Abstract
Chemotherapy and molecularly targeted approaches represent two very different modes of cancer treatment and each is associated with unique benefits and limitations. Both types of therapy share the overarching limitation of the emergence of drug resistance, which prevents these drugs from eliciting lasting clinical benefit. This review will provide an overview of the various mechanisms of resistance to each of these classes of drugs and examples of drug combinations that have been tested clinically. This analysis supports the contention that understanding modes of resistance to both chemotherapy and molecularly targeted therapies may be very useful in selecting those drugs of each class that will have complementing mechanisms of sensitivity and thereby represent reasonable combination therapies.
Introduction
The past decades of research have led to a deep understanding of the intricate mechanisms that regulate tumor growth and development. However, the intrinsic and acquired resistance of tumors to drug treatment remains a fundamental challenge to improving patient outcome. Early efforts to develop effective anticancer drugs resulted in several chemotherapeutics. Although these could potently induce cell death in rapidly dividing cells, they did not effectively discriminate between tumor and normal cells. This necessitated reliance on a therapeutic window where efficacy was maximized, whereas non-tumor toxicity was minimized. For patients with some types of cancer, such as pediatric acute lymphoblastic leukemia, intensive combinations of cytotoxic chemotherapies resulted in cures (1). However, for most cancer patients, these combination approaches have proven to be too toxic or insufficiently effective to warrant their use.
Advances in genomics have ushered in a new era of ‘targeted’ cancer therapies that offers hope to the majority of cancer patients for whom intensive cytotoxic chemotherapy is unlikely to produce a cure. Discovery of the distinct molecular characteristics of cancer cells that make them unique from normal cells has led to the emergence of therapies that selectively target these cancer-related genetic lesions, mostly without the major chemotherapy-induced toxicities. However, it has become apparent that drug resistance to this category of therapy also occurs regardless of drug target and mechanism of action. Despite resistance, we remain clinically dependent upon use of both classes of drugs due to their unique respective benefits for treating cancer. As resistance remains the most critical impediment to success of either drug type, the current challenge is to determine how to rationally and effectively utilize these drugs in combination.
A number of significant scientific, logistical and financial issues pose a serious challenge. Understanding the many mechanisms by which cancers adapt to evade drug therapies and leveraging this knowledge into more effective combinations will require a thoughtful and rigorous integration of pre-clinical models and clinical trials. An empiric approach will not suffice: cancers contain too many targetable mutations and activated signaling pathways to develop sufficiently powered clinical trials to test them all. Combining targeted agents, although fully scientifically justified, poses significant challenges including cost, intellectual property considerations and cumulative toxicities. Have studies to identify mechanisms of intrinsic and acquired resistance to cytotoxic and targeted agents provided potential cues that could guide the next generation of combination therapies? Here, we review the existing considerable data supporting the idea that a deep knowledge of mechanisms of sensitivity and resistance may effectively guide rational combinations of cytotoxic and targeted agents, potentially suggesting a testable set of hypotheses that can be accomplished through well-designed clinical trials integrated with pre-clinical studies (Figure 1).
Fig. 1.
Summary of main points.
This review will compare and contrast drug resistance mechanisms in response to cytotoxic chemotherapy and molecularly targeted therapy across cancer types. We hope to present a case that distinctive mechanisms of resistance to cytotoxic and targeted agents provide a unique opportunity to develop complementary combinations that are less likely to have combined toxicities and more likely to produce clinical benefit. We will emphasize glioblastoma (GB) because of the breadth of genomic knowledge about the disease; however, several other cancer types will be discussed to illustrate generality. Further, the emphasis will be on pathway-specific, not organ-specific, resistance mechanisms and their implications for combination therapy. The focus will be on similarities and differences in the context of issues that are relevant to future treatment decisions and clinical treatment paradigms (2–4).
Resistance to cytotoxic chemotherapy
Chemotherapy uses highly potent chemicals that kill rapidly dividing cells. As most cancer cells are fast growing, tumors are especially sensitive to these drug treatments. Chemotherapy drugs directly interfere with cell division, often at the level of DNA, and are divided into classes based on their mechanism (Table I) (reviewed in ref. 5). Alkylating agents, such as carmustine and temozolomide (TMZ), and platinum compounds, such as cisplatin, directly damage DNA at any phase of the cell cycle, inducing a DNA-damage response that leads to apoptosis. Mitotic inhibitors, such as the taxanes (paclitaxel and docetaxel) and vinca alkaloids (vinblastine and vincristine), prevent cell division by acting on microtubules. Antitumor antibiotics, the major group being the anthracyclines such as doxorubicin, interfere with DNA synthesis by intercalating between DNA strands and, in some cases, also with DNA replication by inhibiting the activity of topoisomerases. Topoisomerase inhibitors, such as irinotecan and topotecan, also act in this way to inhibit DNA replication. Antimetabolites act by substituting for normal constituents of RNA, DNA or other cellular metabolites during the specific phases of the cell cycle in which these molecules are synthesized. Incorporation of these drugs causes macromolecular damage and cell death. Such antimetabolites include the pyrimidine antagonists 5-fluorouracil and capecitabine as well as the folic acid antagonist methotrexate.
Table I.
Cytotoxic chemotherapies
| Cancer type | Standard therapy | Mechanism of action | Combinatorial treatment in clinical trials | Additional cell death inducing action |
|---|---|---|---|---|
| NSCLC (6) | Cisplatin (II, V) | DNA damage | Erlotinib | EGFR-targeted therapy |
| Cervical cancer (7,8) | Cisplatin (II, V) | DNA damage | Topotecan/paclitaxel (III) | Topoisomerase inhibitor/microtubules stabilizer |
| Pancreatic cancer (9,10) | Gemcitabine (V) | Nucleoside analog | Capecitabine (V) | Nucleoside analog |
| Biliary tract cancer (11) | Gemcitabine (V) | Nucleoside analog | Cisplatin (II, V) | DNA damage |
| Pediatric childhood acute lymphoblastic leukemia, acute myelogenous leukemia, CML (12) | Methotrexate (II) | Folate antimetabolites | Rapalogs | MDR reversal |
| Breast cancer (13) | Anthracycline/taxane/ trastuzumab | Mixed | Lapatinib/capecitabine (V) | HER-2/EGFR inhibitor/nucleoside analog |
| Bladder cancer (14) | Cisplatin (II, V) | DNA damage | Gemcitabine (V) | Nucleoside analog |
| Colorectal cancer (15) | Oxaliplatin (II, V) | DNA damage | Capecitabine (V) | Nucleoside analog |
| Cancer type | Standard therapy | Mechanism of action | Overcoming resistance treatment in clinical trials | Mechanism for overcoming resistance |
| GB (16) | TMZ (V) | DNA damage | O6-benzylguanine (V) | O6-alkylguanine-DNA alkyltransferase inhibiton |
| NSCLC (17) | Docetaxel (II, VI) | Microtubules stabilizer | AT-101 | Bcl-2 family members inhibition |
| Chronic lymphocytic leukemia (18) | Standard or monoclonal therapy (II, VI) | Mixed | Navitoclax | Bcl-2 family members inhibition |
Roman numerals (I–VI) indicate groups corresponding to Figure 2. I, pharmacokinetics/drug metabolism and tumor microenvironment; II, efflux pumps; III, drug-inactivating enzymes; IV, acidic vesicle compartments; V, DNA repair; VI, Bcl-2 family member-mediated resistance to apoptosis.
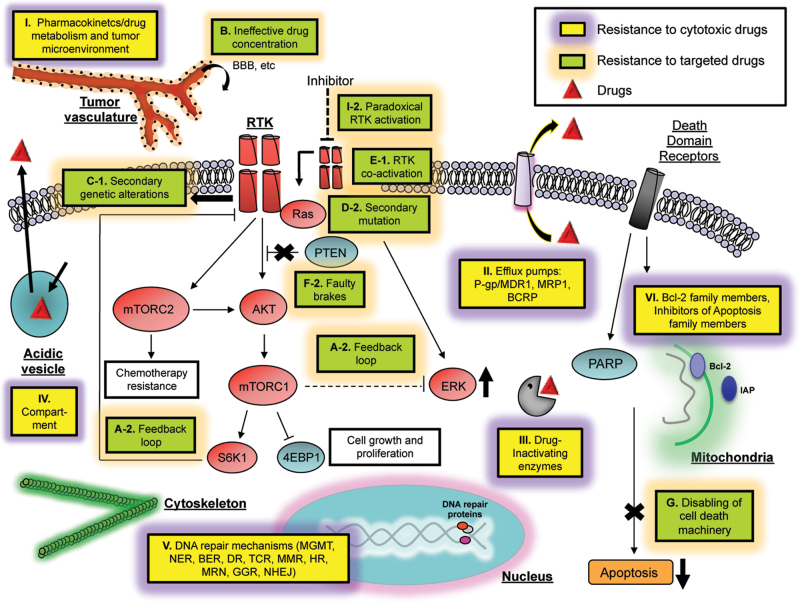
Drug-induced toxicity is a serious limitation because non-cancerous fast-growing cells in the body, such as those in the digestive tract, bone marrow, mucous membranes and hair follicles, are also susceptible to these drugs. Each class of drugs has a specific toxicity profile, and clinical use of the drugs is dependent on achieving an ideal therapeutic index, or maximizing the magnitude of the desired therapeutic effect while limiting toxic side effects. Moreover, although they are potent and effective in inducing cell death, resistance often occurs, thus rendering many of these drugs less clinically effective. Examples of mechanisms of resistance to chemotherapy drugs are shown in Figure 2 and Table I.
Fig. 2.
Cellular mechanisms of resistance to cytotoxic chemotherapies and molecular targeted therapies. Resistance to cytotoxic therapy is commonly a result of failure of the drug to reach its intended target. This is mediated by enhanced activity of drug transporters, induction of emergency response genes to increase the repair of damaged DNA or dampening of mitochondrial-dependent apoptotic cell death. The main mechanisms for resistance to targeted therapies are genetic alteration/mutation of the target itself, persistent activation of downstream signaling pathways, bypass mechanisms such as activation of alternative driver-oncogenes or other downstream signaling pathways or pathway-independent mechanisms such as epigenetic alterations. Yellow boxes represent the major mechanisms of resistance to cytotoxic therapies, and green boxes denote resistance mechanisms to target therapies. Letters and numbers in each box correspond with those in Tables I, II, and III detailing resistance mechanisms.
