Abstract
The unique formation-structure-property attributes and reaction behavior of the thiol-ene “click” reaction have been explored extensively for photochemically and thermally initiated reactions but have been much less explored for redox initiation. Therefore, the objective of this work is to characterize fully the impact of the initiation system, monomer structure, degree of functionalization, and inhibitor level on the redox-mediated thiol-ene polymerization rate and behavior. Moreover, this study confirms the ability of redox initiation to achieve full conversion of desired thiol-ene “click” products for small molecules in solution. For the multifunctional thiol-ene systems, polymerization rate was shown to be comparable to photo- and thermally initiated systems, but with the additional advantages of unlimited depth of cure and mild reaction conditions. Additionally, the network properties of the redox-initiated thiol-ene systems were on par with a photocured material formulated with identical monomers and radical initiating potential. Lastly, control over the polymerization rate and preceding induction period was garnered from the concentration of inhibitor included in the reaction mixture. The mechanism of action of quinone inhibition in redox-mediated thiol-ene polymerizations is shown to depend on both the presence of an aniline reducing agent and the concentration of inhibitor, with quinone concentrations in great excess of oxidizing agent concentrations actually leading to heightened polymerization rates when aniline is present.
Introduction
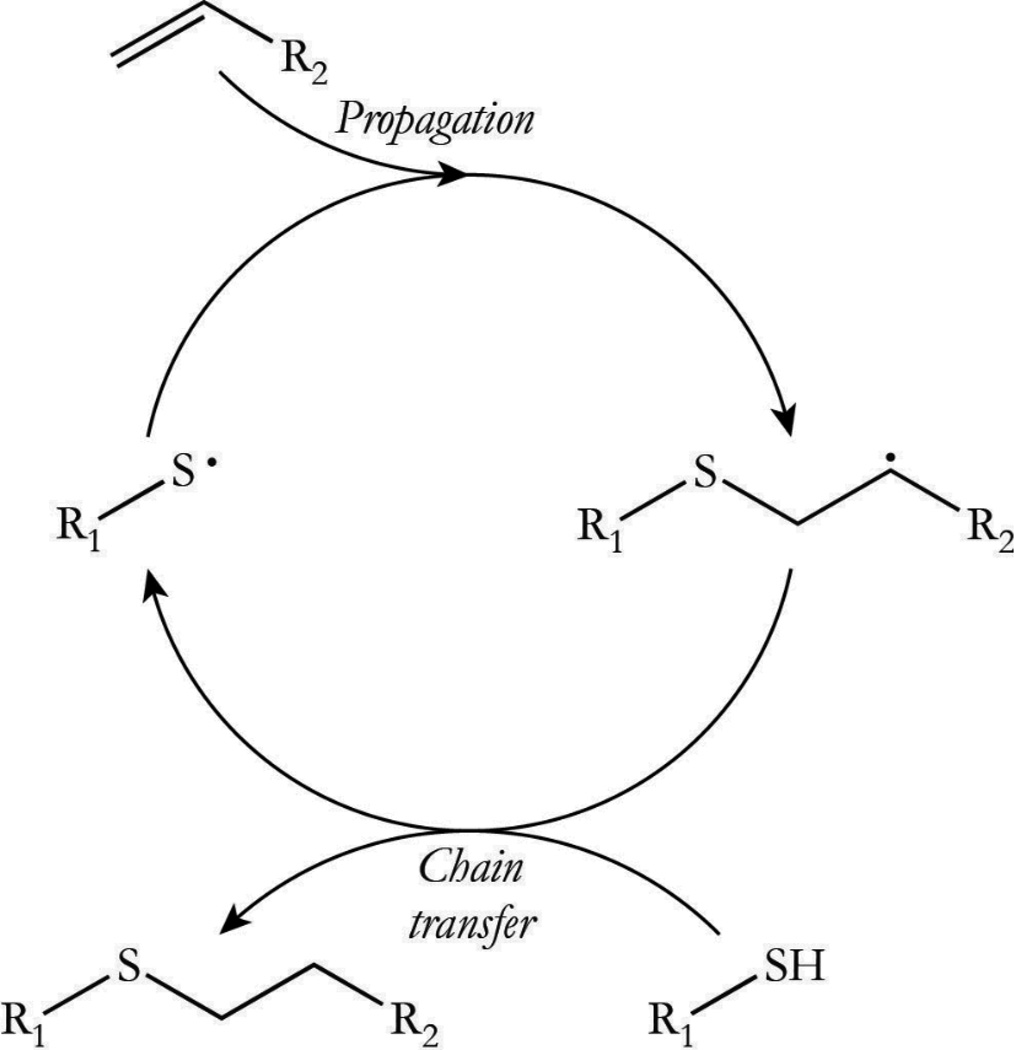
Thiol-ene “click” reactions are well documented with respect to their orthogonality, rapid polymerization rates, and amenability to various modes of initiation.1–4 The addition of thiols to activated olefins, for example, is readily achieved in the presence of a nucleophilic or a basic catalyst with negligible side reactions taking place and quantifiable conversions being attained.5 The thiol-ene reaction is also highly amenable to radical-mediated polymerizations, which are most frequently initiated by photochemical and thermal methodologies.6, 7 The unique reaction behavior and polymer network formation-structure-property attributes achieved in radical-mediated thiol-ene reactions have prompted its thorough investigation with respect to thiol and ene structures and the impact each has on the observed polymerization rate.8–10 Specifically, the thiol-ene polymerization proceeds through an alternating combination of propagation and chain transfer reactions wherein a thiyl radical propagates through a carbon-carbon double bond, generating a carbon-centered radical that abstracts a hydrogen from a thiol to regenerate a thiyl radical (Scheme 1). In polymerizing systems, such behavior delays the gelation of multifunctional monomer systems, which reduces polymerization induced shrinkage and shrinkage stress.11, 12 Accordingly, thiol-ene polymerizations are often used to produce coatings,13 microfluidic devices,14 optical lenses,15 dental materials,16 and holographic diffractive materials,17 among others.
Scheme 1.
Scheme 1. Thiol-Ene Reaction Mechanism. The thiol-ene polymerization proceeds through a cyclic step growth mechanism consisting of alternating propagation/chain transfer steps following initiation and prior to termination. The reaction mechanism assumes ideal conditions in which the alternating steps proceed at the same overall rate.
While the current modes of initiating thiol-ene polymerizations and other thiol-ene reactions have numerous apparent advantages, including, for example, the spatial and temporal control afforded by photopolymerizations, each mechanism also has its associated limitations. The breadth of suitable applications for photochemically and thermally initiated thiol-ene reactions is restricted by their respective capacities to transmit light (or heat) uniformly in opaque systems and withstand the elevated temperatures necessary to produce primary radical species in thermal systems. In the case of photoinitiation, sample geometry and formulation contents must be carefully considered, as optically thick samples, strongly absorbing initiators, and the presence of dyes and/or pigments will create gradients in light intensity and negatively impact network properties, even preventing reaction at the bottom of thick films.6 For functionalization reactions and small molecule production, the need to expose a large volume of reactants to a uniform light intensity can prove impossible, despite attempts to mitigate the non-uniformity with high degrees of mixing. Thermally initiated systems, on the other hand, demand elevated temperatures that for some substrates and circumstances make them unsuitable for biomedical applications or employ initiators that generate gaseous by-products that can compromise the mechanical integrity of the polymerized system. In particular, the standard operation range of a widely used azo initiator, 2,2’-azobisisobutyronitrile (AIBN), is 50–70 °C, and its thermal decomposition results in the evolution of nitrogen gas.18 Clearly, the use of a thermal initiator eliminates the possibility for conducting the reaction at ambient conditions that are desirable for many implementations of this click reaction.
