Abstract
The highly lethal nature of pancreatic cancer and the increasing recognition of high-risk individuals have made research into chemoprevention a high priority. Here, we tested the chemopreventive activity of δ-tocotrienol, a bioactive vitamin E derivative extracted from palm fruit, in the LSL-KrasG12D/+;Pdx-1-Cre pancreatic cancer mouse model. At 10 weeks of age, mice (n = 92) were randomly allocated to three groups: (i) no treatment; (ii) vehicle and (iii) δ-tocotrienol (200mg/kg × 2/day, PO). Treatment was continued for 12 months. Mice treated with δ-tocotrienol showed increased median survival from the onset of treatment (11.1 months) compared with vehicle-treated mice (9.7 months) and non-treated mice (8.5 months; P < 0.025). Importantly, none of the mice treated with δ-tocotrienol harbored invasive cancer compared with 10% and 8% in vehicle-treated and non-treated mice, respectively. Furthermore, δ-tocotrienol treatment also resulted in significant suppression of mouse pancreatic intraepithelial neoplasm (mPanIN) progression compared with vehicle-treated and non-treated mice: mPanIN-1: 47–50% (P < 0.09), mPanIN-2: 6–11% (P < 0.001), mPanIN-3: 3–15% (P < 0.001) and invasive cancer: 0–10% (P < 0.001). δ-Tocotrienol treatment inhibited mutant Kras-driven pathways such as MEK/ERK, PI3K/AKT and NF-kB/p65, as well as Bcl-xL and induced p27. δ-Tocotrienol also induced biomarkers of apoptosis such as Bax and activated caspase 3 along with an increase in plasma levels of CK18. In summary, δ-tocotrienol’s ability to interfere with oncogenic Kras pathways coupled with the observed increase in median survival and significant delay in PanIN progression highlights the chemopreventative potential of δ-tocotrienol and warrants further investigation of this micronutrient in individuals at high risk for pancreatic cancer.
Introduction
Recent advances in clinical oncology and molecular genetics have uncovered chemoprevention opportunities for pancreatic cancer. Most pancreatic cancers arise from the stepwise progression of precursor lesions called pancreatic intraepithelial neoplasms (PanINs), most often initiated by mutations in the Kras oncogene (1,2). Cohorts of individuals who are at significant risk for developing pancreatic cancer have been identified, including those with several first-degree relatives afflicted with the disease (3), individuals with specific genetic mutations such as BRCA2 (4,5) and individuals with premalignant neoplastic cysts in the pancreas (6,7). A new study indicates a long latent phase (more than 12 years) from initiation of a pancreatic tumor to clinical symptoms, allowing ample time to deliver chemopreventative and therapeutic agents (8).
Kras mutations are prevalent (55–60%) in human pancreatic cancer as per Cosmic database (http://www.sanger.ac.uk/genetics/CGP/cosmic/), and mutant Kras has been shown to be required for the initiation and maintenance of pancreatic cancer in animal models (9,10). Furthermore, Kras mutations are associated with poor prognosis and shorter patient survival time (11,12), and tumors harboring Kras are resistant to chemotherapy and antisignaling agents (13,14 ).
Preclinical evaluation of putative chemoprevention agents has been facilitated by the development of genetically engineered mouse models, in which mouse PanINs (mPanINs) can be initiated by oncogenic Kras. One mouse model, KrasG12D, first described by Hingorani et al. (15), has been valuable for studying pancreatic carcinogenesis and its prevention. Targeted endogenous expression of oncogenic KrasG12D to progenitor cells of the murine pancreas during development recapitulates the genetic and histomorphologic aspects of human pancreatic carcinogenesis. In this model, LSL-KrasG12D mice, in which KrasG12D mutation is silenced by an upstream floxed STOP cassette, are crossed with Pdx-1-Cre mice, which express Cre recombinase under the pancreas-specific promoter Pdx-1. Cre recombinase-mediated excision of the STOP cassette in pancreatic progenitor cells leads to physiological expression of KrasG12D in virtually all mature pancreatic cell lineages. Consistent with human pancreatic cancer development, LSL-KrasG12D/+;Pdx-1-Cre mice develop early mPanIN lesions, which progress to advanced mPanINs and eventually to pancreatic cancer. The significance of this model to human pancreatic cancer is further validated by the fact that anticancer drugs that are ineffective in humans are also inactive in this model (16,17) in contrast to the nude-mouse pancreatic xenograft models in which these agents are active (18).