Pharmacokinetics and drug metabolism
Resistance to chemotherapy drugs falls into two categories with respect to the tumor cell: extrinsic and intrinsic. Extrinsic resistance encompasses the failure of drugs to reach their site of action in an active form due to pharmacokinetic/pharmacodynamic reasons, such as a short serum half-life or rapid clearance by the kidneys and/or liver (Figure 2, I (19)). Alterations in endogenous tumor cell gene products involved in drug metabolism also affect their response to chemotherapy. As avenues toward overcoming some of these problems, chemotherapeutics have been conjugated to tumor-targeted antibodies or incorporated within carriers such as liposomes or nanoparticles. These approaches have demonstrated improved drug uptake and decreased drug-induced toxicity to normal tissue through enhancement of specific and efficient delivery to the tumor. Liposomal doxorubicin, for example, was the first encapsulated anticancer drug to gain Food and Drug Administration approval (20) and is now used routinely for the treatment of several tumor types such as multiple myeloma and metastatic breast and ovarian cancer (reviewed in ref. (21)). Substantive clinical evidence shows that liposomal encapsulation does increase tolerability when chemotherapy drugs are used in combination (22). Currently, there are a number of liposomal chemotherapeutic drugs in clinical development, including cisplatin (22) and irinotecan (23), and major efforts are focused on the development of novel, more efficient liposomal formulations. Similarly, antibody–drug conjugates are also under clinical development and exploit tumor-selective antigens to target chemotherapy. Two examples include brentuximab vedotin, which delivers the antimicrotubule agent monomethyl auristatin E to CD-30–positive lymphoma cells (24), and trastuzumab-DM1, which delivers a different antimicrotubule agent to HER-2-overexpressing breast cancer cells (25). In addition, some drugs are dependent upon intracellular activation by metabolic enzymes, such as cytochrome P450. Expression of and genetic polymorphisms in this and other drug-metabolizing enzymes significantly influences plasma levels of active drug and, by extension, drug sensitivity (26) (Figure 2, I).
The tumor microenvironment is another major component that is emerging as a significant mediator of extrinsic drug resistance (Figure 2, I). The abnormal vasculature of tumors often hinders their accessibility to chemotherapeutic drugs, but agents that increase tumor vessel density and perfusion can improve drug distribution and delivery. For example, inhibition of cellular signaling through the Hedgehog pathway leads to depletion of the stromal tissue and more efficient delivery of gemcitabine in a model of pancreatic ductal adenocarcinoma (27). The hypoxic environment of tumors is also known to induce chemoresistance because it inhibits cell proliferation, and most chemotherapy drugs rely on rapid cell division for efficacy. In addition, the transcriptional changes induced by a hypoxic response include increased levels of molecules within tumor cells that contribute to resistance (28). Targeting of hypoxia-inducible factor-1, the major mediator of a hypoxic response, is one approach for blocking tumor hypoxia in an effort to increase drug sensitivity and response (reviewed in ref. 29). Importantly, modulation of the tumor microenvironment at the level of vascularization can also greatly impact hypoxia, which has a dual benefit in increasing both delivery and sensitivity of tumor cells to chemotherapeutic agents.
Drug efflux and decreased cellular uptake
Intrinsic cellular mechanisms of resistance involve processes such as removal of the drug from its site of action by increased efflux or decreased uptake, enzymatic modification/inactivation of the drug and alteration of drug target(s) within the cell. All of these processes represent avenues the cell can exploit to mount multidrug resistance (MDR): the ability of tumor cells to exhibit cross-resistance to several cytotoxic drugs at once (Figure 2, II). MDR was first discovered in the context of the p-glycoprotein family of proteins (30,31). These proteins are components of the adenosine triphosphate-binding cassette transporter efflux pumps that, when expressed in tumor cells, effectively remove drugs from the cell. As a result, chemoresistance occurs due to insufficient intracellular drug concentrations. The specificity of efflux pumps for drugs has a wide spectrum and includes the anthracyclines (e.g. doxorubicin), the vinca alkaloids (e.g. vincristine and vinblastine) and microtubule stabilizing drugs such as paclitaxel (reviewed in ref. 4). Overexpression of p-glycoprotein/MDR1 and other adenosine triphosphate-binding cassette drug transporter genes, such as MRP1 and BCRP, have been shown to be associated with resistance in vitro (32) and clinically with poor response to therapy and shorter overall patient survival (33,34).
Alternatively, some water-soluble drugs depend on cellular energy/nutrient transporters for entrance into cells. Altered expression of these transporters can reduce uptake and resulting intracellular concentration of drugs such as carboplatin (35) and the antifolate methotrexate (36). Moreover, expression and activity of glutathione-S-transferase detoxification enzymes is typically high in MDR cells, further contributing to resistance (37). Expression of these and other related drug-inactivating enzymes represent another cell-intrinsic mechanism of resistance (Figure 2, III).
Several approaches have been taken to re-sensitize cells that have become chemoresistant through efflux-pump-mediated MDR. For example, MDR reversal agents such as verapamil, quinidine and cyclosporine analogs act as direct or indirect/allosteric inhibitors against the efflux pump to prevent drug binding to the pump and, consequently, they block transport of the drug out of the cell (reviewed in ref. 38). Many of these have been tested clinically, but unfortunately have produced marginal benefits due to problems with high toxicity, low efficacy at non-toxic doses and metabolic inactivation (39–45) (reviewed in ref. 46).
Intracellular drug sequestration
Another tactic that tumor cells employ to avoid the cytotoxic effects of some chemotherapeutics is to sequester the drug away from its intracellular site of action. Chemotherapeutic drugs are localized predominantly in specific organelles within the cytoplasm of drug-resistant cells, whereas sensitive cells exhibit more nuclear drug localization and a more general distribution throughout the cytoplasm (47). It is thought that the weak basic charge of many chemotherapy drugs results in increased trapping within acidic vesicles followed by secretion from the cells by normal vesicular transport. Consistent with this model, drug-sensitive cells have been shown to harbor reduced pH gradients across vesicular membranes compared with resistant cells. Furthermore, reversal of drug resistance occurs upon disruption of pH gradients in resistant cells (48) (Figure 2, IV).
DNA repair enzyme-mediated resistance
As cancer cells rely on DNA synthesis to meet their proliferative needs, a large number of chemotherapeutic drugs are DNA damaging agents. DNA damage normally prompts either repair of the damaged DNA or induction of signals for the cell to undergo apoptosis as a means for preventing expansion of cells harboring damaged DNA. The DNA repair machinery is a complex of enzymes whose function is to assure the integrity of DNA strands and ensure repair in the event of damage. DNA damage induces specific damage-type classes of repair enzymes, including direct reversal, transcription-coupled repair, mismatch repair, homologous recombination, Mre11–Rad50–Nbs1 complex, base excision repair, nucleotide excision repair, global genome repair and non-homologous end joining (reviewed in ref. 49).
Tumors utilize various strategies to overcome the DNA damage induced by chemotherapy, many of which rely upon the enzymes that mediate the various forms of DNA repair (Figure 2, V). TMZ, an alkylating agent used for the treatment of GBM, is known to induce cytotoxic cell death through the formation of O(6)-methylguanine adducts in the DNA. The primary mechanism associated with TMZ resistance in patients is the expression of the DNA repair enzyme O(6)-methylguanine-DNA methyltransferase (MGMT) in malignant cells where it repairs specific damage caused by TMZ, thus allowing cancer cell survival. The regulation of MGMT expression has been widely investigated and of particular interest is the methylation status of its promoter, which is often altered in GB patients. MGMT promoter methylation is predictive of improved response to TMZ treatment in glioma patients as compared with patients with unmethylated MGMT promoters (50). However, MGMT is not the only enzyme responsible for TMZ failure in glioma patients. Recently, a base excision repair-associated enzyme called alkylpurine-DNA-N-glycosylase has also been shown to contribute to TMZ resistance (51). Indeed, multiple mechanisms likely collaborate to neutralize cytotoxic therapies making the circumvention of drug resistance particularly challenging.
Defects in apoptosis
All chemotherapeutic drugs, regardless of their specific target or mechanism of action, produce the same cytotoxic end effect in sensitive cells: apoptotic cell death. In fact, the success of chemotherapy is almost completely dependent upon the ability of the cell to undergo apoptosis. This provides the tumor cell with another avenue to chemoresistance mediated through the cellular reprogramming of pathways that are responsible for inducing apoptosis. Inhibition or loss of pro-apoptotic proteins and pathways is often associated with the clinical onset of resistance to chemotherapy, with an important role played by the ATM–Chk2–p53 axis, as exemplified in the case of breast cancer (52). Another important role is played by the Bcl-2 family of proteins. For example, the expression of pro-apoptotic Bim, which inhibits the pro-survival proteins Bcl-2 and Bcl-xL and acts directly at the mitochondria to engage apoptosis-inducing Bax and Bak, appears central for the ability of cells to undergo apoptosis in response to chemotherapeutic drugs (reviewed in ref. 53). In addition, the p53-regulated pro-apoptotic members Puma and Noxa are required for apoptotic cell death in response to DNA damage–inducing agents in lymphoma (54). Many tumors inherently or in response to therapy express high levels of pro-survival proteins such as Bcl-2 and Bcl-xL (53) as well as inhibitor of apoptosis family members such as cIAP, XIAP and ML-IAP (55) (Figure 2, VI). As a result, there is significant effort in overcoming chemotherapy-induced resistance to apoptosis by using agents that are specifically targeted to components of the apoptotic machinery within the cell. A promising therapeutic approach is the use of Bcl-2 homology domain 3 (BH3) mimetic compounds, such as ABT-737, which mimic the function of Bim and inhibit Bcl-xL, Bcl-2 and BclW (56). Pre-clinical studies have clearly demonstrated the ability of these drugs to chemosensitize resistant cells (57) and their clinical efficacy in combination with chemotherapeutics is encouraging (17,18). Resistance to apoptosis is also a predominant theme in tumor cells that have failed to respond to molecularly targeted therapy and represents an area where the distinctions between resistance to chemo- and targeted therapy begin to overlap (Figure 2, VI and G).
To summarize, the dominant intrinsic mechanisms of resistance to cytotoxic chemotherapies include efflux-pump-mediated MDR to remove the agent from cells, activation of DNA repair pathways and failure of the cellular apoptotic machinery, both of which prevent DNA damage from killing tumor cells. Of note, these mechanisms can be at the cell surface, mitochondria or nucleus and none of them are known to be dependent upon activated cancer signaling pathways downstream of oncogene and tumor suppressor mutations, although such altered signaling could regulate their function.
