Abstract
Background:
Obstructive sleep apnea (OSA) is a highly prevalent disorder that has been associated with an increased risk for cardiovascular morbidity, even in children. However, not all children with OSA manifest alterations in endothelial postocclusive hyperemia, an endothelial nitric oxide synthase (eNOS)-dependent response. Since expression of the eNOS gene is regulated by epigenetic mechanisms and OSA may cause epigenetic modifications such as DNA hypermethylation, we hypothesized that epigenetic modifications in the eNOS gene may underlie the differential vascular phenotypes in pediatric OSA.
Methods:
Age-, sex-, ethnicity-, and BMI-matched prepubertal children with polysomnographically confirmed OSA and either normal (OSAn) or abnormal (OSAab) postocclusive hyperemic responses, assessed as the time to attain peak reperfusion flow (Tmax) by laser Doppler flowmetry, were recruited. Blood genomic DNA was assessed for epigenetic modifications in the eNOS gene using pyrosequencing. Children with no evidence of OSA or endothelial dysfunction served as a control group.
Results:
The study comprised 36 children with OSA (11 with OSAab and 25 with OSAn) and 35 children in the control group. Overall, the mean age was 7.5 ± 2.4 years, 65% were boys, and 30% were obese; mean apnea-hypopnea index was 18 ± 8.6/h of sleep for the children with OSA. Tmax was 66.7 ± 8.8 s in the OSAab group and 30.1 ± 8.3 s in the OSAn group (P < .001). Pyrosequencing of the proximal promoter region of the eNOS gene revealed no significant differences in six of the seven CpG sites. However, a CpG site located at position -171 (relative to transcription start site), approximating important transcriptional elements, displayed significantly higher methylation levels in the OSAab group as compared with the OSAn or control groups (81.5% ± 3.5%, 74.8% ± 1.4%, and 74.5% ± 1.7%, respectively; P < .001). eNOS mRNA expression levels were assessed in a separate group of children and were significantly reduced in the OSAab group in comparison with the OSAn group.
Conclusions:
The presence of abnormal eNOS-dependent vascular responses in children with OSA is associated with epigenetic modifications in the eNOS gene.
Obstructive sleep apnea (OSA) is a frequent condition in children primarily related to the presence of adenotonsillar hypertrophy and obesity. In the last decade, pediatric OSA has been identified as a condition independently associated with increased risk for systemic hypertension and cardiovascular disease,1 as well as with lipid alterations and insulin resistance.2
The presence of altered endothelial function is currently viewed as an early risk marker of cardiovascular disease, and is a relatively common occurrence in both adult and pediatric patients with OSA.3–5 Indeed, assessed with a modified hyperemic test after cuff-induced occlusion of the brachial artery, nonobese children aged 6-9 years who were diagnosed with OSA displayed significant increases in the time to attain peak reperfusion flow (Tmax).5 Furthermore, the concurrent presence of obesity and OSA magnified the severity of endothelial dysfunction, thereby reinforcing the assumption that both conditions will impose incremental, long-term cardiovascular risk.6–8 However, not all children with OSA manifest delayed postocclusive reperfusion kinetics, suggesting that in addition to OSA disease severity, both genetic and environmental factors may play roles in the pathophysiology of the functional state of the endothelium.