In contrast to the thermal and photochemical initiation of thiol-ene reactions, little work has been done to initiate these reactions with traditional redox radical initiation systems.19, 20 Radical-mediated redox polymerizations would eliminate the compulsory concern for the electro negativity of the ene moiety, problems associated with the sample geometry and pigmentation, and any problems associated with elevated temperatures. Historically, Fenton’s chemistry has been utilized for the production of hydroxyl radicals capable of initiating polymerization. Although the ferrous iron is the traditional reducing agent, other transition metals, namely Cr2+, V2+, Co2+, and Cu+, have also been employed.18, 21 Likewise, ferrous iron may serve as the reductant for various types of organic peroxides in lieu of hydrogen peroxide.18, 21 More recently, enzyme-mediated approaches to Fenton’s chemistry have been used to develop signal amplification techniques for biorecognition, as well as to produce multilayered poly(ethylene glycol) (PEG) hydrogels and immunoactive barriers.22–24 Still, the use of an enzymatic approach necessitates tight control of medium acidity to optimize enzyme activity and generally requires that the polymerization be conducted in an aqueous environment. Specifically, the glucose oxidase mediated reaction is optimized when performed at a pH of 5.5 and requires the substrate β-D-glucose in addition to a suitable reductant (i.e., Fe2+) to generate the primary hydroxyl radical.25, 26 Though the immobilization of glucose oxidase may enhance its stability, the enzyme must maintain a favorable conformation for its active site to be effective, and the presence of organic solvents or neat monomer may counteract this occurrence.27, 28
Still, neat redox-initiated reactions have been conducted in both the industrial and biological arenas through the combination of an organic peroxide with a tertiary amine. In particular, the benzoyl peroxide (BPO) and N,N-dimethyl-p-toluidine (DMT) redox pair has been used for decades to initiate the free radical polymerization of methacrylate monomers in bone cements and dental resins.29, 30 More recently, the same redox pair has been applied to the fabrication of microfluidic chips and hydrogels, again through the network formation of multifunctional methacrylate monomers.31, 32 In contrast, little work has been conducted on the use of BPO/DMT or similar redox initiating systems in neat thiol-ene formulations despite the abundant advantages of thiol-ene copolymerizations over methacrylate homopolymerizations. Thiol-ene reactions are appropriate for numerous small molecule and functionalization reactions,33, 34 and thiol-ene polymerizations have lower volumetric shrinkage and shrinkage stress, are not susceptible to oxygen inhibition, and proceed at a faster overall polymerization rate than their (meth)acrylate counterparts.11, 35 Thus, the primary objective of this work is to evaluate the initiation potential of BPO/DMT in bulk thiol-ene reactions and polymerizations.
Herein, the effects of the initiation mode on the observed reaction rate and polymer network properties are evaluated. Three traditional thiol monomers of varying electrophicity and degree of functionalization were reacted with a single ene-functionalized monomer to demonstrate the general value of this approach, and the polymerization rates were monitored via real-time FTIR for varying redox initiation conditions and concentrations. A multifunctional thiol was then used to prepare crosslinked networks using both redox and photopolymerization techniques, and the thermomechanical properties of each network were measured. Additionally, the nucleophilicity of the tertiary amine was varied, and the subsequent impact on polymerization rate determined. The role of quinone inhibition on the thiol-ene polymerization was also investigated by gradually increasing the molar fraction of inhibitor relative to BPO in a single thiol-ene formulation. Finally, a redox-mediated thiol-ene substitution reaction in small molecules was evaluated for its potential advantages in a variety of other reaction schemes in which thiol-ene reactions have been used such as polymer substitution, surface modification and bioconjugation.
Experimental
Materials
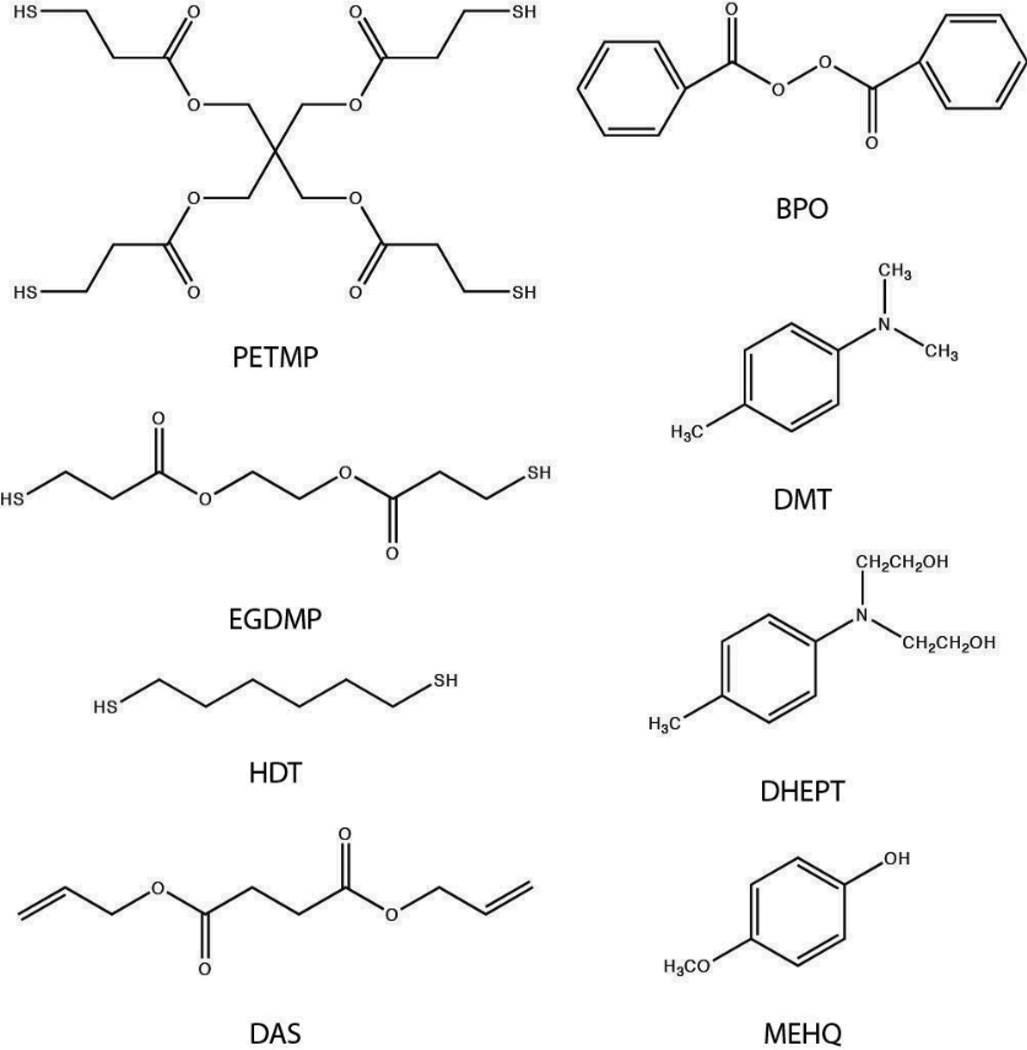
Pentaerythritol tetra-3-mercaptopropionate (PETMP) and glycol di-3-mercaptopropionate (EGDMP) were purchased from Evans Chemetics (Teaneck, NJ). 1,6-hexane dithiol (HDT, ≥97%), diallyl succinate (DAS), N,N-dimethyl-p-toluidine (DMT, ≥98.5%), N,N-bis(2-hydroxyethyl)-p-toluidine (DHEPT, ≥97%), benzoyl peroxide (BPO, ≥97%), 4-vinylpyridine (95%) and monomethyl ether hydroquinone (MEHQ, ≥98%) were purchased from Sigma Aldrich (St. Louis, MO). Irgacure 184 (1-hydroxy-cyclohexyl-phenyl-ketone) was purchased from Ciba Specialty Chemicals. 3-mercaptopropyl trimethoxy silane (95%) was purchased from Gelest, Inc (Morrisville, PA). All products were used without further purification, and the structures of all monomers used in this study are shown in Scheme 2a. Additionally, the structures of initiators and inhibitor are shown in Scheme 2b.
Scheme 2.
The materials discussed in this paper: Monomers (PETMP, EGDMP, HDT, DAS), initiators (BPO, DMT, DHEPT), and inhibitor (MEHQ).
Characterization
Polymerization conversion studies were performed on formulated thiol-ene mixtures in the near IR (Nicolet Magna-IR 750 series II FTIR spectrometer). Sample holders were formed from two glass slides (Corning, 0215 Glass, 0.96–1.06 mm thick) separated by a 130 µm plastic spacer. Conversions of thiol (2570 cm−1, S-H stretch) and allyl (4490 cm−1, C=C stretch) functionalities were monitored in real-time at a collection rate of 5 spectra every 2 seconds and a resolution of 2 cm−1. The standard delay between the completion of sample preparation and the start of FTIR spectra collection was 2.5 min. All experiments were performed in triplicate.