Resistance to molecularly targeted cancer therapy
The cancer treatment field has evolved considerably over the past years with the introduction of targeted therapies that are characterized by higher specificities of action than chemotherapy and which promise the potential for tumor eradication with decreased toxicity. Cancers typically contain multiple mutations, but may develop dependence on a single mutation for growth and survival rendering them preferentially susceptible to targeted inhibition (58,59). Experimental models, and some clinical examples, support the ‘oncogene addiction’ hypothesis, providing compelling rationale for targeted cancer therapy (60,61). The National Cancer Institute defines targeted therapies as drugs that interfere with specific key molecules involved in tumor growth and progression. These compounds comprise small molecules or monoclonal antibodies that block tumor proliferation and may indirectly or directly induce apoptosis. The small molecules penetrate cells to reach their intracellular targets, whereas the monoclonal antibodies are typically directed against cell surface or extracellular antigens. Targeted therapies affecting cell growth signaling include inhibitors of receptor tyrosine kinases (RTKs) and drugs affecting downstream kinases. Aberrant RTK activation has a pivotal role in cancer progression and is due to gene amplification, receptor overexpression, autocrine activation or gain of function mutations (62). Many RTKs and downstream kinases are being targeted by clinically approved agents and include epidermal growth factor receptor (EGFR) (63), HER-2 (64), MET (65), phosphatidylinositol 3-kinase (PI3K) (66), mammalian target of rapamycin (mTOR) (67) and BRAF (68), among others (Tables II and III). As a representative example, hyperactivation of the EGFR/PI3K/Akt/mTOR cascade occurs in up to 50% of GB tumors, which generally respond poorly to standard non-targeted therapies. Clinical trials with small molecule EGFR-selective tyrosine kinase inhibitors (TKIs) have been conducted with improved outcome for a subgroup of patients (63). Specifically, inhibition of EGFR with erlotinib is preferentially effective in GBM patients co-expressing the common constitutively active mutant EGFR (EGFRvIII) and the tumor suppressor phosphatase and tensin homolog (PTEN) (63), demonstrating the necessity to molecularly characterize each tumor for prediction of therapeutic efficacy (69). Further, erlotinib is an inhibitor of EGFR in its active conformation and poorly blocks the EGFR extracellular domain mutants expressed in GB as compared with lapatinib, which inhibits EGFR in its inactive conformation (70). In contrast, non-small cell lung cancers (NSCLCs) primarily express EGFR that is mutated within the kinase, rather than the extracellular, domain and inhibitors such as erlotinib demonstrate remarkable efficacy for NSCLC (70). This highlights the need to carefully pair specific drugs with specific targets, as it is clear that the drug effect can be drastically different based on the specific mutations of the target.
Table II.
Targeted therapies with small molecules
| Cancer type | Small molecule and target | Determinant of sensitivity | Determinant of resistance | Clinical trials to overcome resistance |
|---|---|---|---|---|
| NSCLC (71) | 1) Gefitinib-EGFR (72); 1) Crizotinib-ALK (73) |
EGFR mutations (74) | C) T790M EGFR mutation (72); E) MET amplification (72,75); G) BIM mutations (76) |
EGFR and MET inhibitors (65); Combination with retinoid (71); Combination with chemotherapy (77) |
| GIST (78) | 1) Imatinib-KIT, PDGFRα (78) | C) Types of KIT (78) | CPDGFRα (79) mutations | ND |
| Colorectal cancer (80) | 2) Everolimus-mTOR (80); | PIK3CA mutation, PTEN loss of function (80), V600E BRAF mutation (81) | D) KRAS mutation (80) | |
| 2) Vemurafenib-BRAF (81), olaparib-PARP (82), regorafenib-TKI, cediranib-VEGFR; | E) Increased EGFR expression (81) | Combination with conventional chemotherapy partially successful (83) | ||
| mCRC (83) | Aflibercept-VEGFA/B, PIGF (83) | |||
| CML (84) | 2) Imatinib, nilotinib, dasatinib-BCR-ABL1 (84–86) | BCR-ABL1 mutation (84) | C) Target mutations or amplif ication (87,88); I) extracellular signal-regulated kinase activation (84); I) PI3K, Src, Janus kinases (89,90); G) BIM mutations (76); B) pharmacokinetic failure (91) | Pan-ABL inhibitors (92), treatment with mycophenolic acid (93) |
| Various (66,94) | 2) Sirolimus, everolimus, temsirolimus, PI3K inhibitors, PI3K and mTORk or MEK inhibitors, Akt inhibitors- PI3K/AKT/mTOR pathway (94); | H1047R PIK3CA mutation (94); BIM mutation (preclinical test (95)) |
D) KRAS mutations (94) | Target therapies are in progress |
| Pancreatic cancer, HNSCC (80) | 2) Everolimus-mTOR (80); | PIK3CA mutation, PTEN loss of function (80); | D) KRAS mutation (80) | ND |
| TNBC (96) | 3) Olaparib-PARP (96) | BRCA1/2 mutations (96) | ND | PI3K and PARP inhibitors (96) |
| Breast cancer (64) | 1) Lapatinib, neratinib, afatinib-HER-2 (64); tamoxifen-ER (97); olaparib-PARP (82); MET inhibitors (65) |
PIK3CA mutation (98); PTEN loss of function (80) |
D) PI3K mutations (97); E) Alternative pathway and HER signaling (99); F) Constitutive activation of downstream effectors (64); D) BRCA1 and BRCA2 mutations (82); |
TKI plus chemotherapy (64) |
| GB (100) | 1) Erlotinib-EGFR (101); 1) Lapatinib-EGFR (70); 2) Rapamycin-mTOR (67) |
PTEN expression (101); PTEN loss (67) |
B) drug efflux (102); micro RNA (103); PTEN Y240 (104); pharmacokinetic failure (70); A) Redundant signaling through alternate pathways (67); |
Dual inhibitor PI3K and mTOR (100) |
| Renal cancer (105) | 1) MET antagonists, MET kinase inhibitors-MET (65,105); 2) Temsirolimus-mTOR(106); 2) Everolimus-mTOR(107) |
Hypoxia-inducible factor-1α (106) | Not understood (105) |
New immunotherapeutic strategies are emerging (105); Combination with antibody (107) |
| Ovarian cancer (66) | 2) Temsirolimus-PI3K/AKT/mTOR pathway (98); | PIK3CA and KRAS or BRAF mutations (98) | ND | |
| 3) olaparib-PARP (82); 1) A6-uPAR (108) |
D) BRCA1, BRCA2 mutations (82) | |||
| Cervical cancer, Endometrial cancer (98,109) |
2) Temsirolimus-PI3K/AKT/mTOR pathway(98) | PIK3CA and KRAS or BRAF mutations(98) | D) KRAS mutation(98,109) | Combined inhibition of the RAS/RAF/MEK and PI3K/AKT/mTOR pathways suggested (110) |
| Melanoma (68) | 2) Vemurafenib-mitogen- activated protein kinase dependent and independent pathways (68,111); 2) Everolimus-mTOR (80); Olaparib-PARP (82) |
V600E BRAF mutation, KIT mutation (68); PIK3CA mutation, PTEN loss of function (80) |
D) NRAS-mutant clones (112); H) amplification of MAP3K8 (113); E) increased IGF-1R signaling (114); F) loss of PTEN and G) Bim (115); D) MEK mutations (116); D) BRAF alterations (117,118); E) PDGF overexpression (112); D) BRCA1, BRCA2 mutations (82) |
Combination of BRAF and MEK inhibitors (119,120) |
| DLBCL (121) | Fostamatinib-SYK, enzastaurin-PKCβ (122,123); ruxolitinib-JAK/STATSB1518, alisertib-Aurora A (121); panobinostat-histone deacetylase, bortezomib-proteasome (121,124) | BCR signaling (122) PKCβ over-e xpression (123); Aurora A over- expression (121) |
ND | Therapies still in clinic al trials (124) |
Numbers refer, respectively, to 1), RTK targets; 2), downstream kinase; 3) cell death targets. Letters refer to resistance mechanisms in Figure 2. GIST, gastrointestinal stromal tumor; HNSCC, head and neck squamous cell carcinoma; TNBC, triple negative breast cancer; DLBCL, diffuse large B-cell lymphoma; ND, not determined.
Italicized text in ‘small molecule and target’ column indicates drug names.
Table III.
| Cancer type | Small molecule and target | Determinant of sensitivity | Determinant of resistance | Clinical trials to overcome resistance |
|---|---|---|---|---|
| mCRC (83) | 1) Bevacizumab- VEGF, ramucirumab-VEGFR2 (83) | ND | ND | Combination with conventional chemotherapy partially successful (83) |
| Breast cancer (64) | 1) Trastuzumab, pertuzumab, ertumaxomab- HER-2 (64) | PIK3CA mutation (98) | C) Altered target, E) alternative pathway and HER signaling (99); F) constitutive activation of downstream effectors (64) |
Trastuzumab plus pertuzumab and docetaxel (127), Trastuzumab-DM1 (128); double HER-2 inhibition with antibodies plus small TKI molecules (64) |
| Glioma (125) | 1) Cetuximab- EGFR (125,129) | ND | ND | ND |
| Renal cancer (105) | 1) Bevacizumab- VEGF (107) | ND | ND | New immunotherapeutic strategies are emerging (105); combination with TKI (107) |
| Ovarian cancer (66) | PIK3CA and KRAS or BRAF mutations (98) | D) KRAS mutation (98,109) | ND | |
| Cervical cancer, endometrial cancer (98,109) | 1) Bevacizumab- 2) PI3K/AKT/mTOR pathway (98) |
PIK3CA mutation (98,109) | D) KRAS mutation (98,109) | Combined inhibition of the RAS/RAF/MEK and PI3K/AKT/mTOR pathways suggested (110) |
| Melanoma (68) | 1) Bevacizumab- VEGF inhibitor (130); Ipilimumab-CTLA-4 (131) |
ND | ND | Combination with cancer vaccine and chemotherapy (68,132); combination of ipilimumab and vemurafenib-V600E BRAF mutation inhibitor- in progress (128) |
| DLBCL (121) | Rituximab-CD20 (121) | ND | ND | Aurora A inhibitor, chemotherapy and rituximab (121); Combination with chemotherapy (124,133) |
Numbers refer, respectively, to 1), RTK targets; 2), downstream kinase target. Letters refer to resistance mechanisms represented in Figure 2. DLBCL, diffuse large B-cell lymphoma; ND, not determined.
Detailed review on immunotherapy and targeted therapies combination is available in Vanneman et al. (134).