δ-Tocotrienol, a major bioactive compound found in cereal grains, oats, barley, annatto beans and palm (19), is one of eight lipid-soluble natural vitamin E compounds. Extensive in vitro and in vivo studies have indicated that δ-tocotrienol is a cancer-suppressing bioactive micronutrient that inhibits cell proliferation and induces tumor cell apoptosis. Its anticancer effects have been demonstrated in various cancers, including pancreatic cancer (20–24). Potential mechanisms of δ-tocotrienol anticancer activity include inhibition of oncogenic Ras activation via inhibition of its prenylation through inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase (24–26) and inhibition of downstream oncogenic Ras signaling targets such as NF-kB (22). Here, we used the above-described conditional KrasG12D model to show that δ-tocotrienol increases median survival and delays PanIN progression, demonstrating the chemopreventative potential of this micronutrient.
Materials and methods
Reagents and animals
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. δ-Tocotrienol was obtained from Davos Life Science Ltd (Helios, Singapore). LSL-KrasG12D and PDX-1-Cre mice were obtained from the National Cancer Institute Mouse Models of Human Cancers Consortium (Frederick, MD). All animal studies were approved by our Institutional Animal Care and Use Committee, following the guidelines of the American Association for the Assessment and Accreditation of Laboratory Animal Care.
Conditional KrasG12D mouse model
LSL-KrasG12D and PDX-1-Cre mice were maintained as heterozygous lines and crossed and bred in our institutional vivarium. Tail snips, harvested from offspring of LSL-KrasG12D and PDX-1-Cre mice, were digested overnight and genomic DNA was extracted and estimated using the DNAeasy kit (Qiagen, Gaithersburg, MD), per manufacturer’s instructions.
Genotyping analysis
We used the following PCR primer sequences to detect PDX-1-Cre and LSL-KrasG12D (Integrated DNA Technologies, Coralville, IA): PDX-forward = 5′-CTGGACTACATCTTGAGTTGC-3′, PDX-reverse = 5′-GGTGTACGGTCAGTAAATTTG-3′, Kras-forward = 5′-AGGTAGC CACCATGGCTTGAGTAAGTCTGCA-3′ and Kras-reverse = 5′-CCTTTACA AGCGCACGCAGACTGTAGA-3′. PCR buffer (25 µl) contained 2.0mM MgCl2, 0.2mM deoxynucleoside triphosphate mix, 0.025U/µl DNA Taq polymerase, 0.5 µM primers and 50ng DNA. The reaction was carried out in a PTC-200 thermal cycler [preheat at 94°C for 3min, 35 cycles of (30 s at 94°C, 30 s at 60°C, 30 s at 72°C) and 72°C for 3 min]. PCR products were mixed with 5 µl of loading dye and separated on a 2% agarose gel containing ethidium bromide. The electrophoresis was run for 1h at 100V using Tris base, acetic acid and ethylenediaminetetraacetic acid (TAE) buffer. A 650-bp product (PDX) and a 550-bp product (Kras) were identified using DNA ladder and bands were imaged using AlphaImage analysis.