Nitric oxide (NO) is the major, albeit not the exclusive, mediator underlying the adequacy of the postocclusive hyperemic responses, and its bioavailability is contingent on the enzyme endothelial nitric oxide synthase (eNOS).9,10 It has been recently demonstrated that expression of the gene encoding for eNOS is highly regulated by epigenetic mechanisms including both CpG methylation and histone modifications.11–15 DNA methylation and histone modifications also appear to be involved in the expression of eNOS during endothelial lineage commitment of early endothelial progenitor cells (EPCs).16–18 For instance, the proximal region of the promoter (approximately 740 base pairs upstream from the main transcription start site) was shown to be heavily methylated in early EPCs but not in vascular endothelial cells, an interesting observation considering the role played by EPCs in the vascular phenotype among children with OSA.19 In addition, repressive trimethyl histone H3 (Lys27) was enriched in the eNOS promoter in early EPCs and treatment with 3-deazaneplanocin A, an S-adenosylhomocysteine hydrolase inhibitor that inhibits Ezh2 and acts as an inhibitor of histone H3 (Lys27) trimethylation, and trichostatin A significantly increased eNOS mRNA expression in these cells.16–18 Furthermore, the potential relevance of the methylation state of the eNOS gene has been recently explored in other contextual settings, such as obesity and bone mineral density.20,21 In parallel, we have shown that pediatric OSA is associated with discrete increases in CpG site methylation in inflammatory genes, particularly FOXP3, suggesting that the presence of sleep-disordered breathing may be conducive to the presence of epigenetic alterations of selected genes.22 Consequently, we hypothesized that the presence of vascular dysfunction in children with OSA, as evidenced by delayed, postocclusive, peak hyperemic responses, could be associated with epigenetic changes in the eNOS gene.
Materials and Methods
Subjects
The study was approved by the University of Louisville Human Research Committee (protocol #474.99), and informed consent was obtained from the legal caregiver of each participant. Consecutive, healthy, prepubertal children (aged 4-12 years) from the community who agreed to participate in a sleep and neurocognitive research study at the University of Louisville Pediatric Sleep Medicine Center were recruited to investigate endothelial function. All participants underwent baseline overnight polysomnography and measurement of endothelial function, followed by a fasting blood draw in the morning. Children with the following conditions were excluded: hypertension or undergoing antihypertensive therapies; diabetes; craniofacial, neuromuscular, or defined genetic syndrome; undergoing chronic antiinflammatory therapies; known acute or chronic illness; and undergoing treatment with sympathomimetic agents.
Anthropometry
Children were weighed using the InBody 320 scale (Biospace Co Ltd) and their height (to 0.1 cm) was measured with a stadiometer (Holtain Ltd). BMI was calculated, and BMI z-score was computed using US Centers for Disease Control and Prevention 2000 growth standards23 and online software (www.cdc.gov/epiinfo). A BMI z-score > 1.65 (> 95th percentile) was considered as fulfilling obesity criteria. This and other assessments and tests conducted in this study are discussed in more detail in e-Appendix 1 (389.8KB, pdf) .
Polysomnography
Polysomnography was conducted and scored as previously reported.24 Details are described in e-Appendix 1 (389.8KB, pdf) .
Endothelial Function
Endothelial function was assessed upon awakening from the sleep study in the morning, using a modified hyperemic test after cuff-induced occlusion of the radial and ulnar arteries as previously described.5–7 Briefly, a laser Doppler sensor (Periflux 5000 System; Perimed, AB) was applied over the volar aspect of the hand at the distal metacarpal surface of the first finger, and the hand was gently immobilized. Once cutaneous blood flow over the area became stable, the pressure within an inflatable cuff placed at the forearm and connected to a computer-controlled manometer was raised to 200 mm Hg for 60 s, during which blood flow was reduced to undetectable levels. The cuff was rapidly deflated and the laser Doppler measured hyperemic responses. As previously shown, the Tmax is highly reproducible and representative of the postocclusion hyperemic response, an index of endothelial function.25
Blood Tests
Fasting blood samples were drawn by venipuncture in the morning immediately after endothelial function testing. Serum levels of lipids were assessed with a Flex reagent cartridge (Siemens Healthcare Diagnostics Inc).
Pyrosequencing
Pyrosequencing methylation assays (ADS083-1 and ADS083-2; EpigenDx Inc) were used to quantitatively assess DNA methylation levels in the core promoter of the eNOS gene. Briefly, blood genomic DNA was bisulfate treated using the Zymo DNA Methylation Kit (Zymo Research Corp), and the region of interest was amplified by polymerase chain reaction. The resulting products were then sequenced with the Pyrosequencing PSQ96 HS System (Qiagen NV). The methylation status of each locus was analyzed individually as a T/C single nucleotide polymorphism using QCpG software (Qiagen NV).