Italicized text in ‘small molecule and target’ column indicates drug names.
As shown in Tables II and III, targeted therapy can also involve inhibition of downstream effectors of oncogenic mutations. EGFRvIII-expressing GB cells rely preferentially on the activity of mTORC2, which confers tumorigenicity through activation of the nuclear factor-κB pathway (135). This implies the possibility of suppressing GB growth by targeting mTORC2 and clinical trials are now testing combinatorial therapies designed to achieve more complete inhibition of EGFR/PI3K/Akt/mTOR signaling. The strategy is to inhibit both upstream oncogenic signaling and downstream effectors by administering allosteric mTOR inhibitors in combination with erlotinib, or using dual PI3K/mTOR kinase inhibitors (100,136). However, just as for chemotherapies, the molecularly targeted therapies have thus far achieved clinically relevant efficacy in only a limited number of cases because of the emergence of drug resistance and because the pairing of patients who are genotypically subject to the agent is in its infancy.
Examples of targeted therapies and corresponding mechanisms that cancers invoke to evade treatment are shown in Tables II and III and Figure 2. Resistance to targeted drugs can be classified as intrinsic (primary) or acquired (secondary) and often relies on cellular responses that maintain the signal flux despite effective RTK targeting and maintain signaling through alteration of downstream effectors and/or disable the cell death machinery through compensatory cell survival pathways. Other mechanisms of targeted drug resistance have also been described in the literature and are described.
Maintaining signal flux while targeting RTKs
One of the most common mechanisms of resistance to targeted therapies involves the maintenance of oncogenic signaling through downstream pathways despite efficient target inhibition by a drug (Tables II and III, Figure 2). These include co-activation of other RTKs (Figure 2, E-1), signaling by physiologically regulated RTKs while mutant RTKs are inhibited (Figure 2, I-2), and secondary activating resistance mutations (Figure 2, C-1). EGFR TKIs and the allosteric mTOR inhibitor rapamycin (and its derivatives) have failed to display durable efficacy in gliomas even though the rationale for targeting these molecules has been clearly demonstrated (63,67,137–139). These failures may be explained by the relative ease with which cancers develop compensatory resistance mechanisms to maintain signal flux. In this regard, it was reported that when treated with EGFR inhibitors, other RTKs such as c-MET and/or platelet-derived growth factor receptor (PDGFR) become co-activated, engaging PI3K to maintain downstream pathway activation (140,141). Thus, effective targeted therapy may require combined regimens targeting multiple RTKs. This is consistent with the demonstration that inhibition of AKT induces the expression and phosphorylation of multiple RTKs, primarily due to mTORC1 inhibition and secondary to a FOXO-dependent transcriptional activation of receptor expression (142). Furthermore, pre-clinical studies in GB have shown that upon EGFR inhibition, compensatory signaling is mediated by fibroblast growth factor receptor and SRC family kinases and involves the inactivating phosphorylation of PTEN at Y240 (104).
EGFR kinase domain mutations have been identified in NSCLC patients who are responsive to the reversible EGFR TKIs gefitinib and erlotinib. Tumor responses to these agents are dictated by the presence of EGFR somatic mutations, increased gene copy number and certain clinical and pathological features (72). Despite dramatic responses to such inhibitors in some cases, most patients eventually become resistant and progression of the disease follows. In those tumors with acquired resistance, a second point mutation occurs in exons encoding the kinase domain of EGFR (T790M) in about half of these cases (72,143). Structural and functional analyses revealed that this second mutation confers gefitinib and erlotinib resistance by increasing the affinity of the kinase domain for adenosine triphosphate, thereby decreasing affinity for the TKIs. In addition, a second-site D761Y EGFR mutation reduced the sensitivity of the drug-sensitive L858R EGFR mutant to TKIs in a brain metastasis of NSCLC (144). Irreversible second-generation EGFR inhibitors and those designed to specifically bind the T790M mutant receptor are under clinical development and may overcome second-site mutation-mediated resistance (145,146).
Maintaining the signal through downstream effectors
Another type of acquired drug resistance is achieved by tumor cells through rewiring of downstream signaling pathways thereby conferring faulty brakes on growth checkpoints (Figure 2, F-2), secondary mutations in downstream signaling effectors (Figure 2, D-2) and/or feedback loop activation (Figure 2, A-2). The previously cited demonstration that expression of the constitutively active mutant EGFRvIII sensitized GBs to EGFR inhibitors only in the context of intact PTEN expression and function underscores the combinatorial nature of drug sensitivity. As loss of PTEN function is a common event in GB, many patients expressing EGFRvIII, but without functional PTEN, were resistant to erlotinib (63) due to uncoupled inhibition of EGFR from PTEN-mediated inhibition of downstream PI3K signaling flux (63). Consistent with this, patients whose tumors had high levels of EGFR coupled with low levels of activated AKT were more likely to respond to the TKIs (138). These studies indicate that intact negative regulation of downstream PI3K signaling appears to be critical for effective response to EGFR inhibitors and that efficacy of targeted agents, or such agents with chemotherapy, is likely to require consideration of genetic synthetic lethality or complementation for optimal effect.
The PI3Ks are a family of lipid kinases grouped into three classes with class IA PI3Ks being activated by the binding of a growth factor to its associated cognate RTK. The PIK3CA gene encodes the p85 regulatory and the p110 catalytic subunits of PI3K. Activating mutations in PIK3CA have been found in approximately 25% of primary breast cancers, and these occur almost exclusively in PTEN-positive tumors (147). PIK3CA mutations contribute to increased risk for progression in trastuzumab-treated HER-2-positive breast cancer (148) and PIK3CA exon 20 mutations are also associated with a lack of cetuximab sensitivity in colorectal tumors (149). This suggests that the relationship between mutant PIK3CA and other aberrations affects response to PI3K/AKT/mTOR axis inhibitors and the necessity for further characterization and understanding of which mutation combinations cause sensitization or resistance.
mTOR acts within the canonical PI3K signaling pathway to mediate cell growth and proliferation, and mTOR is an important integrator of several targetable signaling cascades (69). However, studies in patients with recurrent malignant gliomas have failed to demonstrate consistent responses to the mTOR inhibitor rapamycin or its analogs (139,150–152). Paradoxically, rapamycin treatment leads to AKT activation by disrupting the negative feedback loop that normally attenuates PI3K signaling and is associated with significantly shorter time-to-progression (67). This finding suggests that a more effective way to target mTOR signaling and to prevent PI3K pathway reactivation might be to use a dual PI3K/mTOR inhibitor (153). Dual PI3K/mTOR inhibitors may also suppress the ability of mTORC1 to activate extracellular signal-regulated kinase signaling by a PI3K-dependent mechanism, an example of the remarkable plasticity of tumor cells and their capacity for rewiring (154). Additionally, it has been increasingly recognized that rapamycin only partially inhibits the phosphorylation of 4E-BP1, which may further contribute to the resistance to rapamycin treatment in cancer (155).
mTOR inhibition has been explored in pre-clinical studies with breast cancer cells where inhibiting mTORC1/C2 activity with mTOR kinase inhibitors had a biphasic effect on Akt phosphorylation and function (156). Specifically, the mTOR inhibitor induced a temporary inhibition of Akt-Thr308 phosphorylation followed by reactivation through hyperactivation of PI3K signaling, which is mediated by insulin-like growth factor (IGF)-1 receptor/insulin receptor signaling. By preventing the relief of this RTK survival feedback loop with the combinatorial treatment of mTOR and HER-kinase inhibitors, T308 phosphorylation has been shown to be stably inhibited in breast cancer xenograft models, providing a rationale to consider this approach in cancer therapy.
Failure to induce cell death due to compensatory cell survival pathways and other mechanisms of resistance to targeted therapies
The failure to cause cell death due to disabling of the cellular death machinery (Figure 2, G) is a mechanism that has been characterized in chronic myelogenous leukemia (CML) and NSCLC. In CML and EGFR-mutant NSCLC, a germ-line polymorphism results in expression of a novel isoform of the pro-apoptotic Bcl-2 family protein Bim (76). High levels of Bim expression are required to induce apoptosis upon TKI exposure (76). However, because this novel Bim isoform lacks the pro-apoptotic BH3, it is deficient in inducing apoptosis and thus contributes to poor intrinsic clinical response to TKI treatment. For these patients, sensitivity to targeted therapy might be generated using a combination of TKI and BH3 mimetics (76). This highlights the existence of inherited resistance determinants that are shared between different cancer types and are fundamental in guiding new synergistic treatment combinations for specific molecularly defined patients.
Two other examples of mechanisms of resistance to targeted therapies are resistance dependent on differential cell-type sensitivity or on DNA repair pathways. The presence of a small population of cancer cells, defined as cancer stem cells, that have the ability to self-renew and are refractory to conventional drug treatment because of MDR activity (157) or enhanced DNA damage response, has been described ref. 158.
With respect to EGFR inhibition, a selected population of NSCLC and prostate cancer cells develop a chromatin-mediated state of tolerance upon EGFR inhibitor treatment that is dependent on epigenetic regulation of chromatin and IGF-1 signaling. By inhibiting IGF-1 receptor or using chromatin-modifying compounds, this secondary resistance-triggering action can be prevented (159).
More than 80% of endometrioid endometrial cancers are characterized by loss of PTEN function. This deficiency, similarly as found in GB, is generally correlated with resistance to TKI inhibition (101), and, in endometrioid endometrial cancer, with sensitization to poly (ADP ribose) polymerase (PARP) inhibitors (160). This lethal effect in PTEN-mutant endometrioid endometrial cancer has been correlated with genomic instability elicited by defects in homologous recombination DNA repair (160).
All of the examples above present compelling evidence that the best approach to achieving a clinical response to molecularly targeted therapy will result from the utilization of molecular tools to stratify patients and clinical trials to assess the most effective strategy to inhibit both the triggering mutations and the mechanisms that evade drug efficacy. Importantly, several studies using massively parallel sequencing technologies have reported intratumoral genetic heterogeneity in solid cancers (161,162) and revealed that specific mutations, including PIK3CA or PTEN mutations, may only be predominant in a subset of tumor cells in a given cancer (163,164). This observation implies the necessity to develop robust and accurate diagnostic biomarkers to collect representative tumor specimens pre- and post-drug treatment and personalize therapies throughout the disease course.