Dynamic mechanical analysis (DMA) was performed in tension on samples formed by both redox and photoinitiation using a TA Instruments Q800 DMA scanning at 1 °C/min from -40°C to 40°C at a frequency of 1 Hz and a strain maximum of 0.1%. The crosslink density was estimated from the elastic modulus (E’) in the rubbery regime according to rubber elasticity theory.36 The glass transition temperature (Tg) was defined as the temperature corresponding to the maximum in the tan δ curve. Average sample thickness was 300 µm. Photopolymerized specimens were irradiated with a high-pressure mercury vapor short arc lamp (EXFO Acticure 4000) equipped with a 365 nm narrow bandpass filter. Light intensity was measured with a radiometer equipped with a GaAsP detector (International Light IL1400A, model SEL005), a wide bandpass filter (WBS320), and a quartz diffuser (model W).
Results and discussion
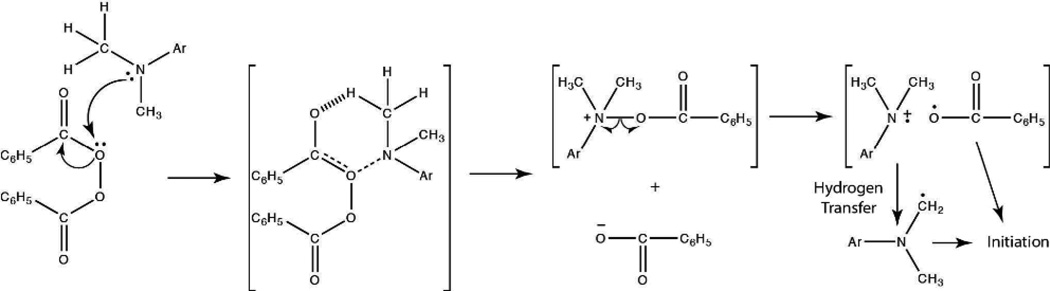
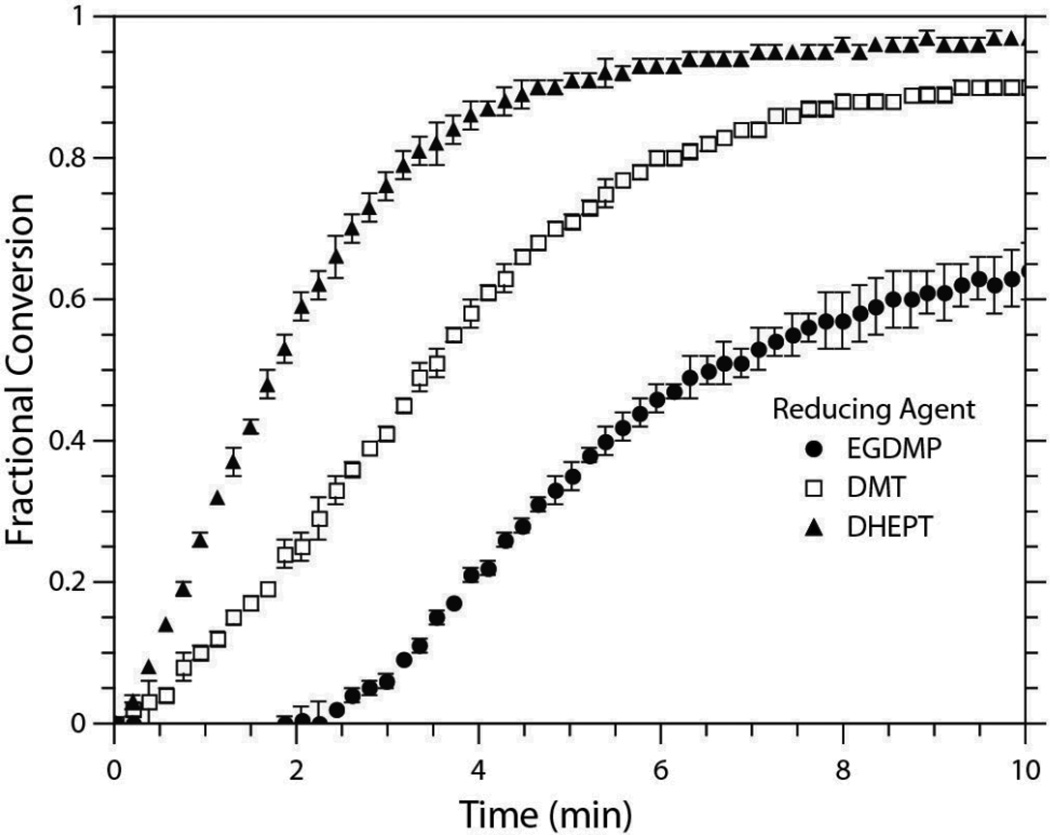
The radical generation mechanism of the BPO/DMT redox system has been well documented by Pryor and Hendrikson to proceed through the formation of a complex intermediate that produces an amine radical cation, a benzoylate anion, and a benzoyloxy radical (Scheme 3).37 Although the benzoyloxy radical is the dominant initiating species, Sato et al. have since demonstrated that the amine radical cation may undergo chain transfer with the benzoylate anion to generate an aminomethyl radical capable of initiating polymerization (Scheme 3).38 The presence of electron-donating substituents on the aniline has further been shown to increase the efficiency of the BPO/amine system.18, 39 Therefore, of the reducing agents employed in this study, formulations containing DHEPT versus DMT would be expected to proceed at more rapid polymerization rates. This tendency is confirmed in Figure 1. When the EGDMP/DAS system is initiated with 0.1 wt% BPO and an equimolar amount of either DHEPT or DMT, the DHEPT system reaches complete conversion in a shorter timeframe.
Scheme 3.
Benzoyl Peroxide/Dimethyl-p-toluidine Initiation Mechanism.
Fig. 1.
Conversion versus time of EGDMP/DAS initiated with three different reducing agents (DMT, DHEPT, EGDMP). All samples were formulated with 0.1 wt% BPO ([BPO]:[Reductant] 1:1) and [BPO]:[MEHQ] of 4:1. Samples were evaluated in triplicate. For clarity, not all data points are included.
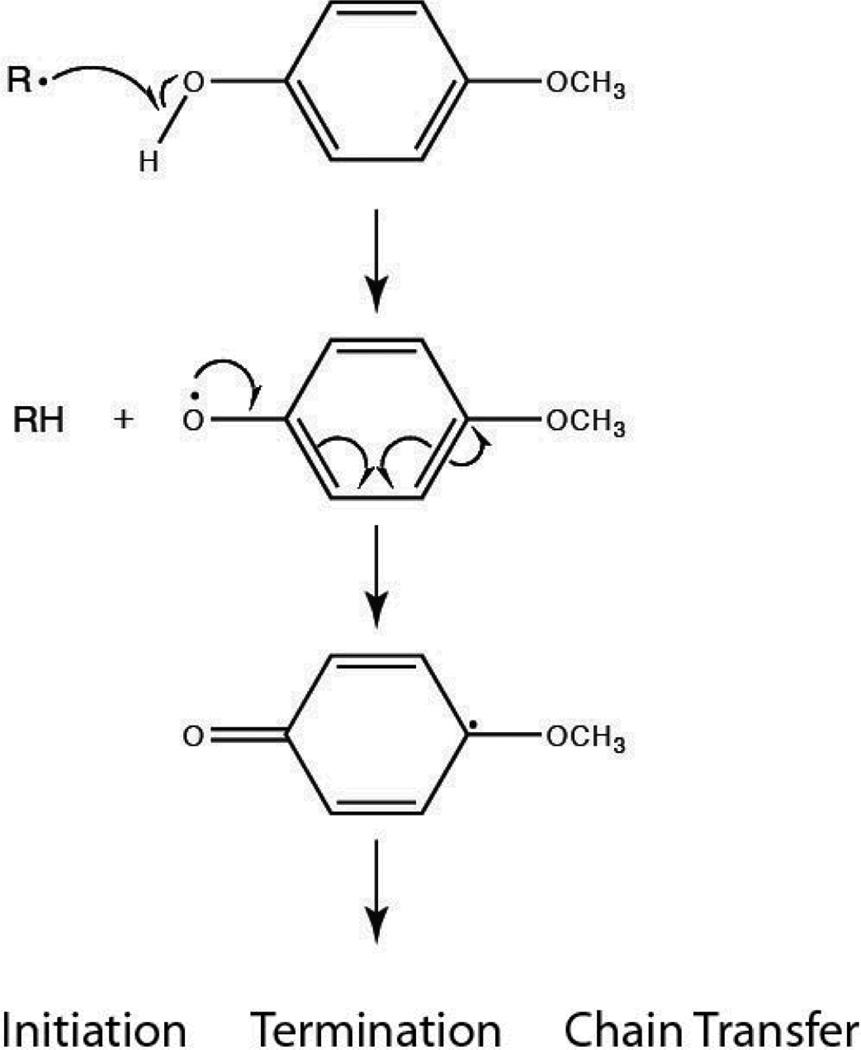
Also shown in Figure 1 is the ability of the thiol functional group in EGDMP to act as the sole reducing agent. Although using only the thiol monomer results in the longest induction period, it has minimal effect on the initial polymerization rate as compared to the DMT initiated system. Such a phenomenon may stem from the natural chain transfer tendency of thiols. Each of the systems presented in Figure 1 was formulated with an inhibitor (MEHQ) at a 4:1 [BPO]:[MEHQ] ratio. The mechanism of MEHQ inhibition (Scheme 4) proceeds through the scavenging of primary benzoyloxy radicals, peroxy radicals, or propagating radicals within the reacting system to form an oxygen-centered MEHQ radical. These radical species then undergo bimolecular termination, which reduces the quantity of initiating radicals and, therefore, lengthens the induction period and slows the polymerization rate. However, with the thiol monomer acting as the reducing agent, no aminomethyl radicals are formed, which, in turn, increases the likelihood of the MEHQ radicals undergoing a chain transfer event with a thiol rather than a termination event with a second radical in solution. Consequently, a longer induction period is observed, as the thiol monomer is a less efficient reducing agent than either of the amine species and benzoyloxy radicals are produced at a slower rate, but the rate of polymerization is minimally impacted since a higher concentration of primary radicals remains to initiate polymerization.