Drug treatments
LSL-KrasG12D/+;Pdx-1-Cre mice were randomized as follows: (i) no treatment control (n = 34); (ii) vehicle (ethanol-extracted olive oil, 1.0ml/kg twice a day by oral gavage; n = 27) and (iii) δ-tocotrienol (200mg/kg twice a day by oral gavage; n = 31), with treatment started at 10 weeks of age and continued for 12 months. The δ-tocotrienol dose was chosen based on reports published previously (22,23). Mouse body weights were recorded twice weekly, mortality was noted and survival curves were plotted. After 12 months of treatment, animals were euthanized and blood was collected in heparin vials, with the entire pancreas harvested. The pancreatic head, neck, body and tail were separated and fixed in buffered formalin for further analyses. Other pancreatic tissues were snap frozen in liquid nitrogen and kept at -80°C for protein extraction and western blot analysis.
Histologic evaluation
Formalin-fixed, paraffin-embedded tissues were sectioned (4 µm) and stained with hematoxylin-eosin. About 10 sections (100 µm apart) from each tissue specimen were evaluated histologically by a single pathologist (B.A.C.) blinded to the experimental groups. mPanIN lesions were classified according to histopathologic criteria as recommended elsewhere (15,27). A representative cross-section of a duct (duct profile) within one lobule was counted as one duct. Care was taken to count structures showing only ductal morphology to prevent counting acinar to ductal metaplasia. To quantify mPanIN lesion progression, the total number of ductal lesions and their grade were determined and the count from one section was used for the analyses. About 100–140 pancreatic ducts of the entire fixed specimen (pancreatic head, neck, body and tail) were analyzed for each animal, with relative proportion of each mPanIN lesion to the overall number of analyzed ducts recorded for each animal.
Immunohistochemistry
Immunohistochemistry was performed using the Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) per manufacturer’s protocol with proprietary reagents. Briefly, slides were deparaffinized on the automated system with EZ Prep solution. Sections were heated for antigen retrieval. For immunohistochemistry, tissue sections were incubated with anticaspase 3 (no. 9661, Cell Signaling, Danvers, MA) at 1:4000 dilution for 60min, antiphosphorylated MEK (pMEK) (no. 2338, Cell Signaling) at 1:100 dilution for 32min, antiphosphorylated ERK (pERK) (no. 4376, Cell Signaling) at 1:200 dilution for 32min and p27 (no. E2604, Spring BioScience, Pleasanton, CA) at 1:250 dilution for 60min. Detection was performed using the Ventana OmniMap kit.
Assessment of immunohistochemical expression
All stained tissues were examined by one independent observer (B.A.C.). Caspase 3-stained tissues were assessed for expression in non-neoplastic areas and mPanIN. Percent expression was recorded for each area and then averaged for each mouse. Intensity of staining in pERK- and pMEK-stained tissue cores was assessed as 0 (absent), 1+ (weak), 2+ (moderate) and 3+ (strong). Percentage of cells expressing pERK and pMEK was also recorded for each duct counted, with each duct then assigned a score comprising product of the intensity and the percentage of positive cells. Scores were averaged for each area for each mouse. Percentage of p27 expression, expressed both in perinuclear and in nuclear locations, was recorded for each mouse within each duct, with locations noted and averages rendered for each area. In addition, a low-power assessment of degree of overall staining intensity was assessed.
CK18 enzyme-linked immunosorbent assay
Heparinized blood from mice was centrifuged at 5000 r.p.m. for 5min, and plasma was carefully isolated and stored at -80°C until analysis. The apoptosis marker cytokeratin-18 (CK18) was assayed using the M30-Apoptosense ELISA kit (PEVIVA, Bromma, Sweden).
Western blot analysis
Proteins were extracted from pancreatic tumor tissues using M-PER lysis buffer containing protease inhibitors/ethylenediaminetetraacetic acid (Thermo Scientific, Rockford, IL). Extracted proteins (40 µg) were resolved on 12.5% sodium dodecyl sulfate–polyacrylamide running gel and 5% stacking gel. Proteins were then electrotransferred onto nitrocellulose membranes. After membranes were blocked in 5% non-fat powdered milk for 1h, they were washed and treated with antibodies to PARP1, NF-κB/p65, Bcl-XL, Bax, p27Kip1 and β-actin (1:1000) overnight at 4°C (Santa Cruz Biotechnology, Santa Cruz, CA, and Cell Signaling). After washing, blots were incubated with horseradish peroxidase–conjugated secondary antibody IgG (1:5000) for 1h at room temperature. The washed blot was then treated with SuperSignal West Pico chemiluminescent substrate (Pierce) for positive antibody reaction. Membranes were exposed to Kodak X-ray film for visualization and densitometric quantization of protein bands using AlphaEaseFC software (Alpha Innotech).