A more recent addition to the realm of targeted therapy is the drug crizotinib, an inhibitor of the RTKs anaplastic lymphoma kinase (ALK) (165) and c-Met (165,166). It has been approved and shown to be efficacious for the treatment of NSCLC patients carrying the specific fusion gene involving EML4 and ALK, namely EML4-ALK (73,167), and is entered in clinical trials for anaplastica large-cell lymphoma (NCT00585195). It was also shown to be effective in treating a patient with a myofibroblastic tumor (168). Despite initial enthusiasm, resistance mechanisms have been described, such as ligand overexpression to overcome receptor signaling blockade (166), poor blood–brain barrier penetration (169), mutations in the ALK protein that inhibit crizotinib action (170–172) or paracrine signaling activating additional survival pathways (173). A novel ALK inhibitor, LDK378, capable of overcoming resistance to crizotinib is now in phase II clinical trials (NCT01685138 and NCT01685060). A recent mouse neuroblastoma model expressing an ALK mutation highlighted mTOR as an alternate target to block in overcoming crizotinib resistance (174). All of this suggests that, even for the most contemporary targeted agents, monotherapy is unlikely to produce long-term benefit.
To summarize, the dominant mechanisms of intrinsic resistance to targeted therapies involve maintained signal flux to downstream signaling effectors as a consequence of: second-site resistance mutations or amplification of drug targets, loss of downstream pathway suppressors, secondary genetic alterations and feedback loops or co-activation of other upstream effectors such as other altered RTKs. Moreover, the mechanisms of resistance are largely cytoplasmic; they engage the signaling cascades that are not the core components of, but may actually impact the drug efflux-MDR system, the intrinsic apoptotic circuitry and DNA repair pathways. In conclusion, a rigorous analysis of resistance mechanisms may suggest a set of complementary combination cytotoxic plus targeted therapy approaches.
Therapeutic combination of cytotoxic and molecular targeted agents
Drug resistance is perhaps the predominant factor limiting the clinical success of cytotoxic chemotherapy as well as molecular targeted therapy. The complexity of cancer cells and tumors prevents the success of either type of agent alone or combinations of the same types of drug. Each therapeutic approach hinges upon an essential dependency: on DNA synthesis and/or cell division in the case of chemotherapy and on the targeted pathway or oncogene in the case of molecular targeted therapy. Like their dependencies and mechanisms of action, resistance is reflective of the type of drug. Resistance to cytotoxic therapy is commonly a result of failure of the drug to reach its intended target in an active form within a rapidly dividing cell. In contrast, the major mechanisms for resistance to targeted therapy are genetic alteration/mutation of the target itself, persistent activation of downstream signaling pathways, bypass mechanisms such as activation of alternative oncogenes or other downstream signaling pathways, or pathway-independent mechanisms such as epigenetic alterations or epithelial-to-mesenchymal transition (175,176). Analogous to the concept of genetic complementation or synthetic lethality, the combination of these two classes of drugs clinically would appear to be promising. However, in order to glean the most information, combinations should be approached rationally and not in the opportunistic and less-informed way in which combinations have been tested and failed to demonstrate clinical benefit. In illustrating this point, it is useful to consider examples of combinations that have demonstrated clinical efficacy (or that have failed) as a way to inform the development of future treatment paradigms.
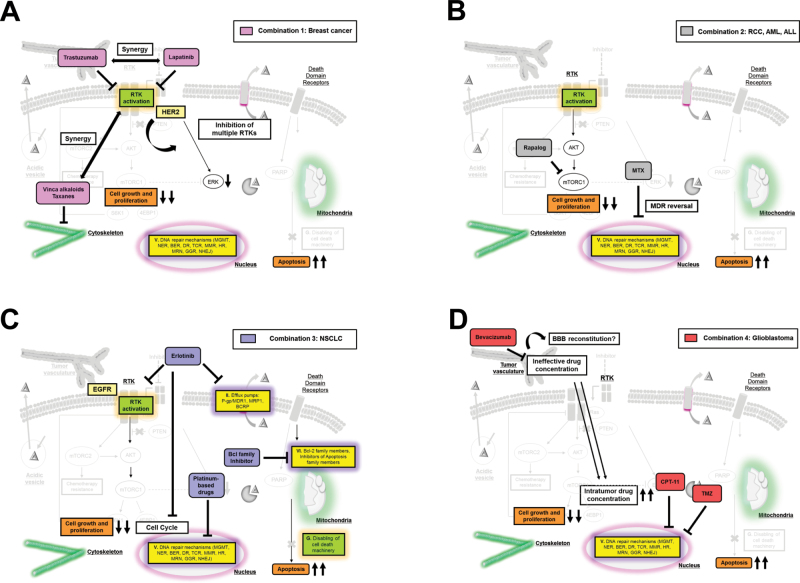
The treatment of advanced HER-2-positive breast cancer offers a good example of recently introduced protocols that have markedly improved the progression-free survival of patients (Figure 3A). Conventional clinical practice for HER-2-positive breast cancer until recently was based on sequential chemotherapies and/or antihormonal treatments (64). Targeted therapies in breast cancer are mainly directed against HER-2 that is overexpressed or amplified in 15–20% of cases (64) and involves HER-2 inhibition by monoclonal antibodies, immunochemotherapy and small molecules (Tables II and III). However, the acquisition of acquired resistance to HER-2-targeted therapies after initial response remains a daunting clinical problem (Tables II and III). The introduction of simultaneous multitargeted therapies also presents logistic limitations for cancer treatment suggesting that a more achievable approach might entail the combination of single targeted therapy together with chemotherapy or immunochemotherapy (Tables II and III, Figure 3A (64)). Indeed, single agent trastuzumab in combination with chemotherapy demonstrated increased patient response rates compared with single agents alone (Figure 2A (177)). In addition, immunochemotherapy with trastuzumab-DM1 demonstrated higher progression-free survival than chemotherapy combined with TKI in a randomized phase III clinical trial (128). Phase III trials in which several HER family blockers were used simultaneously with chemotherapy also have proven to be more effective than trastuzumab alone (127). New TKIs are also being tested and could potentially enhance the outcome for breast cancer patients (64).
Fig. 3.
Examples of rational combinations of chemotherapies and targeted therapies. Chemo- and targeted therapies are interrelated in that the limitations of one approach can be overcome by corresponding benefits of the other. Several combinations of chemotherapy and targeted therapy for different types of cancers are depicted by corresponding color boxes. (A) Combinations of HER-2 targeted therapies and chemotherapies for breast cancers demonstrate synergistic effects as well as inhibition of multiple RTKs. (B) Rapalog and methotrexate combinations for renal cancers and leukemias improve their effects through MDR reversal. (C) Erlotinib induces G1-arrest and inhibition of efflux pumps, which can synergize with chemotherapeutics, and sequential administration of EGFR TKIs following chemotherapy provides a greater effect than concurrent administration. Also, resistance to chemo- and targeted therapies converges on an inability to undergo apoptosis, and pharmacological inhibition of pro-apoptotic members including the Bcl-2 family could overcome chemotherapy drug resistance. (D) Addition of bevacizumab can promote the efficacy of combined cytotoxic agents through the improved delivery of cytotoxic agents via normalization of the tumor vasculature. On the contrary, it could reconstitute the blood–brain barrier in GBM, preventing the diffusion of anticancer drugs.
The breast cancer example is one of several demonstrating that complementing chemo- and targeted therapy can be an effective approach that minimizes the toxicity limitations of chemotherapy. Another case in point is mTOR inhibition using rapalogs in combination with methotrexate for hematological malignancies such as acute myelogenous leukemia and acute lymphocytic leukemia (Figure 3B) where improvements in survival have been achieved (12,178). For acute promyelocytic leukemia, degradation of the oncogenic fusion gene PML-RARα is mediated by treatment with retinoic acid and arsenic and produces disease remission (179,180) and upon relapse, combination with chemotherapy induced complete remission of the disease (181,182).
Although chemotherapy drugs are extremely potent in their ability to induce apoptosis of rapidly dividing cells, it has been shown that cell types exist within the tumor that are not susceptible. Cancer stem cells tend to be slow-growing components of a tumor and are therefore less likely to be affected by these drugs (183) resulting in tumor re-growth following chemotherapy. Combining chemotherapy with a targeted agent directed against the specific pathways responsible for cancer stem cell survival represents a potential remedy to this problem.
Importantly, resistance to both chemo- and targeted therapy converges on an inability to successfully undergo apoptosis. As discussed previously, pharmacological inhibition of pro-apoptotic members of the Bcl-2 family of proteins as a strategy for overcoming chemotherapy drug resistance has been well characterized at the pre-clinical level. The Bcl-2/Bcl-xL dual inhibitor ABT-263 (Navitoclax; Abbott Laboratories/Genentech) has been used in phase I clinical trials for lymphoid malignancies (18,184) and in a phase II trial for small cell lung cancer (185) (Figure 3C). Although this is a promising approach, efficacy still needs to be established for each of the cancer types because another Bcl-2 family inhibitor, AT-101, failed in clinical trials for small cell lung cancer (186), in NSCLC in combination with docetaxel (17) and in metastatic castration-resistant prostate cancer (187).
Another issue that must be taken into careful consideration when contemplating combinations for clinical use is that chemotherapy and molecularly targeted therapy can interact with each other in both positive and detrimental manners. For example, bevacizumab (Avastin; Genentech), a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), can improve the efficacy of combined cytotoxic agents (188,189) through following several mechanisms: (i) normalization of the tumor vasculature culminating in the efficient delivery of cytotoxic agents to the tumor, (ii) prevention of rapid tumor cell re-population after cytotoxic therapies and (iii) augmentation of the antivascular effects of chemotherapeutic agents (190). However, to the contrary, it also has the ability to hinder the effect of the combination therapy by normalizing blood vessels and reconstituting the blood–brain barrier in brain tumors, thereby preventing the diffusion of anticancer drugs (Figure 3D (191)).