Data Analysis
All numerical data were subjected to statistical analysis using independent Student t tests or analysis of variance followed by post-hoc tests (Tukey) as appropriate, using SPSS software, version 18.0 (IBM). A P value < .05 was considered significant.
Results
There were 35 children in the control group and 36 children with OSA, of whom 11 had marked delays in postocclusive hyperemic responses (OSAab) as shown by a significant increase in Tmax in these children (Table 1). As would be expected, the respiratory measures of the overnight polysomnogram demonstrated significant alterations in all children with OSA, but no differences emerged between those with OSAab and those with OSA but normal hyperemic responses (OSAn). Children with OSA had overall higher diastolic BP in the morning after sleep when compared with the control group, and their serum lipids were also higher (Table 1). However, these measurements were similar between the OSAn and OSAab groups. Tmax values are provided for the three groups in Table 1.
Table 1.
—General Characteristics of Children With OSA With and Without Endothelial Dysfunction and of Healthy Control Subjects
| Characteristics | OSAn (n = 25) | OSAab (n = 11) | Control Subjects (n = 35) |
| Age, y | 7.3 ± 2.2 | 7.5 ± 2.5 | 7.4 ± 1.8 |
| Male sex, % | 60 | 64 | 71 |
| White ethnicity, % | 47.1 | 47.0 | 67.9 |
| BMI z score | 1.34 ± 0.31 | 1.47 ± 0.36 | 1.22 ± 0.26 |
| Systolic BP, mm Hg | 109.2 ± 8.2 | 108.8 ± 10.7 | 103.4 ± 7.6 |
| Diastolic BP, mm Hg | 64.7 ± 8.1 | 65.2 ± 7.5 | 60.9 ± 6.2a |
| AHI, events/ h | 17.8 ± 7.1 | 20.2 ± 8.8 | 0.6 ± 0.3a |
| SaO2 nadir, % | 77.5 ± 11.7 | 76.1 ± 8.9 | 91.6 ± 3.7a |
| Total cholesterol, mg/dL | 174.9 ± 36.1 | 178.3 ± 37.8 | 159.4 ± 19.3a |
| HDL cholesterol, mg/dL | 48.2 ± 13.5 | 47.4 ± 14.9 | 54.0 ± 10.3a |
| LDL cholesterol, mg/dL | 114.1 ± 36.2 | 118.5 ± 22.9 | 93.8 ± 19.1a |
| Triglycerides, mg/dL | 106.2 ± 43.5 | 102.5 ± 45.7 | 82.7 ± 31.3a |
| Peak hyperemic response,b s | 30.1 ± 8.3c | 66.7 ± 8.8 | 27.8 ± 6.1a |
Data are mean ± SD. AHI = apnea-hypopnea index; HDL = high-density lipoprotein; LDL = low-density lipoprotein; OSAab = obstructive sleep apnea with delays in postocclusive hyperemic response; OSAn = obstructive sleep apnea with normal hyperemic response; SaO2 = hemoglobin oxygen saturation.
P < .01, control subjects vs OSA.
Peak hyperemic response time < 45 s or > 45 s was used to determine whether a subject had normal or abnormal endothelial function.7
P < .02, OSAab vs OSAn groups.