Statistical analysis
For continuous variables (e.g. body weight gain, number of normal ducts and protein expression), data (mean ± standard error mean) were analyzed statistically using one-way analysis of variance (ANOVA) with Duncan’s multiple range tests for pairwise comparison among treatment groups using SAS statistical software. Kaplan–Meier method and log-rank test were used to generate survival curves and test their difference. Bonferroni method was used to correct P values for pairwise comparisons.
Results and discussion
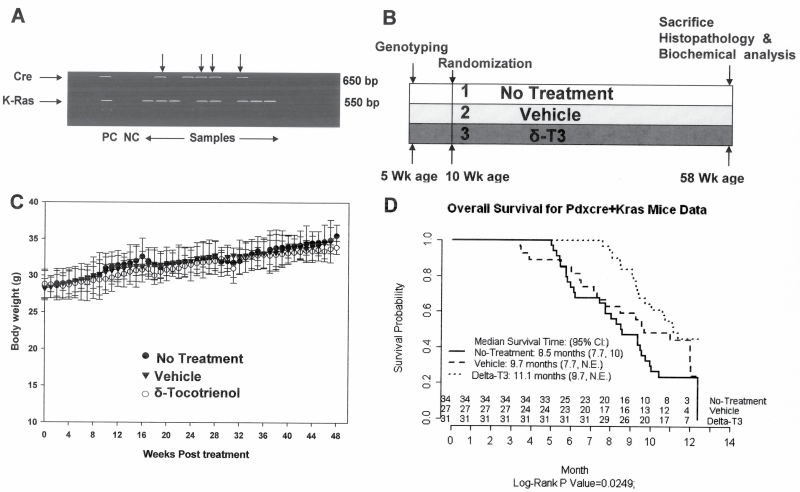
Although the antitumor activity of δ-tocotrienol has been previously reported, its chemopreventive potential for pancreatic cancer is not known. In this study, we evaluated the effects of this micronutrient on the development and progression of pancreatic tumors in a mouse pancreatic cancer model that is driven by mutant Kras and that faithfully recapitulates human pancreatic carcinogenesis. LSL-KrasG12D/+;Pdx-1-Cre mice were randomized to non-treated, vehicle-treated and δ-tocotrienol-treated groups (Figure 1B). Genotypes were confirmed by analyses of genomic DNA (Figure 1A), and no differences in body weight among all three groups were observed during the entire study period (Figure 1C). Mice were killed when they developed symptoms of terminal pancreatic cancer such as cachexia, abdominal distension and/or labored breathing. Death predictably occurs within 24–72h after such symptoms appear (28). All of the mice were confirmed at necropsy to have pancreatic cancer. Survival analysis by log-rank test showed statistically significant differences in survival curves among the three groups (P = 0.025). Specifically, median survival beginning from start of treatment at 10 weeks of age was 11.1 months in mice treated with δ-tocotrienol, significantly longer than in controls (8.5 months; P = 0.016, after Bonferroni correction of P value for pairwise comparison; Figure 1D).
Fig. 1.
δ-Tocotrienol (δ-T3) increases median survival of Kras mice. (A) Genotyping of Pdx-1-Cre and KrasG12D offspring by PCR. Top arrows indicate double-positive genes. (B) Experimental design. (C) Body weights over 12 months by treatment group. Body weight gain was not significantly altered by δ-tocotrienol treatment (by ANOVA). Points, means; bars, standard error (n = 27–34). (D) Kaplan–Meier survival curves. Survival curves were significantly different among the three groups by log-rank test (P = 0.025). CI, confidence interval. Pairwise comparison with Bonferroni correction of P value showed significant survival increase by δ-tocotrienol treatment versus no treatment (P = 0.016).
Consistent with the above survival data, histopathological examination (Figure 2) showed that δ-tocotrienol-treated mice had a significant delay in progression of mPanIN lesions versus no treatment or vehicle-treated animals (P < 0.001). Only 24% of pancreatic ducts in control and 28% in vehicle-treated animals appeared normal, whereas 44% of pancreatic ducts in δ-tocotrienol-treated animals were normal. Whereas non-treated and vehicle-treated mice had on average 12–15% mPanIN-2 and mPanIN-3, δ-tocotrienol-treated mice had only 2–5%. Furthermore, δ-tocotrienol-treated mice harbored no invasive carcinoma, whereas non-treated and vehicle-treated mice had an average of 8% and 10%, respectively. Our results showed significant suppression of mPanIN progression and inhibition of carcinoma (mPanIN-1: 47–50%, P < 0.09; mPanIN-2: 6–11%, P < 0.001; mPanIN-3: 3–15%, P < 0.001; invasive cancer: 0–10%, P < 0.001) in δ-tocotrienol-treated mice versus that shown in controls (Figure 2B and C). To our knowledge, this is the first report showing that the genetically predetermined progression of mPanIN lesions to invasive cancer in the conditional KrasG12D mouse model can be attenuated by a natural vitamin.
Fig. 2.
δ-Tocotrienol delays PanIN progression to invasive cancer. (A) Histological representation (hemotoxylin–eosin) of mPanIN lesions in transgenic KrasG12D mice. NML, normal pancreatic ducts; InvCA, invasive cancer. (B) δ-Tocotrienol treatment significantly decreased progression of mPanIN-2 and mPanIN-3 lesions and increased number of normal ducts compared with no treatment or vehicle (*P < 0.05, **P < 0.001). Points, means; bars, standard error (n = 5–7). (C) Percentage of invasive carcinoma in each treatment group. Points, means; bars, standard error (n = 5–7). *P < 0.001 versus vehicle or no treatment groups. All statistical analyses were performed using ANOVA with Duncan test.
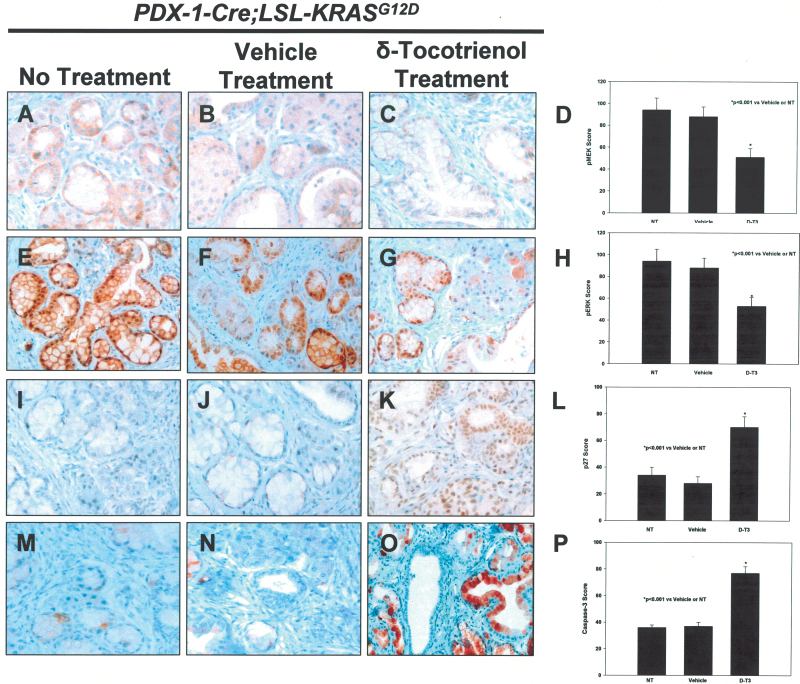
The ability of mutant Kras to induce malignant transformation probably depends on its ability to persistently activate downstream effectors such as Akt and MEK. We therefore reasoned that the ability of δ-tocotrienol to prolong median survival and delay PanIN progression may be mediated, at least in part, by interfering with these oncogenic pathways. To this end, we determined the effects of δ-tocotrienol on pMEK, pERK, phosphorylated-AKT (pAKT) and NF-kB using both immunohistochemistery and western blot approaches. mPanIN regions harbored high levels of pMEK and pERK in LSL-KrasG12D/+;Pdx-1-Cre control and vehicle-treated animals. In contrast, markedly decreased pMEK and pERK levels were observed in mice treated with δ-tocotrienol (Figure 3A–C and E–G). Staining intensities of pMEK and pERK were significantly reduced in δ-tocotrienol-treated animals compared with that shown in controls (P < 0.001; Figure 3D and H). Because it was shown previously that the activated Ras-Raf-MEK-ERK