For advanced NSCLC, the standard treatment has long been a platinum-based two-drug chemotherapy regimen. However, disease progression following acute response is common. Because a large proportion of NSCLC patients have tumors that overexpress or harbor mutated EGFR, there has been a clinical effort to incorporate the use of EGFR TKIs with chemotherapy for treatment of this disease (Figure 3C). The EGFR TKI erlotinib (Tarceva®) was approved for patients as second-line treatment following failure of chemotherapy or as a maintenance therapy following completion of chemotherapy based on successful clinical trials (192,193). This combination of EGFR TKIs with concurrent cytotoxic chemotherapy was then tested in four randomized phase III clinical trials (194–197), all of which failed to demonstrate a benefit of the combination compared with chemotherapy alone. Pre-clinical data suggest that pharmacodynamic reasons are to blame for the failure of this combination approach. Studies have shown that the G1-arrest induced by erlotinib antagonizes chemotherapy drugs that depend on a progressing cell cycle for efficacy (198). Thus, the prediction would be that sequential administration of EGFR TKIs following chemotherapy provides a greater effect than concurrent administration (199–201). In fact, a phase II trial demonstrated that treatment-naïve NSCLC patients receiving erlotinib on days 15–28 of a 4 week cycle following chemotherapy had significantly better response rates and longer progression-free survival than patients receiving a placebo (202). Interestingly, the Tarceva OR CHemotherapy (TORCH) randomized phase III trial, which was designed to test first-line erlotinib followed by cisplatin–gemcitabine at progression versus the inverse sequence, demonstrated conclusively that erlotinib first followed by chemo was inferior compared with chemo followed by erlotinib (203). These clinical data suggest a rational combination treatment paradigm in which patients are treated with first-line sequential chemotherapy followed by erlotinib. This illustrates the point that clinical success of combining EGFR-targeted agents with chemotherapy is likely to be dependent on a mechanistic understanding of how the drugs are likely to influence one another as well as selection of a patient population that would be predicted to respond to the targeted therapy component of the combination. Given the similarity of action on different targets achieved by the various targeted agents, it can be anticipated that this point is a generalizable one.
Future perspectives
There are many still unanswered questions that confound the combined chemotherapy-molecularly targeted agent approach. For example, it is clear that dose scheduling is a critical component of the success of certain combinations. Should combinations be administered up-front or sequentially and if so, in what order? Does resistance develop more, or less, quickly in response to combinations compared with single agents? Pre-clinical and clinical data would suggest that for toxicity and pharmacodynamic reasons, agents should be administered sequentially and chemotherapy should precede molecularly targeted therapy, but will this be tumor-type and even patient-specific? Ultimately, the approach may even depend on the goal of combination therapy: to prevent resistance from occurring or to be able to successfully treat and clinically manage resistant tumors.
Rapidly evolving technology will provide a platform for even more sophisticated methods of rationally combining chemotherapy and targeted therapy. Molecular network analysis, both computationally and in cells and tumor tissue, could provide a way to identify synergistic strategies for combining drugs and identify new vulnerabilities created upon the emergence of drug resistance. The ever-expanding data of cancer genomes will likely play a role in the design and rationalization of combination therapies by providing insight into the best sequence and combinations of therapies for which specific patients will benefit the most. For example, molecular subgroups of GB based on The Cancer Genome Atlas data provide information not only on the targetable mutations they possess but also on their sensitivity to the chemotherapeutic treatment (204). Moreover, it is becoming increasingly clear that the metabolic pathways that are of critical importance in influencing tumor biology represent novel targets for therapy. Combining chemotherapy and targeted therapy does not negate, but rather underscores, the requirement for patient stratification according to biomarkers for response to the targeted therapy. As a consequence, a personalized medicine approach is of critical importance and becoming increasingly more feasible than ever before.
Overall, there are many layers of complexity underlying successful clinical implementation of what appears to be a very rational and simple biological concept. Toxicity, dose scheduling and molecular heterogeneity are issues that surround drug efficacy and resistance. Combining traditional cytotoxic chemotherapy drugs with molecularly targeted agents represents a formidable challenge but also a distinct opportunity. The future of the approach requires a conjoint consideration of what is learned by clinical failures as well as successes, driven by mechanistic knowledge of therapeutic sensitivity and resistance to guide the design of rational future clinical trials with the goal of establishing novel and more effective treatment paradigms.
Funding
The James S. McDonnell Foundation (to F.F.); National Institutes of Health (P01-CA95616 to W.K.C. and F.F., F32NS066519 to J.W., RO1-73831 and CA119347 to P.S.M.); American-Italian Cancer Foundation (to C.Z.); The National Foundation for Cancer Research (Fellow award to W.K.C.); The Ben and Catherine Ivy Foundation (P.S.M.); The European Commission (PIOF-GA-2010–271819 to B.G.).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ALK
anaplastic lymphoma kinase
- BH3
Bcl-2 homology domain 3
- CML
chronic myelogenous leukemia
- EGFR
epidermal growth factor receptor
- GB
glioblastoma
- IGF
insulin-like growth factor
- MDR
multidrug resistance
- MGMT
O(6)-methylguanine-DNA methyltransferase
- mTOR
mammalian target of rapamycin
- NSCLC
non-small cell lung cancers
- PARP
poly (ADP ribose) polymerase
- PDGF
platelet-derived growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog
- RTKs
receptor tyrosine kinases
- TMZ
temozolomide
- TKIs
tyrosine kinase inhibitors.
References
- 1. Pui C.H, et al. (2012). Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood, 120, 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garraway L.A, et al. (2012). Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov., 2, 214–226 [DOI] [PubMed] [Google Scholar]
- 3. González-Neira A. (2012). Pharmacogenetics of chemotherapy efficacy in breast cancer. Pharmacogenomics, 13, 677–690 [DOI] [PubMed] [Google Scholar]
- 4. Gottesman M.M, et al. (2002). Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer, 2, 48–58 [DOI] [PubMed] [Google Scholar]
- 5. Chabner B.A, et al. (2005) Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer, 5, 65–72 [DOI] [PubMed] [Google Scholar]
- 6. Ettinger D.S, et al. ; NCCN (National Comprehensive Cancer Network) (2012) Non-small cell lung cancer. J. Natl. Compr. Canc. Netw., 10, 1236–1271 [DOI] [PubMed] [Google Scholar]
- 7. Rose P.G, et al. ; Gynecologic Oncology Group (2007) Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol., 25, 2804–2810 [DOI] [PubMed] [Google Scholar]
- 8. Pectasides D, et al. (2008) Chemotherapy for recurrent cervical cancer. Cancer Treat. Rev., 34, 603–613 [DOI] [PubMed] [Google Scholar]
- 9. Cunningham D, et al. (2009) Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol., 27, 5513–5518 [DOI] [PubMed] [Google Scholar]
- 10. Hung S.W, et al. (2012). Overcoming nucleoside analog chemoresistance of pancreatic cancer: a therapeutic challenge. Cancer Lett., 320, 138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valle J, et al. ; ABC-02 Trial Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med., 362, 1273–1281 [DOI] [PubMed] [Google Scholar]
- 12. Barrett D, et al. (2012). Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Paediatr. Drugs, 14, 299–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geyer C.E, et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med., 355, 2733–2743 [DOI] [PubMed] [Google Scholar]
- 14. Sternberg C.N, et al. (2013) ICUD-EAU International Consultation on Bladder Cancer 2012: chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur. Urol., 63, 58–66 [DOI] [PubMed] [Google Scholar]
- 15. Haller D.G, et al. (2011) Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J. Clin. Oncol., 29, 1465–1471 [DOI] [PubMed] [Google Scholar]
- 16. Quinn J.A, et al. (2009) Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J. Clin. Oncol., 27, 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ready N, et al. (2011). Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J. Thorac. Oncol., 6, 781–785 [DOI] [PubMed] [Google Scholar]
- 18. Roberts A.W, et al. (2012). Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol., 30, 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danesi R, et al. (2001) Pharmacogenetic determinants of anti-cancer drug activity and toxicity. Trends Pharmacol. Sci., 22, 420–426 [DOI] [PubMed] [Google Scholar]
- 20. Treat J, et al. (1990) Antitumor activity of liposome-encapsulated doxorubicin in advanced breast cancer: phase II study. J. Natl. Cancer Inst., 82, 1706–1710 [DOI] [PubMed] [Google Scholar]
- 21. Slingerland M, et al. (2012). Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today, 17, 160–166 [DOI] [PubMed] [Google Scholar]
- 22. Stathopoulos G.P, et al. (2010). Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann. Oncol., 21, 2227–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batist G, et al. (2009). Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin. Cancer Res., 15, 692–700 [DOI] [PubMed] [Google Scholar]
- 24. Younes A, et al. (2012). Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol., 30, 2183–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burris H.A, et al. (2011). Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol., 29, 398–405 [DOI] [PubMed] [Google Scholar]
- 26. Ingelman-Sundberg M. (2004). Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol. Sci., 25, 193–200 [DOI] [PubMed] [Google Scholar]
- 27. Olive K.P, et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science, 324, 1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson W.R, et al. (2011). Targeting hypoxia in cancer therapy. Nat. Rev. Cancer, 11, 393–410 [DOI] [PubMed] [Google Scholar]
- 29. Wang R, et al. (2011). Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr. Med. Chem., 18, 3168–3189 [DOI] [PubMed] [Google Scholar]
- 30. Kartner N, et al. (1983). Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science, 221, 1285–1288 [DOI] [PubMed] [Google Scholar]
- 31. Chen C.J, et al. (1986). Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell, 47, 381–389 [DOI] [PubMed] [Google Scholar]
- 32. Cole S.P, et al. (1992). Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science, 258, 1650–1654 [DOI] [PubMed] [Google Scholar]
- 33. Leith C.P, et al. (1999). Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood, 94, 1086–1099 [PubMed] [Google Scholar]
- 34. Trock B.J, et al. (1997). Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J. Natl. Cancer Inst., 89, 917–931 [DOI] [PubMed] [Google Scholar]
- 35. Shen D.W, et al. (2000). Decreased accumulation of [14C]carboplatin in human cisplatin-resistant cells results from reduced energy-dependent uptake. J. Cell. Physiol., 183, 108–116 [DOI] [PubMed] [Google Scholar]
- 36. Shen D, et al. (1998). Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer Res., 58, 268–275 [PubMed] [Google Scholar]
- 37. Hayes J.D, et al. (1995). The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol., 30, 445–600 [DOI] [PubMed] [Google Scholar]
- 38. Robert J, et al. (2003). Multidrug resistance reversal agents. J. Med. Chem., 46, 4805–4817 [DOI] [PubMed] [Google Scholar]
- 39. Belpomme D, et al. (2000). Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann. Oncol., 11, 1471–1476 [DOI] [PubMed] [Google Scholar]
- 40. Warner E, et al. (1998). Phase II study of dexverapamil plus anthracycline in patients with metastatic breast cancer who have progressed on the same anthracycline regimen. Clin. Cancer Res., 4, 1451–1457 [PubMed] [Google Scholar]
- 41. Rowinsky E.K, et al. (1994). Phase I and pharmacodynamic study of the topoisomerase I-inhibitor topotecan in patients with refractory acute leukemia. J. Clin. Oncol., 12, 2193–2203 [DOI] [PubMed] [Google Scholar]
