CK8
cytokeratin 8
- DAPI
4′,6-diamidino-2-phenylindole
- GFP
green fluorescent protein
- HOSE
human ovarian surface epithelial
- IF
immunofluorescence
- IHC
immunohistochemistry
- LEF1
lymphoid enhancer factor 1
- OEA
ovarian endometrioid adenocarcinoma
- OSE
ovarian surface epithelium
- pH3
phospho-histone H3
- TCF1
T-cell factor 1.
References
- 1. Cho K.R, et al. (2009). Ovarian cancer. Annu. Rev. Pathol., 4, 287–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bast R.C, Jr, et al. (2009). The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer, 9, 415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aoki K, et al. (2007). Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J. Cell Sci., 120, 3327–3335 [DOI] [PubMed] [Google Scholar]
- 4. Tanwar P.S, et al. (2011). Mammalian target of rapamycin is a therapeutic target for murine ovarian endometrioid adenocarcinomas with dysregulated Wnt/β-catenin and PTEN. PLoS ONE, 6, e20715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goss K.H, et al. (2000). Biology of the adenomatous polyposis coli tumor suppressor. J. Clin. Oncol., 18, 1967–1979 [DOI] [PubMed] [Google Scholar]
- 6. Hu P.J, et al. (2012). Ovarian steroid cell tumor with biallelic adenomatous polyposis coli inactivation in a patient with familial adenomatous polyposis. Genes Chromosomes Cancer, 51, 283–289 [DOI] [PubMed] [Google Scholar]
- 7. Galle T.S, et al. (1999). Causes of death in familial adenomatous polyposis. Scand. J. Gastroenterol., 34, 808–812 [DOI] [PubMed] [Google Scholar]
- 8. Kulkarni K.P, et al. (2011). Familial adenomatous polyposis with concurrent metastatic appendiceal carcinoid and ovarian cystoadenoma. Pediatr. Blood Cancer, 57, 702–703 [DOI] [PubMed] [Google Scholar]
- 9. Catasus L, et al. (2004). Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum. Pathol., 35, 1360–1368 [DOI] [PubMed] [Google Scholar]
- 10. Wu R, et al. (2007). Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell, 11, 321–333 [DOI] [PubMed] [Google Scholar]
- 11. Lague M.N, et al. (2008). Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis, 29, 2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. You S, et al. (2006). Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int. J. Exp. Pathol., 87, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang S, et al. (2009). Expression of activated PIK3CA in ovarian surface epithelium results in hyperplasia but not tumor formation. PLoS ONE, 4, e4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mullany L.K, et al. (2011). Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene, 30, 3522–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Auersperg N, et al. (2001). Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr. Rev., 22, 255–288 [DOI] [PubMed] [Google Scholar]
- 16. Cheng W, et al. (2005). Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat. Med., 11, 531–537 [DOI] [PubMed] [Google Scholar]
- 17. Lane D.B, et al. (2004). HOXA10 expression in endometrial adenocarcinoma. Tumour Biol., 25, 264–269 [DOI] [PubMed] [Google Scholar]
- 18. Jamin S.P, et al. (2002). Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat. Genet., 32, 408–410 [DOI] [PubMed] [Google Scholar]
- 19. Kuraguchi M, et al. (2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet., 2, e146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanwar P.S, et al. (2011). Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer Res., 71, 1584–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanwar P.S, et al. (2010). Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol. Reprod., 82, 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drapkin R, et al. (2005). Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res., 65, 2162–2169 [DOI] [PubMed] [Google Scholar]
- 23. Reya T, et al. (2003). A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature, 423, 409–414 [DOI] [PubMed] [Google Scholar]
- 24. Fan H.Y, et al. (2009). Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res., 69, 6463–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oshima M, et al. (1995). Evidence against dominant negative mechanisms of intestinal polyp formation by Apc gene mutations. Cancer Res., 55, 2719–2722 [PubMed] [Google Scholar]
- 26. Faux M.C, et al. (2004). Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J. Cell Sci., 117, 427–439 [DOI] [PubMed] [Google Scholar]