signaling pathway regulates the cell-cycle inhibitor p27Kip1 in pancreatic cancer cells (29), we evaluated the effect of δ-tocotrienol treatment on p27Kip1. Consistent with inhibition of the Raf-MEK-ERK signaling pathway, p27Kip1 was significantly induced in δ-tocotrienol-treated animals compared with controls (P < 0.001; Figure 3I–L). Western blots were performed to confirm the effects of δ-tocotrienol treatment on oncogenic Kras signaling pathways in the pancreatic tissues of the LSL-KrasG12D/+;Pdx-1-Cre mice. δ-Tocotrienol treatment significantly inhibited pAKT, pERK and NF-kB, which are well-known downstream effectors of oncogenic Kras (Figure 4A). Furthermore, consistent with inhibition of these effectors, downstream targets of pERK (i.e. p27Kip1) and those of NF-kB (i.e. Bax) were significantly induced by δ-tocotrienol treatment, whereas levels of the prosurvival protein Bcl-xL were decreased (Figure 4A and B). Earlier studies have also demonstrated that tocotrienol inhibited NF-kB-related pro-inflammatory cytokines (30) and Stat3 pathway (31), as well as induced cell-cycle inhibitor p21 expression (32). One of the established anticancer effects of δ-tocotrienol is the selective induction of apoptosis of neoplastic cells (22). We confirmed this effect in mPanIN lesions by observing more intense immunostaining of cleaved/activated caspase 3 in δ-tocotrienol-treated animals than in controls (P < 0.001, Figure 3M–P). Interestingly, we also detected significantly increased plasma levels of CK18, a surrogate marker of circulating apoptotic epithelial cells, in δ-tocotrienol-treated animals compared with that shown in controls (Figure 4C; P < 0.001), indicating that oral intake of δ-tocotrienol at 400mg/kg/day achieved tissue levels that were sufficient to induce apoptosis of epithelial cells that were shed into the circulation.
Fig. 3.
δ-Tocotrienol decreases pMEK and pERK levels and induces levels of p27Kip1 and activated caspase 3. Effect of no treatment (A), vehicle (B) and δ-tocotrienol (C) for 12 months on pMEK immunostaining in pancreatic tissues of LSL-KrasG12D/+;Pdx-1-Cre mice. (D) Semiquantitative analysis (histogram) shows δ-tocotrienol significantly inhibited pMEK compared with control (*P < 0.001). Effect of no treatment (E), vehicle (F) and δ-tocotrienol (G) for 12 months on pERK immunostaining. (H) δ-tocotrienol treatment significantly inhibited pERK versus control (*P < 0.001). Effect of no treatment (I), vehicle (J) and δ-tocotrienol (K) on p27Kip1 immunostaining. (L) δ-tocotrienol significantly (*P < 0.001) increased p27Kip1 levels versus control. Effect of no treatment (M), vehicle (N) and δ-tocotrienol (O) on cleaved caspase-3 immunostaining. (P) δ-tocotrienol treatment significantly (*P < 0.001) increased cleaved caspase-3 levels versus control. Bars (means) ± standard error (n = 5). All statistical analyses were performed using ANOVA with Duncan test.