- 42. Wishart G.C, et al. (1994). Quinidine as a resistance modulator of epirubicin in advanced breast cancer: mature results of a placebo-controlled randomized trial. J. Clin. Oncol., 12, 1771–1777 [DOI] [PubMed] [Google Scholar]
- 43. Tsimberidou A, et al. (2003) Gemtuzumab, fludarabine, cytarabine, and cyclosporine in patients with newly diagnosed acute myelogenous leukemia or high-risk myelodysplastic syndromes. Cancer., 97, 1481–1487 [DOI] [PubMed] [Google Scholar]
- 44. List A.F, et al. (2001). Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood, 98, 3212–3220 [DOI] [PubMed] [Google Scholar]
- 45. Gruber A, et al. (2003). A phase I/II study of the MDR modulator Valspodar (PSC 833) combined with daunorubicin and cytarabine in patients with relapsed and primary refractory acute myeloid leukemia. Leuk. Res., 27, 323–328 [DOI] [PubMed] [Google Scholar]
- 46. Szakács G, et al. (2006). Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov., 5, 219–234 [DOI] [PubMed] [Google Scholar]
- 47. Hindenburg A.A, et al. (1989). Intracellular distribution and pharmacokinetics of daunorubicin in anthracycline-sensitive and -resistant HL-60 cells. Cancer Res., 49, 4607–4614 [PubMed] [Google Scholar]
- 48. Altan N, et al. (1998) Defective acidification in human breast tumor cells and implications for chemotherapy. J. Exp. Med., 187, 1583–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tell G, et al. (2010) Targeting DNA repair proteins for cancer treatment. Cell. Mol. Life Sci., 67, 3569–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hegi M.E, et al. (2004). Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res., 10, 1871–1874 [DOI] [PubMed] [Google Scholar]
- 51. Agnihotri S, et al. (2012) Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J. Clin. Invest., 122, 253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Knappskog S, et al. (2012). Low expression levels of ATM may substitute for CHEK2 /TP53 mutations predicting resistance towards anthracycline and mitomycin chemotherapy in breast cancer. Breast Cancer Res., 14, R47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Adams J.M, et al. (2007). The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene, 26, 1324–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Happo L, et al. (2010). Maximal killing of lymphoma cells by DNA damage-inducing therapy requires not only the p53 targets Puma and Noxa, but also Bim. Blood, 116, 5256–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fulda S, et al. (2012). Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov., 11, 109–124 [DOI] [PubMed] [Google Scholar]
- 56. Ni Chonghaile T, et al. (2008). Mimicking the BH3 domain to kill cancer cells. Oncogene, 27 (suppl. 1), S149–S157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kutuk O, et al. (2008). Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res., 68, 7985–7994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weinstein I.B. (2002). Cancer. Addiction to oncogenes–the Achilles heal of cancer. Science, 297, 63–64 [DOI] [PubMed] [Google Scholar]
- 59. Weinstein I.B, et al. (2008). Oncogene addiction. Cancer Res., 68, 3077–3080 [DOI] [PubMed] [Google Scholar]
- 60. Weinstein I.B, et al. (2006). Mechanisms of disease: oncogene addiction–a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol., 3, 448–457 [DOI] [PubMed] [Google Scholar]
- 61. Sharma S.V, et al. (2007). Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev., 21, 3214–3231 [DOI] [PubMed] [Google Scholar]
- 62. Casaletto J.B, et al. (2012). Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer, 12, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mellinghoff I.K, et al. (2005). Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med., 353, 2012–2024 [DOI] [PubMed] [Google Scholar]
- 64. Nielsen D.L, et al. (2013). Efficacy of HER2-targeted therapy in metastatic breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Breast, 22, 1–12 [DOI] [PubMed] [Google Scholar]
- 65. Gherardi E, et al. (2012). Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer, 12, 89–103 [DOI] [PubMed] [Google Scholar]
- 66. Weigelt B, et al. (2012). Genomic determinants of PI3K pathway inhibitor response in cancer. Front. Oncol., 2, 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cloughesy T.F, et al. (2008). Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med., 5, e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Flaherty K.T, et al. (2012). From genes to drugs: targeted strategies for melanoma. Nat. Rev. Cancer, 12, 349–361 [DOI] [PubMed] [Google Scholar]
- 69. Masui K, et al. (2012). Review: molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol. Appl. Neurobiol., 38, 271–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vivanco I, et al. (2012). Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov., 2, 458–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tsao A.S, et al. (2012). Clinical outcomes and biomarker profiles of elderly pretreated NSCLC patients from the BATTLE trial. J. Thorac. Oncol., 7, 1645–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kobayashi S, et al. (2005). EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med., 352, 786–792 [DOI] [PubMed] [Google Scholar]
- 73. Kwak E.L, et al. (2010). Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med., 363, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang X, et al. (2008). Molecular predictors of EGFR-TKI sensitivity in advanced non-small cell lung cancer. Int. J. Med. Sci., 5, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sequist L.V, et al. (2011). Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med., 3, 75ra26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ng K.P, et al. (2012). A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat. Med., 18, 521–528 [DOI] [PubMed] [Google Scholar]
- 77. Jänne P.A, et al. (2012). Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J. Clin. Oncol., 30, 2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heinrich M.C, et al. (2003). Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol., 21, 4342–4349 [DOI] [PubMed] [Google Scholar]
- 79. Antonescu C.R. (2011). The GIST paradigm: lessons for other kinase-driven cancers. J. Pathol., 223, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Di Nicolantonio F, et al. (2010). Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J. Clin. Invest., 120, 2858–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Prahallad A, et al. (2012). Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature, 483, 100–103 [DOI] [PubMed] [Google Scholar]
- 82. Fong P.C, et al. (2009). Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med., 361, 123–134 [DOI] [PubMed] [Google Scholar]
- 83. Sun W. (2012). Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J. Hematol. Oncol., 5, 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Packer L.M, et al. (2011). Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell, 20, 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Druker B.J, et al. (2001). Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med., 344, 1031–1037 [DOI] [PubMed] [Google Scholar]
- 86. Kantarjian H.M, et al. (2010). Optimizing therapy for patients with chronic myelogenous leukemia in chronic phase. Cancer, 116, 1419–1430 [DOI] [PubMed] [Google Scholar]
- 87. O’Hare T, et al. (2012). Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat. Rev. Cancer, 12, 513–526 [DOI] [PubMed] [Google Scholar]
- 88. Mahon F.X, et al. (2000). Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood, 96, 1070–1079 [PubMed] [Google Scholar]
- 89. Esposito N, et al. (2011). SHP-1 expression accounts for resistance to imatinib treatment in Philadelphia chromosome-positive cells derived from patients with chronic myeloid leukemia. Blood, 118, 3634–3644 [DOI] [PubMed] [Google Scholar]
- 90. Burchert A, et al. (2005). Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia, 19, 1774–1782 [DOI] [PubMed] [Google Scholar]
- 91. Apperley J.F. (2007). Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol., 8, 1018–1029 [DOI] [PubMed] [Google Scholar]
- 92. O’Hare T, et al. (2011) Targeting the BCR-ABL signaling pathway in therapy-resistant Philadelphia chromosome-positive leukemia. Clin. Cancer Res., 17, 212–221 [DOI] [PubMed] [Google Scholar]
- 93. Chen L, et al. (2007). Recent development of IMP dehydrogenase inhibitors for the treatment of cancer. Curr. Opin. Drug Discov. Devel., 10, 403–412 [PubMed] [Google Scholar]
- 94. Janku F, et al. (2013) PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res., 73, 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Faber A.C, et al. (2011). BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov., 1, 352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ibrahim Y.H, et al. (2012) PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov., 2, 1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lai Y.L, et al. (2008). PIK3CA exon 20 mutation is independently associated with a poor prognosis in breast cancer patients. Ann. Surg. Oncol., 15, 1064–1069 [DOI] [PubMed] [Google Scholar]
- 98. Janku F, et al. (2012). PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J. Clin. Oncol., 30, 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Karamouzis M.V, et al. (2009). Targeting MET as a strategy to overcome crosstalk-related resistance to EGFR inhibitors. Lancet Oncol., 10, 709–717 [DOI] [PubMed] [Google Scholar]
- 100. Nghiemphu P.L, et al. (2012). A dose escalation trial for the combination of erlotinib and sirolimus for recurrent malignant gliomas. J. Neurooncol., 110, 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mellinghoff I.K, et al. (2007) PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin. Cancer Res., 13(2 Pt 1), 378–381 [DOI] [PubMed] [Google Scholar]
- 102. de Vries N.A, et al. (2012). Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest. New Drugs, 30, 443–449 [DOI] [PubMed] [Google Scholar]
- 103. Weller M. (2011). Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med. Wkly., 141, w13210 [DOI] [PubMed] [Google Scholar]
- 104. Fenton T.R, et al. (2012). Resistance to EGF receptor inhibitors in glioblastoma mediated by phosphorylation of the PTEN tumor suppressor at tyrosine 240. Proc. Natl. Acad. Sci. U.S.A., 109, 14164–14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Elfiky A.A, et al. (2012). Novel molecular targets for the therapy of renal cell carcinoma. Discov. Med., 13, 461–471 [PubMed] [Google Scholar]
- 106. Mahalingam D, et al. (2010) Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels. Clin. Cancer Res., 16, 141–153 [DOI] [PubMed] [Google Scholar]
- 107. Hainsworth J.D, et al. (2010). Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J. Clin. Oncol., 28, 2131–2136 [DOI] [PubMed] [Google Scholar]
- 108. Ghamande S.A, et al. (2008). A phase 2, randomized, double-blind, placebo-controlled trial of clinical activity and safety of subcutaneous A6 in women with asymptomatic CA125 progression after first-line chemotherapy of epithelial ovarian cancer. Gynecol. Oncol., 111, 89–94 [DOI] [PubMed] [Google Scholar]
- 109. Janku F, et al. (2011). PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol. Cancer Ther., 10, 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Slomovitz B.M, et al. (2012) The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res., 18, 5856–5864 [DOI] [PubMed] [Google Scholar]
- 111. Chapman P.B, et al. ; BRIM-3 Study Group (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med., 364, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nazarian R, et al. (2010). Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature, 468, 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Johannessen C.M, et al. (2010). COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature, 468, 968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Villanueva J, et al. (2010). Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell, 18, 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Paraiso K.H, et al. (2011). PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res., 71, 2750–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Emery C.M, et al. (2009). MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl. Acad. Sci. U.S.A., 106, 20411–20416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Poulikakos P.I, et al. (2011). RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature, 480, 387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shi H, et al. (2012). Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat. Commun., 3, 724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Greger J.G, et al. (2012). Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol. Cancer Ther., 11, 909–920 [DOI] [PubMed] [Google Scholar]
- 120. Flaherty K.T, et al. (2012). Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med., 367, 1694–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Roschewski M, et al. (2012) Diffuse large B cell lymphoma: molecular targeted therapy. Int. J. Hematol., 96, 552–561 [DOI] [PubMed] [Google Scholar]