- 27. Maeda D, et al. (2011). β-Catenin (CTNNB1) S33C mutation in ovarian microcystic stromal tumors. Am. J. Surg. Pathol., 35, 1429–1440 [DOI] [PubMed] [Google Scholar]
- 28. Ohishi Y, et al. (2011). Nuclear localization of E-cadherin but not beta-catenin in human ovarian granulosa cell tumours and normal ovarian follicles and ovarian stroma. Histopathology, 58, 423–432 [DOI] [PubMed] [Google Scholar]
- 29. Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 30. Harada N, et al. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J., 18, 5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laury A.R, et al. (2011). A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol., 35, 816–826 [DOI] [PubMed] [Google Scholar]
- 32. Miller C, et al. (1998). Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development, 125, 3201–3211 [DOI] [PubMed] [Google Scholar]
- 33. Crum C.P, et al. (2007). Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin. Med. Res., 5, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karst A.M, et al. (2010). Ovarian cancer pathogenesis: a model in evolution. J. Oncol., 2010, 932371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dubeau L. (2008). The cell of origin of ovarian epithelial tumours. Lancet Oncol., 9, 1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dinulescu D.M, et al. (2005). Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med., 11, 63–70 [DOI] [PubMed] [Google Scholar]
- 37. Connolly D.C, et al. (2003). Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res., 63, 1389–1397 [PubMed] [Google Scholar]
- 38. Flesken-Nikitin A, et al. (2003). Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res., 63, 3459–3463 [PubMed] [Google Scholar]
- 39. Chiang Y.C, et al. (2008). Synchronous primary cancers of the endometrium and ovary. Int. J. Gynecol. Cancer, 18, 159–164 [DOI] [PubMed] [Google Scholar]
- 40. Tanwar P.S, et al. (2009). Constitutive activation of beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol. Reprod., 81, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roh M.H, et al. (2010). High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod. Pathol., 23, 1316–1324 [DOI] [PubMed] [Google Scholar]
- 42. Cho K.R. (2009). Ovarian cancer update: lessons from morphology, molecules, and mice. Arch. Pathol. Lab. Med., 133, 1775–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palacios J, et al. (1998). Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res., 58, 1344–1347 [PubMed] [Google Scholar]
- 44. Memarzadeh S, et al. (2010). Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc. Natl Acad. Sci. U.S.A., 107, 17298–17303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Persad S, et al. (2001). Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J. Cell Biol., 153, 1161–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao H, et al. (2005). Overexpression of the tumor suppressor gene phosphatase and tensin homologue partially inhibits wnt-1-induced mammary tumorigenesis. Cancer Res., 65, 6864–6873 [DOI] [PubMed] [Google Scholar]
- 47. Wright K, et al. (1999). Beta-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int. J. Cancer, 82, 625–629 [DOI] [PubMed] [Google Scholar]
- 48. Wu R, et al. (2001). Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res., 61, 8247–8255 [PubMed] [Google Scholar]
- 49. Arango N.A, et al. (2005). Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol., 288, 276–283 [DOI] [PubMed] [Google Scholar]
- 50. Deutscher E, et al. (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev. Biol., 307, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mericskay M, et al. (2004). Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development, 131, 2061–2072 [DOI] [PubMed] [Google Scholar]
- 52. Parr B.A, et al. (1998). Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature, 395, 707–710 [DOI] [PubMed] [Google Scholar]
- 53. Moreno-Bueno G, et al. (2002). Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene, 21, 7981–7990 [DOI] [PubMed] [Google Scholar]
- 54. Taylor H.S, et al. (1997). A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol. Reprod., 57, 1338–1345 [DOI] [PubMed] [Google Scholar]
- 55. Bei L, et al. (2011). HoxA10 activates CDX4 transcription and Cdx4 activates HOXA10 transcription in myeloid cells. J. Biol. Chem., 286, 19047–19064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pilon N, et al. (2006). Cdx4 is a direct target of the canonical Wnt pathway. Dev. Biol., 289, 55–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.