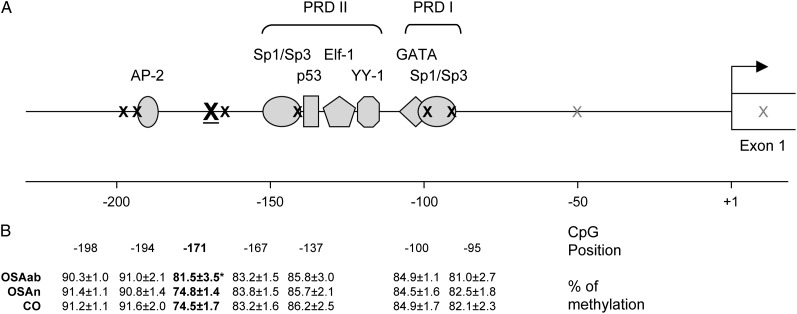
Pyrosequencing analyses of blood genomic DNA identified a hypermethylated CpG site in the core promoter region of the eNOS gene (-171, relative to the transcription start site26,27) in the OSAab group as compared with the OSAn or control groups (81.5% ± 3.5%, 74.8% ± 1.4%, and 74.5% ± 1.7%, respectively; P < .001) (Fig 1). Meanwhile, six adjacent CpG sites were similarly methylated and there was no significant difference in overall methylation levels within this region among the three groups(85.4% ± 1.5%, 84.8% ± 1.5%, and 84.8% ± 2.2%, respectively).
Figure 1.
Sequence characteristics and OSA-induced hypermethylation in the proximal promoter of the human eNOS gene. A, CpG dinucleotide positions and their relationship with the transcriptional machinery in the eNOS promoter. B, Methylation levels of seven CpG sites within the eNOS promoter among the three groups of children. Data are mean ± SE; *P < .001 vs OSAn or CO. Sequence details are derived from Karantzoulis-Fegaras et al26 and Marsden et al.27 Numbering is relative to the transcription start site (bent arrow). Underlined X = differentially methylated CpG; black X = nondifferentially methylated CpG; gray X = unexamined CpG. CO = control group; eNOS = endothelial nitric oxide synthase; OSAab = obstructive sleep apnea with delays in postocclusive hyperemic response; OSAn = obstructive sleep apnea with normal hyperemic response; PRD = positive regulatory domain.
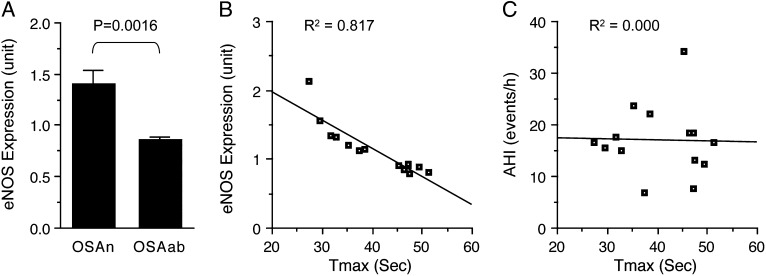
To clarify whether hypermethylation of the eNOS promoter and impaired postocclusive hyperemic response in the OSAab group were associated with reduced eNOS expression, we assessed eNOS mRNA expression levels in peripheral blood mononuclear cells in seven nonobese children with OSAab (Tmax > 45 s) and seven matched children with OSAn (Tmax < 45 s) (Table 2) in a cDNA array study. We found that eNOS mRNA levels were significantly reduced in the OSAab group in comparison with the OSAn group (Fig 2A). More interestingly, endothelial function, as indicated by Tmax, was inversely correlated with eNOS expression levels, but not with the severity of the apnea-hypopnea index in these patients (Fig 2B, 2C).
Table 2.
—General Characteristics of Children Included in the cDNA Array Study
| Characteristics | OSAn (n = 7) | OSAab (n = 7) |
| Age, y | 7.0 ± 1.0 | 7.1 ± 1.1 |
| Male sex, % | 57 | 57 |
| AHI, events/ h | 16.7 ± 5.5 | 17.3 ± 8.4 |
| Peak hyperemic response, s | 33.2 ± 4.1 | 47.8 ± 2.0a |
Data are mean ± SD. See Table 1 legend for expansion of abbreviations.
P < .001 vs OSAn group.
Figure 2.