- 122. Friedberg J.W, et al. (2010). Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood, 115, 2578–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Robertson M.J, et al. (2007). Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol., 25, 1741–1746 [DOI] [PubMed] [Google Scholar]
- 124. Foon K.A, et al. (2012). Novel therapies for aggressive B-cell lymphoma. Adv. Hematol., 2012, 302570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Scott A.M, et al. (2012). Antibody therapy of cancer. Nat. Rev. Cancer, 12, 278–287 [DOI] [PubMed] [Google Scholar]
- 126. Pardoll D.M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer, 12, 252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Baselga J, et al. ; CLEOPATRA Study Group (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med., 366, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Blackwell K.L, et al. (2012) Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane. ASCO Meeting Abstracts, 30, LBA1 [Google Scholar]
- 129. Neyns B, et al. (2009). Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann. Oncol., 20, 1596–1603 [DOI] [PubMed] [Google Scholar]
- 130. Varker K.A, et al. (2007). A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann. Surg. Oncol., 14, 2367–2376 [DOI] [PubMed] [Google Scholar]
- 131. Hodi F.S, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med., 363, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Robert C, et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med., 364, 2517–2526 [DOI] [PubMed] [Google Scholar]
- 133. Coiffier B, et al. (2010). Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood, 116, 2040–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vanneman M, et al. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer, 12, 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tanaka K, et al. (2011). Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov., 1, 524–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Thomas H.E, et al. (2012). mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci. Transl. Med., 4, 139ra84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Haas-Kogan D.A, et al. (2005). Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J. Natl. Cancer Inst., 97, 880–887 [DOI] [PubMed] [Google Scholar]
- 138. Kreisl T.N, et al. (2009). A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J. Neurooncol., 92, 99–105 [DOI] [PubMed] [Google Scholar]
- 139. Reardon D.A, et al. (2006). Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin. Cancer Res., 12(3 Pt 1)860–868 [DOI] [PubMed] [Google Scholar]
- 140. Stommel J.M. (2007). Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science, 318, 287–290 [DOI] [PubMed] [Google Scholar]
- 141. Huang P.H, et al. (2007) Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc. Natl. Acad. Sci. U.S.A., 104, 12867–12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chandarlapaty S, et al. (2011). AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell, 19, 58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gazdar A.F. (2009). Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene, 28 (suppl. 1), S24–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Balak M.N, et al. (2006). Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin. Cancer Res., 12, 6494–6501 [DOI] [PubMed] [Google Scholar]
- 145. Miller V.A, et al. (2012). Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol., 13, 528–538 [DOI] [PubMed] [Google Scholar]
- 146. Zhou W, et al. (2009). Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature, 462, 1070–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Saal L.H, et al. (2005). PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res., 65, 2554–2559 [DOI] [PubMed] [Google Scholar]
- 148. Berns K, et al. (2007). A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell, 12, 395–402 [DOI] [PubMed] [Google Scholar]
- 149. De Roock W, et al. (2010). Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol., 11, 753–762 [DOI] [PubMed] [Google Scholar]
- 150. Chang S.M, et al. ; North American Brain Tumor Consortium and the National Cancer Institute (2005) Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest. New Drugs, 23, 357–361 [DOI] [PubMed] [Google Scholar]
- 151. Galanis E, et al. ; North Central Cancer Treatment Group (2005) Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol., 23, 5294–5304 [DOI] [PubMed] [Google Scholar]
- 152. Reardon D.A, et al. (2010). Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol., 96, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fan Q.W, et al. (2006). A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell, 9, 341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Carracedo A, et al. (2008). Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest., 118, 3065–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Thoreen C.C, et al. (2009). An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem., 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rodrik-Outmezguine V.S, et al. (2011). mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov., 1, 248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Trumpp A, et al. (2008). Mechanisms of disease: cancer stem cells–targeting the evil twin. Nat. Clin. Pract. Oncol., 5, 337–347 [DOI] [PubMed] [Google Scholar]
- 158. Bao S, et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 444, 756–760 [DOI] [PubMed] [Google Scholar]
- 159. Sharma S.V, et al. (2010). A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell, 141, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dedes K.J, et al. (2010). PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci. Transl. Med., 2, 53ra75 [DOI] [PubMed] [Google Scholar]
- 161. Turner N.C, et al. (2012). Genetic heterogeneity and cancer drug resistance. Lancet Oncol., 13, e178–e185 [DOI] [PubMed] [Google Scholar]
- 162. Yap T.A, et al. (2012). Intratumor heterogeneity: seeing the wood for the trees. Sci. Transl. Med., 4, 127ps10 [DOI] [PubMed] [Google Scholar]
- 163. Gerlinger M, et al. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med., 366, 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Shah S.P, et al. (2012). The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature, 486, 395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Christensen J.G, et al. (2007). Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther., 6(12 Pt 1)3314–3322 [DOI] [PubMed] [Google Scholar]
- 166. Kentsis A, et al. (2012). Autocrine activation of the MET receptor tyrosine kinase in acute myeloid leukemia. Nat. Med., 18, 1118–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Camidge D.R, et al. (2012). Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol., 13, 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Butrynski J.E, et al. (2010) Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med., 363, 1727–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Costa D.B, et al. (2011) CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J. Clin. Oncol., 29, e443–e445 [DOI] [PubMed] [Google Scholar]
- 170. Choi Y.L, et al. ; ALK Lung Cancer Study Group (2010) EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med., 363, 1734–1739 [DOI] [PubMed] [Google Scholar]
- 171. Sasaki T, et al. (2010). The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res., 70, 10038–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Heuckmann J.M, et al. (2011) ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin. Cancer Res., 17, 7394–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yamada T, et al. (2012) Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells. Clin. Cancer Res., 18, 3592–3602 [DOI] [PubMed] [Google Scholar]
- 174. Berry T, et al. (2012). The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell, 22, 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Byers L.A, et al. (2013). An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res., 19, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhang Z, et al. (2012). Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet., 44, 852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Gasparini G, et al. (2007). Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res. Treat., 101, 355–365 [DOI] [PubMed] [Google Scholar]
- 178. Chapuis N, et al. (2010). Perspectives on inhibiting mTOR as a future treatment strategy for hematological malignancies. Leukemia, 24, 1686–1699 [DOI] [PubMed] [Google Scholar]
- 179. Zhu J, et al. (2007). RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell, 12, 23–35 [DOI] [PubMed] [Google Scholar]
- 180. Zhang Y, et al. (2013) Long-term efficacy and safety of arsenic trioxide for first-line treatment of elderly patients with newly diagnosed acute promyelocytic leukemia. Cancer, 119, 115–125 [DOI] [PubMed] [Google Scholar]
- 181. Martinelli G, et al. (1998). Disappearance of PML/RAR alpha acute promyelocytic leukemia-associated transcript during consolidation chemotherapy. Haematologica, 83, 985–988 [PubMed] [Google Scholar]
- 182. Sanz M.A, et al. PETHEMA and HOVON Groups (2010) Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood, 115, 5137–5146 [DOI] [PubMed] [Google Scholar]
- 183. Reya T, et al. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–111 [DOI] [PubMed] [Google Scholar]
- 184. Wilson W.H, et al. (2010). Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol., 11, 1149–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rudin C.M, et al. (2012) Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res., 18, 3163–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Baggstrom M.Q, et al. ; Mayo Phase 2 Consortium; California Consortium (2011) A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J. Thorac. Oncol., 6, 1757–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sonpavde G, et al. (2012). Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann. Oncol., 23, 1803–1808 [DOI] [PubMed] [Google Scholar]
- 188. Mathieu V, et al. (2008). Combining bevacizumab with temozolomide increases the antitumor efficacy of temozolomide in a human glioblastoma orthotopic xenograft model. Neoplasia, 10, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Volk L.D, et al. (2008). Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia, 10, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kerbel R.S. (2006). Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science, 312, 1171–1175 [DOI] [PubMed] [Google Scholar]
- 191. Claes A, et al. (2008). Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization. Mol. Cancer Ther., 7, 71–78 [DOI] [PubMed] [Google Scholar]
- 192. Shepherd F.A, et al. ; National Cancer Institute of Canada Clinical Trials Group (2005) Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med., 353, 123–132 [DOI] [PubMed] [Google Scholar]
- 193. Reck M, et al. (2010). Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J. Thorac. Oncol., 5, 1616–1622 [DOI] [PubMed] [Google Scholar]
- 194. Giaccone G, et al. (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. J. Clin. Oncol., 22, 777–784 [DOI] [PubMed] [Google Scholar]
- 195. Herbst R.S, et al. (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J. Clin. Oncol., 22, 785–794 [DOI] [PubMed] [Google Scholar]
- 196. Herbst R.S, et al. ; TRIBUTE Investigator Group (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol., 23, 5892–5899 [DOI] [PubMed] [Google Scholar]
- 197. Gatzemeier U, et al. (2007) Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J. Clin. Oncol., 25, 1545–1552 [DOI] [PubMed] [Google Scholar]
- 198. Davies A.M, et al. (2006). Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin. Lung Cancer, 7, 385–388 [DOI] [PubMed] [Google Scholar]
- 199. Li T, et al. (2007) Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin. Cancer Res., 13, 3413–3422 [DOI] [PubMed] [Google Scholar]
- 200. Solit D.B, et al. (2005). Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continuous dosing for sensitizing tumors to paclitaxel. Clin. Cancer Res., 11, 1983–1989 [DOI] [PubMed] [Google Scholar]
- 201. Mahaffey C.M, et al. (2007). Schedule-dependent apoptosis in K-ras mutant non-small-cell lung cancer cell lines treated with docetaxel and erlotinib: rationale for pharmacodynamic separation. Clin. Lung Cancer, 8, 548–553 [DOI] [PubMed] [Google Scholar]
- 202. Mok T.S, et al. (2009) Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J. Clin. Oncol., 27, 5080–5087 [DOI] [PubMed] [Google Scholar]
- 203. Gridelli C, et al. (2012) First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J. Clin. Oncol., 30, 3002–3011 [DOI] [PubMed] [Google Scholar]
- 204. Verhaak R.G, et al. ; Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17, 98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]