Scheme 4.
Monomethylether hydroquinone (MEHQ) Inhibition Mechanism.
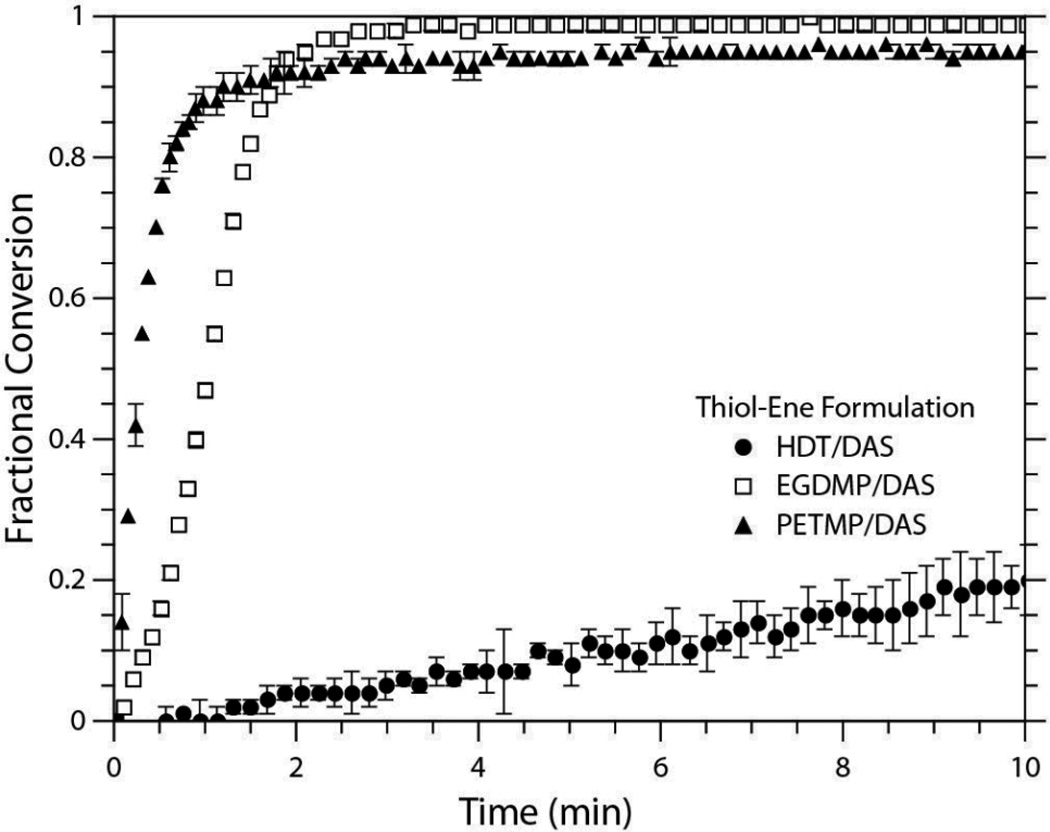
A second factor impacting both the polymerization rate and final conversion is the chemical structure of the thiol monomer. Traditionally, electron-withdrawing substituents increase the reaction rate, as they reduce the electrophilic tendencies of the thiyl radicals and drive propagation through surrounding vinyl monomers. Additionally, the degree of functionalization of the thiol monomer can dramatically impact the rate of polymerization with more highly functional thiols generally more reactive than lower functionality thiols. In particular, the use of multifunctional monomer species lead to network formation, and as the system transitions from a liquid to a gelled state, radical diffusion becomes limited by viscosity effects, and the dominant form of radical movement transitions from translational diffusion through the reaction medium to propagation through a neighboring vinyl monomer. Still, multifunctional thiol or ene monomers with rigid structures may lead to the formation of a glassy network. In such cases, radicals can become trapped within the network as it vitrifies, thereby lowering the final degree of polymerization or reaction yield for small molecules.
The systems used in this study all employed the same diallyl monomer as their vinyl component but varied in the structure of the thiol component that was used. Specifically, alkyl and propionate functionalities were evaluated. Since the propionate moiety offers a much higher electron-withdrawing effect than the six-carbon chain, it was expected to display a much greater polymerization rate under identical reaction conditions. In order to investigate the impact of radical mediated redox polymerization on thiol-ene networks, a multifunctional thiol propionate was also tested. As discussed previously, the rate of polymerization is expected to increase with functionalization, and these expected trends are realized in Figure 2. When the three systems are loaded with 0.2 wt% of BPO ([BPO]:[DMT] of 1:1), both propionate systems exhibit significantly faster rates of polymerization than the alkyl system, despite their higher concentrations of MEHQ. The PETMP/DAS and EGDMP/DAS formulations required a 25 mol% inhibition of BPO (i.e., [BPO]:[MEHQ] of 4:1) just to delay the reaction start so that it did not proceed significantly prior to FTIR collection, whereas the HDT/DAS formulation contained a much lower fraction of inhibitor ([BPO]:[MEHQ] of 10:1).
Fig. 2.
Conversion versus time plots of the three thiol-ene systems evaluated in this study. All samples were formulated with 0.2 wt% BPO and 1:1 [BPO]:[DMT]. The PETMP and EGDMP samples contained 4:1 [BPO]:[MEHQ] and the HDT samples contained 10:1 [BPO]:[MEHQ]. All experiments were conducted in triplicate. For clarity, not all data points are shown.