Fig. 4.
δ-Tocotrienol decreases levels of pAKT, pMEK, pERK, NF-kB and Bcl-xL and increases levels of p27Kip1, Bax and CK18. (A) Western blot of pAKT, pMEK and pERK, p27Kip1, NF-κB (p65) and its associated gene product (Bcl-xL, an antiapoptotic protein), apoptosis markers (PARP1 cleavage) and Bax (a proapoptotic protein) in the pancreas of KrasG12D mice per treatment group over 12 months. δ-Tocotrienol treatment inhibited pAKT, pMEK, pERK, NF-κB and Bcl-xL expression and induced p27Kip1 and PARP1 cleavage and Bax expression compared with control. (B) δ-Tocotrienol significantly inhibited pAKT (*P < 0.048), pMEK (*P < 0.001) and pERK (*P < 0.001) expression and significantly induced p27Kip1 (*P < 0.001) in pancreatic tumor tissues versus control. Bars (means) ± SE (n = 3–5). (C) δ-Tocotrienol resulted in significantly increased CK18 (*P < 0.001) versus vehicle or no treatment. Bars (means) ± standard error (n = 5–7). All statistical analyses were performed using ANOVA with Duncan test.
Our data clearly suggest that δ-tocotrienol is a significant mediator of mutant Kras-induced pancreatic carcinogenesis, possibly by disruption of Kras signaling. Our observation is consistent with recent reports in which compounds that disrupt Kras-associated signaling pathways, such as the epidermal growth factor receptor inhibitor gefitinib (33) and the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin (34), delayed progression of mPanIN in transgenic mice in which oncogenic Kras expression in pancreatic ductal cells was driven by the p48-Cre promoter (34). Thus, strategies aimed at inhibiting oncogenic Kras signaling pathways may have tumor-preventative potential in pancreatic cancer development.
In conclusion, our study shows that oral intake of δ-tocotrienol delayed the progression of mPanIN lesions and ultimately decreased the incidence of invasive pancreatic cancer, thereby prolonging survival of LSL-KrasG12D/+;Pdx-1-Cre mice. A major concern in chemoprevention is potential toxicity associated with prolonged treatment. We show a new chemoprevention approach that specifically targets oncogenic Kras-transformed pancreatic cells for apoptosis using a vitamin without toxicity. Further studies using this model will elucidate whether this chemopreventive effect can be enhanced by coupling δ-tocotrienol with other bioactive food components or targeting agents. Translational studies in early phase clinical trials will validate the biomarkers of δ-tocotrienol activity that were observed in this study.
Funding
National Cancer Institute (1RO1 CA-129227-01A1).
Acknowledgement
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Conflict of Interest Statement: M. P. Malafa and S. M. Sebti have patent interest, which is licensed to BioGene Life Science, a Singapore based company. They have no ownership interest in BioGene. No potential conflicts of interest were disclosed by the other authors.
Glossary
Abbreviations:
- ANOVA
analysis of variance
- mPanIN
mouse pancreatic intraepithelial neoplasm
- pAKT
phosphorylated-AKT
References
- 1. Hong S.M, et al. (2012). Genome-wide somatic copy number alterations in low-grade PanINs and IPMNs from individuals with a family history of pancreatic cancer. Clin. Cancer Res., 18, 4303–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanda M, et al. (2012). Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology, 142, 730–733.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hruban R.H, et al. (2010). Update on familial pancreatic cancer. Adv. Surg., 44, 293–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skoulidis F, et al. (2010). Germline Brca2 heterozygosity promotes Kras(G12D) -driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell, 18, 499–509 [DOI] [PubMed] [Google Scholar]
- 5. Al-Sukhni W, et al. (2012). Identification of germline genomic copy number variation in familial pancreatic cancer. Hum. Genet., 131, 1481–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthaei H, et al. (2011). Cystic precursors to invasive pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol., 8, 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu J, et al. (2011). Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. U.S.A., 108, 21188–21193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canto M.I, et al. (2012). Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology, 142, 796–804; quiz e14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collins M.A, et al. (2012). Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest., 122, 639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ying H, et al. (2012). Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149, 656–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S.T, et al. (2011). Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol. Cancer Ther., 10, 1993–1999 [DOI] [PubMed] [Google Scholar]
- 12. Lee J, et al. (2007). Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer, 109, 1561–1569 [DOI] [PubMed] [Google Scholar]
- 13. Grana T.M, et al. (2002). Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer Res., 62, 4142–4150 [PubMed] [Google Scholar]
- 14. Kullmann F, et al. (2011). KRAS mutation in metastatic pancreatic ductal adenocarcinoma: results of a multicenter phase II study evaluating efficacy of cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line therapy. Oncology, 81, 3–8 [DOI] [PubMed] [Google Scholar]
- 15. Hingorani S.R, et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell, 4, 437–450 [DOI] [PubMed] [Google Scholar]
- 16. Olive K.P, et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science, 324, 1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Provenzano P.P, et al. (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21, 418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hertel L.W, et al. (1990). Evaluation of the antitumor activity of gemcitabine (2’,2’-difluoro-2’-deoxycytidine). Cancer Res., 50, 4417–4422 [PubMed] [Google Scholar]
- 19. Aggarwal B.B, et al. (2010). Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem. Pharmacol., 80, 1613–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji X, et al. (2012). Delta-tocotrienol suppresses Notch-1 pathway by upregulating miR-34a in nonsmall cell lung cancer cells. Int. J. Cancer, 131, 2668–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ling M.T, et al. (2012). Tocotrienol as a potential anticancer agent. Carcinogenesis, 33, 233–239 [DOI] [PubMed] [Google Scholar]
- 22. Husain K, et al. (2011). Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol. Cancer Ther., 10, 2363–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Husain K, et al. (2009). Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology, 83, 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hussein D, et al. (2009). d-δ-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas, 38, e124–e136 [DOI] [PubMed] [Google Scholar]
- 25. Song B.L, et al. (2006). Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J. Biol. Chem., 281, 25054–25061 [DOI] [PubMed] [Google Scholar]
- 26. Shin-Kang S, et al. (2011). Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic. Biol. Med., 51, 1164–1174 [DOI] [PubMed] [Google Scholar]
- 27. Funahashi H, et al. (2007). Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras(G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res., 67, 7068–7071 [DOI] [PubMed] [Google Scholar]
- 28. Hingorani S.R, et al. (2005). Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell, 7, 469–483 [DOI] [PubMed] [Google Scholar]
- 29. Gysin S, et al. (2005). Pharmacologic inhibition of RAF–>MEK–>ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res., 65, 4870–4880 [DOI] [PubMed] [Google Scholar]
- 30. Ahn K.S, et al. (2007). Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem., 282, 809–820 [DOI] [PubMed] [Google Scholar]
- 31. Kannappan R, et al. (2010). Gamma-Tocotrienol but not gamma-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J. Biol. Chem., 285, 33520–33528 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Samant G.V, et al. (2010). Anti-proliferative effects of gamma-tocotrienol on mammary tumour cells are associated with suppression of cell cycle progression. Cell Prolif., 43, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohammed A, et al. (2010). The epidermal growth factor receptor inhibitor gefitinib prevents the progression of pancreatic lesions to carcinoma in a conditional LSL-KrasG12D/+ transgenic mouse model. Cancer Prev. Res. (Phila)., 3, 1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohammed A, et al. (2012). Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int. J. Cancer, 131, 1951–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]