Association of eNOS expression with endothelial function. A, eNOS mRNA levels in peripheral blood mononuclear cells were significantly lower in children with OSAab than in those children with OSAn. Data are mean ± SE; n = 7 for each group. B, eNOS expression levels were inversely correlated with postocclusive hyperemic responses in these children. C, Conversely, the severity of the AHI was not correlated with postocclusive hyperemic responses. AHI = apnea-hypopnea index; Tmax = the time to attain peak reperfusion flow. See Figure 1 legend for expansion of other abbreviations.
Discussion
Our findings indicate that hypermethylation of CpG dinucleotides in the core promoter region of the eNOS gene is associated with and likely responsible for reduced eNOS bioactivity and impaired peripheral vascular function in pediatric patients with OSA. Before we discuss the potential implications of our findings, we will mention some of the strengths and potential limitations of this study. First and foremost, the use of the laser Doppler technique for assessment of vascular responses following cuff-induced arterial occlusion not only permits reproducible determination of the kinetics of postischemic reperfusion but also serves as an accurate reporter of NO-mediated physiologic recruitment of the microvasculature.7,28 Indeed, the mechanisms that have been suggested to contribute to the process of postocclusive hyperemic responses include increase in the vasodilator NO following increases in shear stress associated with the increased blood flow after an ischemic period,29 and likely the accumulation of metabolic vasodilators such as adenosine and prostaglandins.30 The selection of the arterial occlusion time at 60 s rather than 5 min, which is typically used in most ischemic challenges in adult studies, was dictated by the priority of ensuring the children’s comfort during testing, thus preventing motion-induced artifacts and preserving the validity of the tests. Further, all studies were conducted in the fasting state at the same time of the day to minimize potential confounders introduced by differences in meal content and timing relative to the testing, and also to ensure that circadian variation in endothelial function would not play a role. In addition, we excluded children with a variety of diagnoses that can be associated with endothelial dysfunction.31 Notwithstanding, we did not assess the impact of treatment on the reversibility of Tmax abnormalities and eNOS hypermethylation in the current cohort, and such studies will definitely be required to better ascertain the interactive dynamics of epigenetic and vascular changes in the context of pediatric sleep apnea.
As in previous studies, we found that only a proportion of children with OSA manifested prolongations of Tmax beyond the normative range. In such studies, obesity, severity of OSA, the degree of concurrent inflammatory responses, and recruitment of EPCs to the circulation emerged as significant contributors to the phenotypic variance in microvascular alterations in children with sleep apnea.7,8,19,32 Here, we identify the presence of a specific epigenetic alteration in the eNOS gene promoter that links exclusively to children who also manifest reduced eNOS expression as well as altered microvascular responses following occlusion. Although the role of DNA methylation in regulating eNOS gene expression in various tissues has been known for a few years,33–36 current findings suggest for the first time an epigenetic linkage, via the eNOS gene, between a disease (ie, OSA) and a morbid consequence of this disease, namely vascular dysfunction. In this regard, it is important to point out that methylation levels in the eNOS promoter reported in this study are from whole blood cells, and therefore differences between the OSAab and the OSAn/control groups represent a substantial underestimate of those changes affecting eNOS-expressing cells between these groups. This is because most blood cells are eNOS-nonexpressing cells in which all CpG sites within the eNOS proximal promoter are already 100% methylated under normal conditions13 such that no difference is expected between the OSAab and OSAn/control groups in those cells. In contrast, in eNOS-expressing cells, the eNOS promoter is likely void of any methylation in the OSAn/control group, while substantially hypermethylated in the OSAab group. Accordingly, differences between these groups may be considerable, but are nevertheless masked by the large background from eNOS-nonexpressing cells.