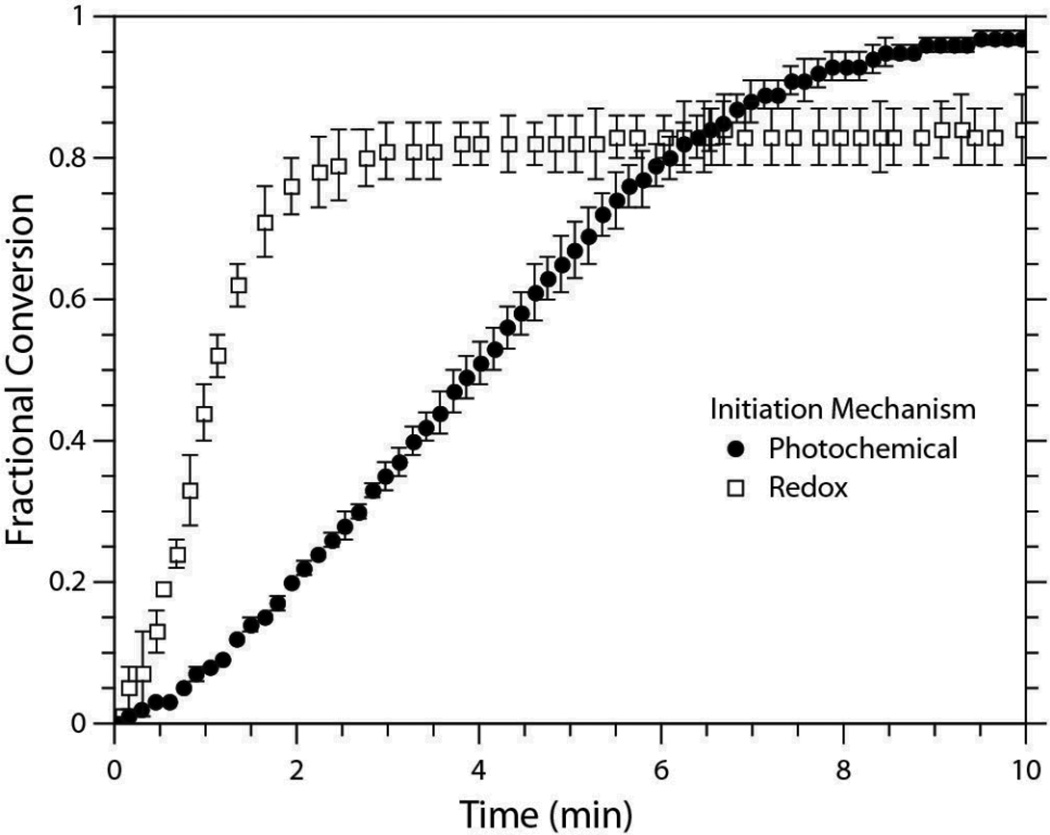
Polymerization rates also follow the expected trend with respect to the degree of functionalization; polymerization rate for the tetrafunctional propionate thiol is greater than that of the difunctional propionate. Closer examination of the conversion versus time plot for the PETMP/DAS system (Figure 3) further reveals that the network formation leads to an increase in polymerization rate with rising fractional conversion (i.e., the system exhibits classical autoacceleration behavior that arises because of diffusion limited termination in radical polymerizations).18 Moreover, a comparison of redox and photoinitiated PETMP/DAS systems formulated with an equivalent primary radical potential and sample thicknesses (300 µm) exhibited similar trends in initial conversion versus time slopes. Obviously, the tendency to undergo autoacceleration must be attributed to both the initiation and polymerization mechanismss. However, the equilibration in reaction conditions did not result in equivalent rates of polymerization or final conversions. The redox-initiated system clearly displayed a faster rate of polymerization, but this occurrence is easily justified by the low light intensity (1.0 mW/cm2) used to form the photoinitiated networks and would readily be reversed at high light intensities. Still, the photoinitiated system proceeded to a higher extent of reaction. The presence of a high level of inhibitor in the PETMP/DAS redox formulation may have played a role in limiting the reaction. As discussed previously, MEHQ can act as a chain transfer agent, particularly from peroxy radicals formed from the combination of molecular oxygen with carbon-centered radicals. The oxygen-centered radical on MEHQ can then reposition itself into tertiary radical capable of terminating other radicals within the reaction medium, thereby reducing the number of radicals available for initiation and/or propagation (Scheme 4).40 When the inhibitor was completely removed from the formulation, the induction period was reduced such that the reaction kinetics could not be monitored in real-time, and the final conversion at 10 min increased noticeably (from 83% to 90%) but remained below the final conversion of the photocured samples (97%).
Fig. 3.
Conversion versus time plot of the PETMP/DAS system polymerized by two initiation mechanisms, redox and photo. The redox samples were formulated with 0.2 wt% BPO, 1:1 [BPO]:[DMT], and 4:1 [BPO]:[MEHQ]. The photo samples contained 0.08 wt% IR184 and were irradiated for 10 min with 1.00 mW/cm2 UV light (365 nm). Sample thickness was 300 µm for both initiation mechanisms.
The differences between the redox and photoinitiated PETMP/DAS networks are further illustrated through minor differences that arise in their thermomechanical properties (Table 1). The redox-initiated systems produced a lower rubbery modulus, which is a consequence of their lower extents of reaction and subsequently lower crosslink densities. The redox system formulated with an inhibitor displayed a 35% reduction in crosslink density, while the neat redox system showed only a 26% reduction from the photoinitiated formulation. Similarly the glass transition temperature in the redox systems is lower than the photo system by 4 K regardless of inhibition level. The glass transition is also directly related to crosslink density and the associated mobility reduction; thus, the reduced reaction extent leads to the redox-initiated system having a lower network property than for the photoinitiated samples. Even though the redox system without inhibitor reached a higher extent of reaction than the system with inhibitor, both systems were less reacted than the photopolymerized system after 10 min. The lower fractional conversion in the uninhibited system may stem from limited working time preventing uniform, ideal films from being made.
Table 1.
Thermomechanical values (avg ± st. dev) for the PETMP/DAS system polymerized by redox and photoinitiation. Average sample thickness was 300 µm. DMA testing was performed in triplicate.
| Initiation Mechanism | E’ (MPa)a | Tg(°C)b | Final Conversion |
|---|---|---|---|
| Light | 7.2 ± 2 | −13 ± 1 | 97 ± 1 |
| Redox (with MEHQ) | 5.5 ± 1 | −17 ± 2 | 83 ± 4 |
| Redox (no MEHQ) | 6.3 ± 0 | −17 ± 0 | 90 ± 2 |
Reported at 25 °C.
Taken as the max tan δ
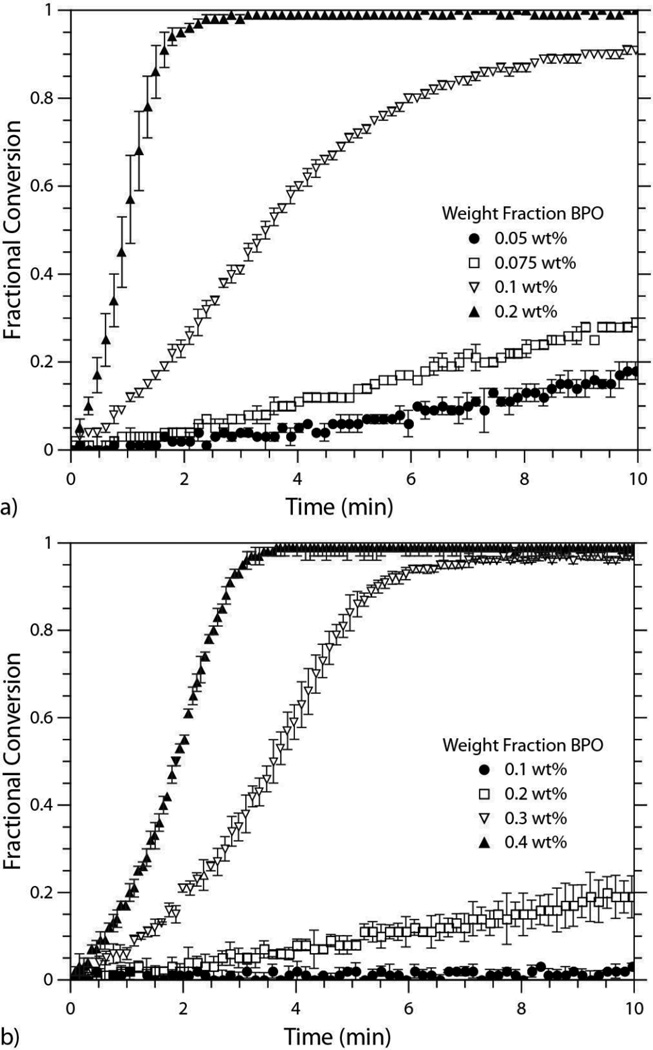
Beyond the comparison of a single thiol-ene formulation polymerized via redox and photoinitiation mechanisms, a thorough investigation of the effect of BPO concentration on the polymerization rate in two different thiol-ene formulations was conducted. The first sytstem utilized the alkyl dithiol, HDT, while the second system contained the propionate dithiol, EGDMP. Both systems were copolymerized with a diallyl monomer (DAS). As noted previously in Figure 2, the propionate system reacts much faster than the alkyl system at identical BPO levels, and this trend persists when the concentration of BPO is systematically varied within the two systems (Figures 4a and 4b). The nucleophicity inherent to the EGDMP monomer leads to a thiol that acts as both a better reducing agent and as a superior chain transfer agent. Consequently, the perpetually faster rates of polymerization in the propionate system achieved by redox initiation, as well as by traditional photoinitiation, are of little surprise.3
Fig. 4.
Conversion versus time plots for the a) EGDMP/DAS system and the b) HDT/DAS system polymerized via redox initiation at 4 BPO concentrations. a) EGDMP/DAS formulated with 0.05 wt%, 0.075 wt%, 0.1 wt%, and 0.2 wt% of BPO and 1:1 [BPO]:[DMT]. The 0.05 wt% and 0.075 wt% samples contained 10:1 [BPO]:[MEHQ], while the 0.1 wt% and 0.2 wt% samples contained 4:1 [BPO]:[MEHQ]. b) HDT/DAS formulated with 0.1 wt%, 0.2 wt%, 0.3 wt%, and 0.4 wt% BPO, 1:1 [BPO]:[DMT], and 10:1 [BPO]:[MEHQ].
The ability of the thiol in the propionate monomer to serve as an efficient reducing agent in conjunction with the aniline (DMT) that is added in equal molar amounts relative to the BPO, allows the EGDMP/DAS system to reach a threshold concentration of primary radicals at a much lower BPO fraction relative to the HDT/DAS system. The EGDMP/DAS formulation ramps from moderate to rapid rates of polymerization between 0.075 wt% and 0.1 wt% BPO. The adiabatic temperature rise associated with the thiol-ene reaction is estimated to be 122 K (assuming ΔHrxn equals 250 mJ/mg)41 and a temperature rise associated with more rapid initiation and limited time for heat transfer is likely responsible for the demonstrated sensitivity of the polymerization rate on redox system concentration. It is possible that the redox system is undergoing side reactions that extinguish primary radicals, e.g., by inhibitors intentionally added or otherwise present, to such a degree that adequate initiating species for rapid polymerization rates are only seen at high initial loads. This facet was recognized in the PETMP/DAS network forming system, as well; even in the absence of inhibitor, full monomer conversion was not seen. Furthermore, the jump in polymerization rate required additional primary radical inhibition to enable the entire polymerization to be captured in real-time by FTIR. At the lower end (≤ 0.075 wt% BPO), the [BPO]:[MEHQ] was 10:1, but was decreased to 4:1 at the higher end (≥ 0.1 wt%). Alternatively, the HDT/DAS system did not achieve the more rapid rates of polymerization until the 0.3 wt% BPO level, and even at the highest BPO concentration, the 10:1 [BPO]:[MEHQ] ratio gave a sufficient induction period for complete FTIR evaluation.
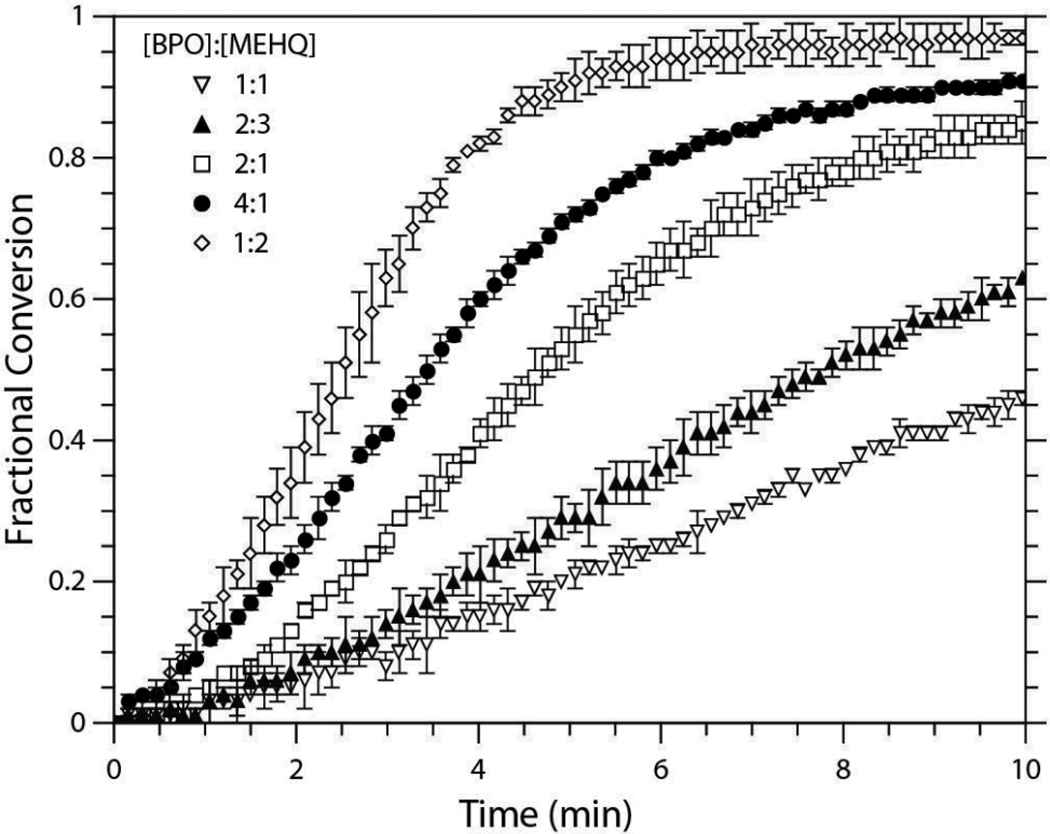
The need for a higher degree of BPO inhibition with increasing polymerization rate lead to further investigations of the impact of MEHQ on the thiol-ene polymerization mechanism, the results of which are shown in Figure 5. When the EGDMP/DAS system was formulated with 0.1 wt% of BPO, the [BPO]:[MEHQ] ratio was varied through an excess of each terminus (i.e., from [BPO]:[MEHQ] ratios of 4:1 to 1:2). The increase in MEHQ concentration from 25 mol% to 50 mol% of the formulation BPO concentration provided a longer induction period and slightly reduced the initial rate of polymerization; however, when doubling the [MEHQ] a second time such that equal molar quantities of MEHQ and BPO were present, a significant reduction in initial polymerization rate is seen. Interestingly, though, as the molar fraction of MEHQ exceeds that of BPO, the polymerization rate actually begins to rise, and the fastest polymerization rate for the EGDMP/DAS samples occurs when the ratio of [BPO]:[MEHQ] is 1:2.
Fig. 5.
Conversion versus time for the EGDMP/DAS system formulated with varying concentrations of MEHQ relative to the 0.1 wt% BPO and 1:1 [BPO]:[DMT]. Five [BPO]:[MEHQ] levels were used (4:1, 2:1, 1:1, 2:3, and 1:2).
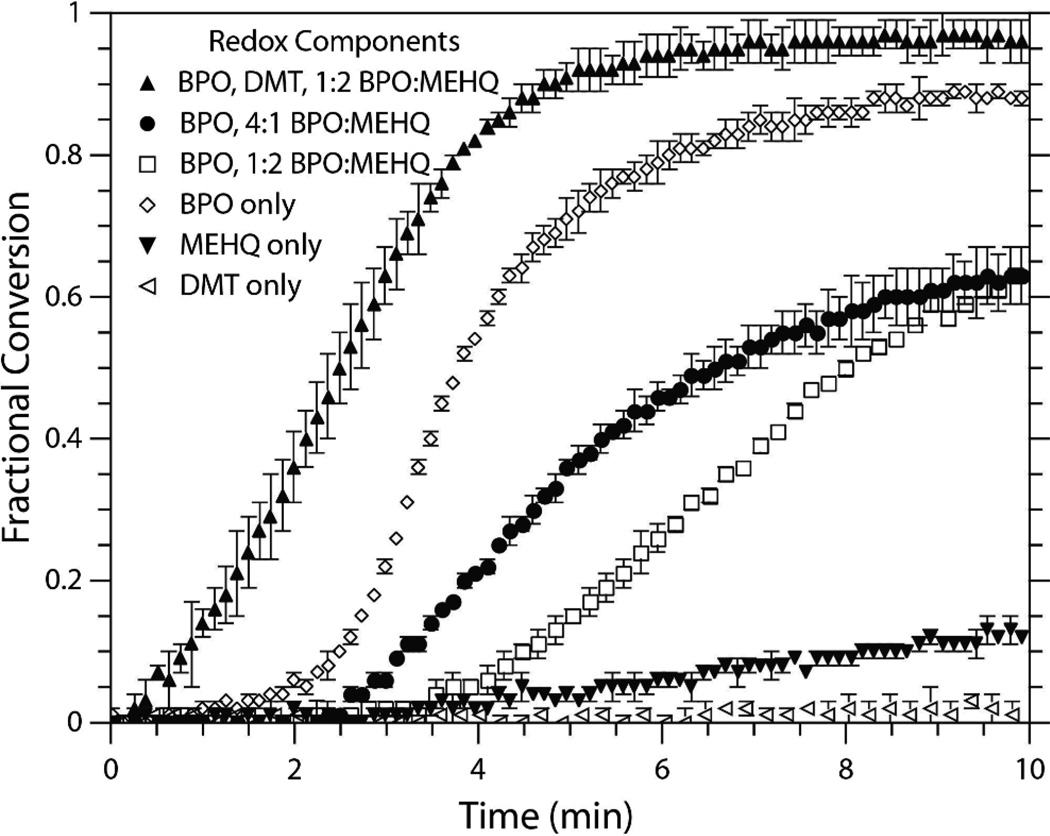
The rise in rate of polymerization with excess [MEHQ]:[BPO] quantities lead to the investigation of MEHQ-centered radicals as an initiating species in thiol-ene redox reactions. With increasing amounts of MEHQ relative to BPO, the propensity of the MEHQ radicals to undergo chain transfer with thiols or directly initiate polymerization may outweigh their propensity to undergo bimolecular termination. As a result, the rate of polymerization proceeds as though a higher initial fraction of initiator (i.e., BPO) were included in the reaction mixture. The validity of this claim is realized in Figures 6 and 7. In the absence of an oxidizing agent, negligible polymerization is seen; however, minimal but measurable conversion is noted when the amine reducing agent is also removed (Figure 6). Likely the amine merely serves as a termination point for MEHQ-centered radicals, such that in its absence, chain transfer events with thiols predominate, and polymerization can proceed. As indicated in Figure 6, the inclusion of an oxidizing agent with three different MEHQ fractions produces a significant rise in the polymerization rate. Still, when no amine is present, the lowered efficiency in benzoyloxy radical production by the thiol monomer leads to an inverse correlation between [MEHQ] and the rate of polymerization. However, the system formulated with a large excess of MEHQ (□) shows continued polymerization after ten minutes, whereas the system with an excess of BPO (●) reaches a plateau in fractional conversion. Thus, the MEHQ radical species may be participating in chain transfer events with thiol monomers rather than just acting in termination, such that radicals are remaining active but are effectively delayed for initiation.
Fig. 6.
Conversion versus time of the EGDMP/DAS system formulated with varying initiating species and inhibitor fractions. First, the combination of DMT and MEHQ ([DMT]:[MEHQ] 1:2), using only MEHQ (0.1 wt%), using BPO and MEHQ ([BPO]:[MEHQ] 4:1 and 1:2), using only BPO (0.1 wt%), and using all three species ([BPO]:[DMT] 1:1, [BPO]:[MEHQ] 1:2).
Fig. 7.
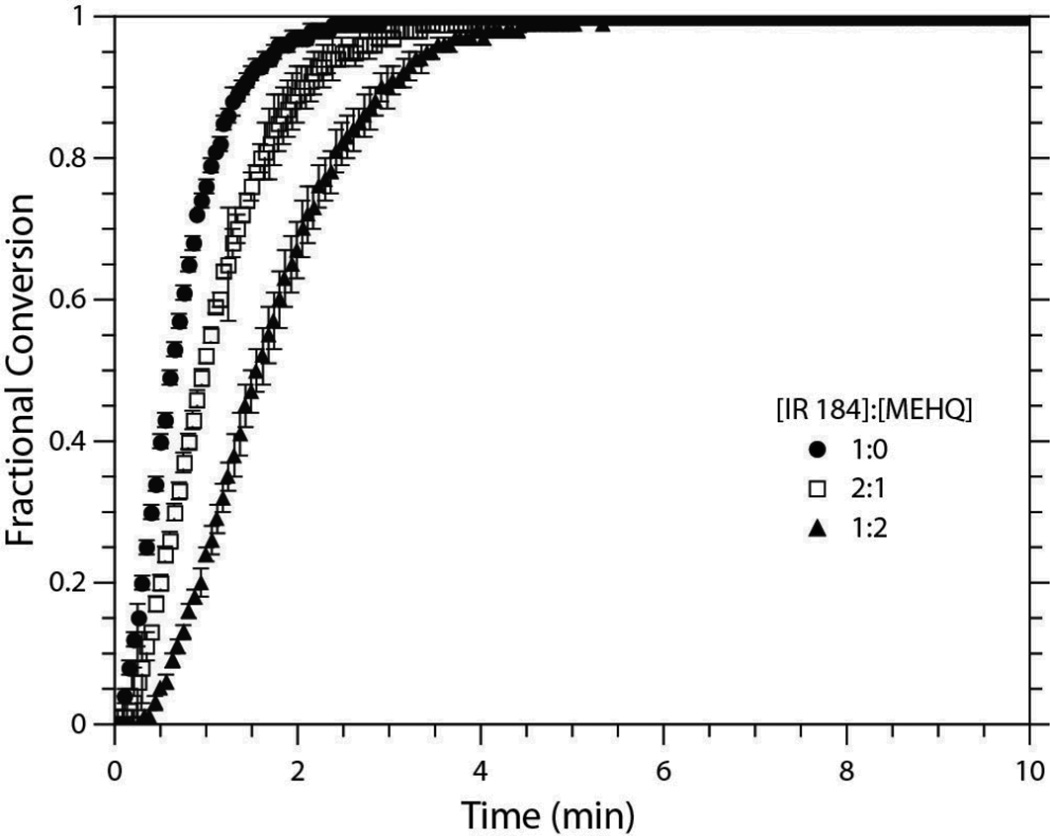
Conversion versus time for EGDMP/DAS formulated with 0.1 wt% IR 184 and three concentrations of MEHQ relative to IR 184 (0 mol%, 25 mol%, and 200 mol%). All systems were irradiated for 10 minutes at an intensity of 1.0 mW/cm2.
When the amine reducing agent is added to the reaction mixture the rate of polymerization greatly exceeds that observed without any inhibition. In such cases, the combined rapid production of benzoyloxy radicals with the propensity for excess MEHQ radicals to initiate polymerization directly or chain transfer with thiol monomers overcomes the traditional tendency for MEHQ to continually slow reaction rates. The need for these two combined features is further evidenced by Figure 7, where light is used to initiate the thiol-ene polymerization in the presence of varying MEHQ concentrations. Here, MEHQ acts to slow the polymerization rate continuously between low and high concentrations, indicating that termination of MEHQ radicals is favored under all these photoinitiated conditions. Thus, the behavior of MEHQ in thiol-ene polymerizations is dependent on concentration and initiation mode and only serves as an initiator at excess concentrations in the presence of an oxidizing agent.
The final component of this study evaluated the use of redox-initiated thiol-ene reactions for the coupling/functionalization of small molecules in solution. These model compounds were evaluated as a means for determining the suitability of redox initiated thiol-ene reactions for coupling reactions as regularly used for polymer conjugation, polymer functionalization, dendrimer synthesis, surface modification, biomolecule functionalization, etc. One complexity of those reactions is that the thiol and ene functional group concentrations are often much lower than in conventional bulk thiol-ene polymerizations. Thus, a much higher ratio of initiator to thiol (albeit usually a photoinitiator) is often used.
Here, varying initiator to thiol ratios were used in the model compound studies to demonstrate that high initiator levels are adverse to the click nature of the reaction, particularly when reacting dilute thiol and ene functional groups. In particular, the reaction of thiols with enes was carried out in methanol at two BPO levels, 1:50 BPO:SH and 1:1 BPO:SH. Specifically 3-mercaptopropyl trimethoxy silane was reacted with 4-vinyl pyridine in a 1:1 SH:C=C ratio and the functional group conversion was confirmed by 1H NMR. The distribution of products formed was then determined by mass spectroscopy. When BPO was truly used as an initiator (i.e., 1:50 BPO:SH), full conversion of the ene and thiol moieties was seen, and the intended “click” product (P) was the dominant species formed, 1H NMR (400 MHz, MeOD,™): 8.44 (ddd, J=19.0, 4.6, 1.7, 2H), 7.32 (ddd, J=4.5, 1.6, 0.5, 2H), 3.55 (d, J=1.0, 9H), 3.35 (t, J=7.9, 2H), 2.85-2.76 (m, 2H), 1.72-1.6 (m, 2H), 0.91-0.68 (m, 2H). Still, even at this relatively low initiator concentration, minor fractions of thiol-thiol, vinyl-vinyl, and amine-amine bimolecular termination products were observed by mass spectroscopy. However, when the radical initiators were mixed in 1:1 molar ratios with the reactants, the reaction with the thiol and ene functionalities yielded several small molecules associated with termination products, initiator fragment addition and other various side reactions that come into play at high ratios of initiator to reactants. 1H NMR (400 MHz, MeOD,™): 8.47-8.39 (m, 2H), 8.02-7.94 (m, 5H), 7.58-7.47 (m, 3H), 7.46-7.36 (m, 6H), 7.02-6.92 (m, 2H), 6.78-6.59 (m, 2H), 6.06 (dd, J=17.6, 0.6, 1H), 5.51 (dd, J=10.9, 0.6, 1H), 3.56-3.42 (m, 9H), 3.31 (s, 3H), 2.80 (d, J=1.1, 4H), 3.31 (s, 3H), 2.80 (d, J=1.1, 4H), 2.68-2.61 (m, 2H), 2.55-2.52 (m, 1H), 2.28-2.13 (m, 4H), 1.80-1.48 (m, 2H), 0.88-0.36 (m, 3H). Moreover, an abundance of undesirable products was formed and in higher fractions than the intended product (P). Of the major products formed were vinyl-vinyl (V), thiol-thiol (T), and vinyl-oxygen (O) terminated species, which were observed in a 7:10:11:20 P:V:T:O ratio by mass spectroscopy analysis. This approach shows both the value of the redox initiation approach for thiol-ene click reactions but also demonstrates the critical need to always maintain the concentration of the radical source (i.e., the initiator) much lower than the concentration of the thiol and ene functional groups.