It seems that OSA-induced hypermethylation of the eNOS promoter may occur in selective locations, while overall methylation levels within the region remain unchanged. Indeed, the CpG pair at -171 seems particularly vulnerable, whereas adjacent CpGs are devoid of OSA-induced hypermethylation. The latter includes the two pairs within the Sp1/Sp3 binding site of the PRD I, which has been shown to act as the core element of the promoter, and the pair within the Sp1/Sp3 binding site of the PRD II, which also contains binding sites for Elf-1, YY-1, and p53 and is believed to act as an important enhancer.26 Since hypermethylation of these CpG dinucleotides would conceivably lead to more severe repression of eNOS expression and, in turn, more severe vascular dysfunction, mechanisms that keep these CpG dinucleotides from being hypermethylated in the context of OSA are important and warrant further investigation. It is possible that frequent and steady interactions of these DNA sequences with transcription factors limit the access of methyltransferase and consequently prevent hypermethylation of CpG sites embedded in these sequences in eNOS-expressing cells.
It is unclear how hypermethylation of the CpG pair at -171 leads to the repressed expression of eNOS identified in the OSAab children. Since sequences immediately upstream and downstream of this CpG do not constitute any known binding site for transcription factors, its modification could not have acted through direct interference of DNA-protein interaction at this location. However, it is possible that such modification may have resulted in alteration of chromatin structure in the adjacent area that restricted access of transcription factors to the area and/or prevented nucleoprotein complex formation.37 Indeed, this CpG pair is only about 20 base pairs from the important PRD II and 15 base pairs from a putative AP-2 binding site (Fig 1). Alternatively, the increasingly methylated CpG could recruit transcriptional repressors, such as the methyl-binding proteins MeCP, MBD, and the recently discovered Kaiso zinc finger domain proteins. These proteins provide a protein scaffold that recruits other factors that mediate transcriptional repression, including histone deacetylases.38,39
In summary, epigenetic changes in the eNOS gene may account for an important component of the variance in endothelial dysfunction associated with pediatric OSA and lead to reduced expression of eNOS. Further studies examining the reversibility and long-term implications of these epigenetic changes appear warranted.
Supplementary Material
Online Supplement
Acknowledgments
Author Contributions: Dr Kheirandish-Gozal had full access to the data and will vouch for the integrity of the data analysis.
Dr Kheirandish-Gozal: contributed to experimental procedures performed during the study, data analysis and interpretation, and writing the manuscript,
Dr Khalyfa: contributed to experimental procedures performed during the study and reviewed and approved the manuscript.
Dr Gozal: contributed to study conception and design, data analysis and interpretation, and writing the manuscript.
Dr Bhattacharjee: contributed to experimental procedures performed during the study and reviewed and approved the manuscript.
Dr Wang: contributed to study conception and design, data analysis and interpretation, and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kheirandish-Gozal has received a grant on montelukast in treatment of pediatric sleep apnea from Merck & Co, Inc. Dr Gozal has received an investigator-initiated grant from ResMed Corp on urine biomarkers in sleep apnea. All other authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- NO
nitric oxide
- OSA
obstructive sleep apnea
- OSAab
obstructive sleep apnea with delays in postocclusive hyperemic response
- OSAn
obstructive sleep apnea with normal hyperemic response
- Tmax
the time to attain peak reperfusion flow
Footnotes
Funding/Support: Drs Kheirandish-Gozal and Gozal are supported by National Institutes of Health (NIH) [Grant K12 HL-090003 to Dr Kheirandish-Gozal and Grant HL-65270 to Dr Gozal].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51(5):416-433 [DOI] [PubMed] [Google Scholar]
- 2.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177(10):1142-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somers VK, White DP, Amin R, et al. ; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology ; American Heart Association Stroke Council ; American Heart Association Council on Cardiovascular Nursing ; American College of Cardiology Foundation Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In Collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080-1111 [DOI] [PubMed] [Google Scholar]
- 4.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102(21):2607-2610 [DOI] [PubMed] [Google Scholar]