Conclusions
Though bulk redox initated thiol-ene polymerizations have received little exploration, the work conducted in this study indicates that it is a viable, highly controllable mode of initiation. The polymerization rate is determined by the nucleophicity of the reducing agent as well as the thiol monomer, which can serve as the primary reducing agent in the absence of a secondary species. Furthermore, networks formed via redox polymerization display negligible differences from their photopolymerized counterparts, yet they can be formed without regard to sample thickness or pigmentation. Small molecule functionalization by the thiol-ene reaction is also highly amenable and appropriate by redox-mediated initiation. As indicated in this study, incorporation of trace fractions of a redox pair can lead to full conversion and coupling of reactants in solution. Moreover, the redox-mediated technique could prove invaluable in surface functionalization or immobilization of desired species, which are typically conducted in bulk aqueous environments.
The redox-mediated polymerization discussed in this paper has also demonstrated features that afford control over the reaction starting point and ensuing rate. Namely, the amount of inhibiting species relative to initiating species can be titrated to provide a designated induction period; in the presence of an aniline reducing agent, quinone lengthens the observed induction period and lowers the initial rate of polymerization for concentrations at or below that of the oxidizing agent. However, as the concentration of the quinone exceeds the oxidizing agent, the rate of polymerization begins to rise and eventually surpass the rate of polymerization seen at very low inhibitor fractions. Still the desired level of inhibition is dictated by the structure of the thiol species, with propionate thiols requiring higher fractions of inhibition for induction periods equivalent to alkyl thiols with identical initiator loadings
Acknowledgements
We acknowledge funding from the National Institute of Health (NIH, grant number: DE018233) and from the Septodont Corporation.
References
- 1.Hoyle CE, Bowman CN. Angew. Chem.-Int. Ed. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 2.Uygun M, Tasdelen MA, Yagci Y. Macromol. Chem. Phys. 2010;211:103–110. [Google Scholar]
- 3.Hoyle CE, Lowe AB, Bowman CN. Chem. Soc. Rev. 2010;39:1355–1387. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 4.Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Chem. Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mather BD, Viswanathan K, Miller KM, Long TE. Progress in Polymer Science. 2006;31:487–531. [Google Scholar]
- 6.Bowman CN, Kloxin CJ. Aiche J. 2008;54:2775–2795. [Google Scholar]
- 7.Cook WD, Chen F, Pattison DW, Hopson P, Beaujon M. Polymer International. 2007;56:1572–1579. [Google Scholar]
- 8.Reddy SK, Cramer NB, Bowman CN. Macromolecules. 2006;39:3673–3680. [Google Scholar]
- 9.Li Q, Zhou H, Hoyle CE. Polymer. 2009;50:2237–2245. [Google Scholar]
- 10.Cramer NB, Reddy SK, O'Brien AK, Bowman CN. Macromolecules. 2003;36:7964–7969. [Google Scholar]
- 11.Hoyle CE, Lee TY, Roper T. J. Polym. Sci. Part A-Polym. Chem. 2004;42:5301–5338. [Google Scholar]
- 12.Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Polymer. 2011;52:3295–3303. doi: 10.1016/j.polymer.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucker-Schwartz AK, Farrell RA, Garrell RL. J. Am. Chem. Soc. 2011;133:11026–11029. doi: 10.1021/ja202292q. [DOI] [PubMed] [Google Scholar]
- 14.Good BT, Reddy SK, Davis RH, Bowman CN. Sensors and Actuators B. 2007;120:473–480. [Google Scholar]
- 15.Matsukawa K, Fukuda T, Watase S, Goda H. J. Photopolym Sci. Technol. 2010;23:115–119. [Google Scholar]
- 16.Carioscia JA, Lu H, Stanbury JW, Bowman CN. Dental Materials. 2005;21:1137–1143. doi: 10.1016/j.dental.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Hata E, Mitsube K, Momose K, Tomita Y. Optical Materials Express. 2011;1:207–222. [Google Scholar]
- 18.Odian GG. Principles of Polymerization. 4th ed. Hoboken, NJ: Wiley; 1933. [Google Scholar]
- 19.Jansen JF, Kraeger RI. Vinyl Ester Resin Compositions. 2010 [Google Scholar]
- 20.Unterweger B, Stoisser T, Leitgeb S, Birner-Grunberger R, Nidetzky B. Bioconjugate Chemistry. 2012;23:1406–1414. doi: 10.1021/bc2006847. [DOI] [PubMed] [Google Scholar]
- 21.Sarac AS. Progress in Polymer Science. 1999;24:1149–1204. [Google Scholar]
- 22.Berron BJ, Johnson LM, Ba X, McCall JD, Alvey NJ, Anseth KS, Bowman CN. Biotechnology and Bioengineering. 2011;108:1521–1528. doi: 10.1002/bit.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson LM, DeForest CA, Pendurti A, Anseth KS, Bowman CN. Acs Applied Materials & Interfaces. 2010;2:1963–1972. doi: 10.1021/am100275n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hume PS, Bowman CN, Anseth KS. Biomaterials. 2011;32:6204–6212. doi: 10.1016/j.biomaterials.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson QH, Massey V, Swoboda BEP. Journal of Biological Chemistry. 1964;239:3927–3934. [PubMed] [Google Scholar]
- 26.Wilson R, Turner APF. Biosensors & Bioelectronics. 1992;7:165–185. [Google Scholar]
- 27.Betancor L, Lopez-Gallego F, Hidalgo A, Alonso-Morales N, Dellamora-Ortiz G, Guisan JM, Fernandez-Lafuente R. Journal of Biotechnology. 2006;121:284–289. doi: 10.1016/j.jbiotec.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield PF, Kittrell JR, Lawrence RL. Analytical Biochemistry. 1975;65:109–124. doi: 10.1016/0003-2697(75)90497-2. [DOI] [PubMed] [Google Scholar]
- 29.Oldfield FF, Yasuda HK. Journal of Biomedical Materials Research. 1999;44:436–445. doi: 10.1002/(sici)1097-4636(19990315)44:4<436::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Sideridou ID, Achilias DS, Kostidou NC. J. Appl. Polym. Sci. 2008;109:515–524. [Google Scholar]
- 31.Chen J, Lin YH, Chen G. Electrophoresis. 2007;28:2897–2903. doi: 10.1002/elps.200700071. [DOI] [PubMed] [Google Scholar]
- 32.Mabilleau G, Cincu C, Basle MF, Chappard D. Journal of Raman Spectroscopy. 2008;39:767–771. [Google Scholar]
- 33.Herczynska L, Lestel L, Boileau S, Chojnowski J, Polowinski S. Eur. Polym. J. 1999;35:1115–1122. [Google Scholar]
- 34.Seo JH, Shin D-S, Mukundan P, Revzin A. Colloids and Surfaces B: Biointerfaces. 2012;98:1–6. doi: 10.1016/j.colsurfb.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Dental Materials. 2005;21:1129–1136. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Flory PJ. Principles of Polymer Chemistry. Ithaca, NY: Cornell University Press; 1953. [Google Scholar]
- 37.Pryor WA, Hendrickson WH. Tetrahedron Lett. 1983;24:1459–1462. [Google Scholar]
- 38.Sato T, Kita S, Otsu T. Makromolekulare Chemie-Macromolecular Chemistry and Physics. 1975;176:561–571. [Google Scholar]
- 39.Sideridou ID, Achilias DS, Karava O. Macromolecules. 2006;39:2072–2080. [Google Scholar]
- 40.Becker H, Vogel H. Chem. Eng. Technol. 2006;29:1227–1231. [Google Scholar]
- 41.Clark T, Kwisnek L, Hoyle CE, Nazarenko S. J. Polym. Sci. Part A-Polym. Chem. 2009;47:14–24. [Google Scholar]