- 5.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116(20):2307-2314 [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee R, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatr. 2010;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Gozal D. Circulating microparticles in children with sleep disordered breathing. Chest. 2011;140(2):408-417 [DOI] [PubMed] [Google Scholar]
- 9.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2-H12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish JE, Marsden PA. Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol Life Sci. 2006;63(2):144-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fish JE, Yan MS, Matouk CC, et al. Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J Biol Chem. 2010;285(2):810-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan Y, Fish JE, D’Abreo C, et al. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279(33):35087-35100 [DOI] [PubMed] [Google Scholar]
- 14.Fish JE, Matouk CC, Rachlis A, et al. The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem. 2005;280(26):24824-24838 [DOI] [PubMed] [Google Scholar]
- 15.Gan Y, Shen YH, Wang J, et al. Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. J Biol Chem. 2005;280(16):16467-16475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagarkova MA, Volchkov PY, Philonenko ES, Kiselev SL. Efficient differentiation of hESCs into endothelial cells in vitro is secured by epigenetic changes. Cell Cycle. 2008;7(18):2929-2935 [DOI] [PubMed] [Google Scholar]
- 17.Ohtani K, Vlachojannis GJ, Koyanagi M, et al. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res. 2011;109(11):1219-1229 [DOI] [PubMed] [Google Scholar]
- 18.Rössig L, Urbich C, Brühl T, et al. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J Exp Med. 2005;201(11):1825-1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(1):92-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey KM, Sheppard A, Gluckman PD, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60(5):1528-1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey NC, Lillycrop KA, Garratt E, et al. Evaluation of methylation status of the eNOS promoter at birth in relation to childhood bone mineral content. Calcif Tissue Int. 2012;90(2):120-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Bhattacharjee R, Khalyfa A, et al. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185(3):330-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Centers for Disease Control and Prevention, National Health Center for Statistics Growth charts. US Centers for Disease Control and Prevention website. www.cdc.gov/growthcharts. Accessed July 2, 2012.
- 24.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741-753 [DOI] [PubMed] [Google Scholar]
- 25.Wahlberg E, Olofsson P, Swedenborg J, Fagrell B. Changes in postocclusive reactive hyperaemic values as measured with laser Doppler fluxmetry after infrainguinal arterial reconstructions. Eur J Vasc Endovasc. 1995;9(2):197-203 [DOI] [PubMed] [Google Scholar]
- 26.Karantzoulis-Fegaras F, Antoniou H, Lai SL, et al. Characterization of the human endothelial nitric-oxide synthase promoter. J Biol Chem. 1999;274(5):3076-3093 [DOI] [PubMed] [Google Scholar]
- 27.Marsden PA, Heng HH, Scherer SW, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268(23):17478-17488 [PubMed] [Google Scholar]
- 28.Medow MS, Taneja I, Stewart JM. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol Heart Circ Physiol. 2007;293(1):H425-H432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250(6 Pt 2):H1145-H1149 [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol. 2007;585(Pt 1):295-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valle Jiménez M, Estepa RM, Camacho RMM, Estrada RC, Luna FG, Guitarte FB. Endothelial dysfunction is related to insulin resistance and inflammatory biomarker levels in obese prepubertal children. Eur J Endocrinol. 2007;156(4):497-502 [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35(4):843-850 [DOI] [PubMed] [Google Scholar]
- 33.Jamal A, Man HSJ, Marsden PA. Gene regulation in the vascular endothelium: why epigenetics is important for the kidney. Semin Nephrol. 2012;32(2):176-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Advani A, Huang QL, Thai K, et al. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol. 2011;178(5):2205-2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirodkar AV, Marsden PA. Epigenetics in cardiovascular disease. Curr Opin Cardiol. 2011;26(3):209-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan MSC, Matouk CC, Marsden PA. Epigenetics of the vascular endothelium. J Appl Physiol. 2010;109(3):916-926 [DOI] [PubMed] [Google Scholar]
- 37.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25(10):1010-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogdanović O, Veenstra GJ. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118(5):549-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cyr AR, Domann FE. The redox basis of epigenetic modifications: from mechanisms to functional consequences. Antioxid Redox Signal. 2011;15(2):551-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement