Abstract
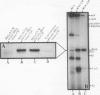
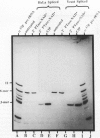
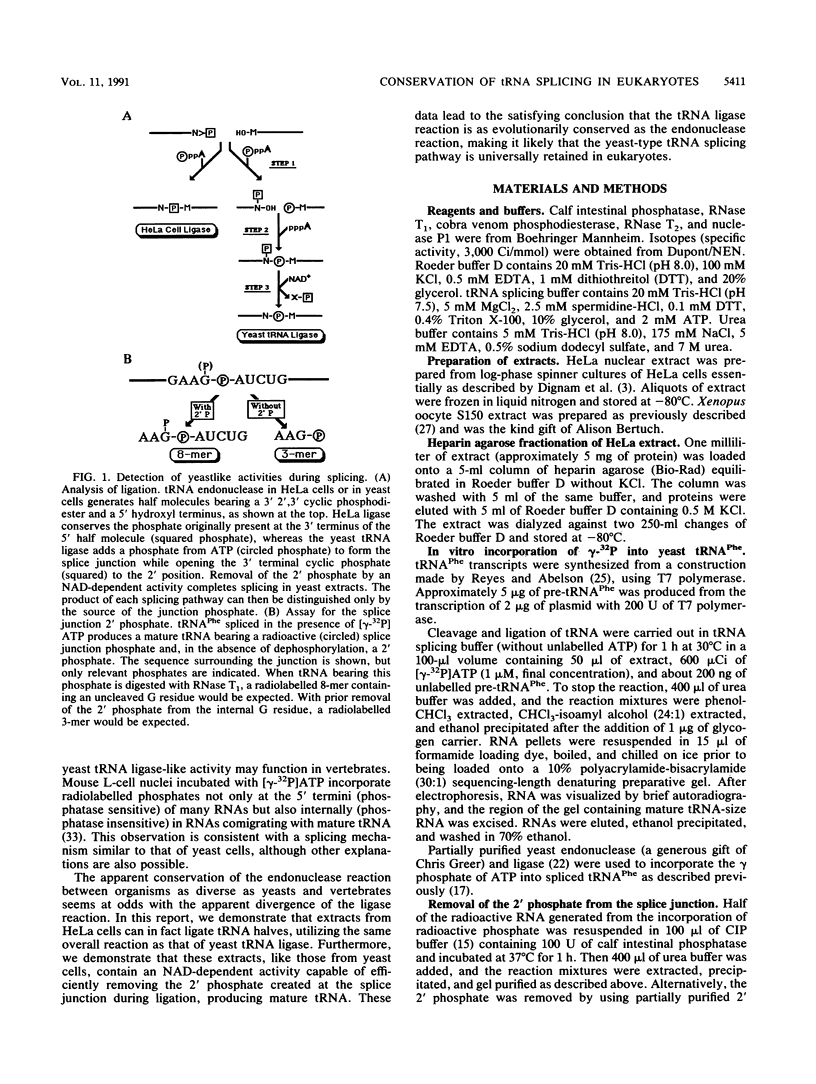
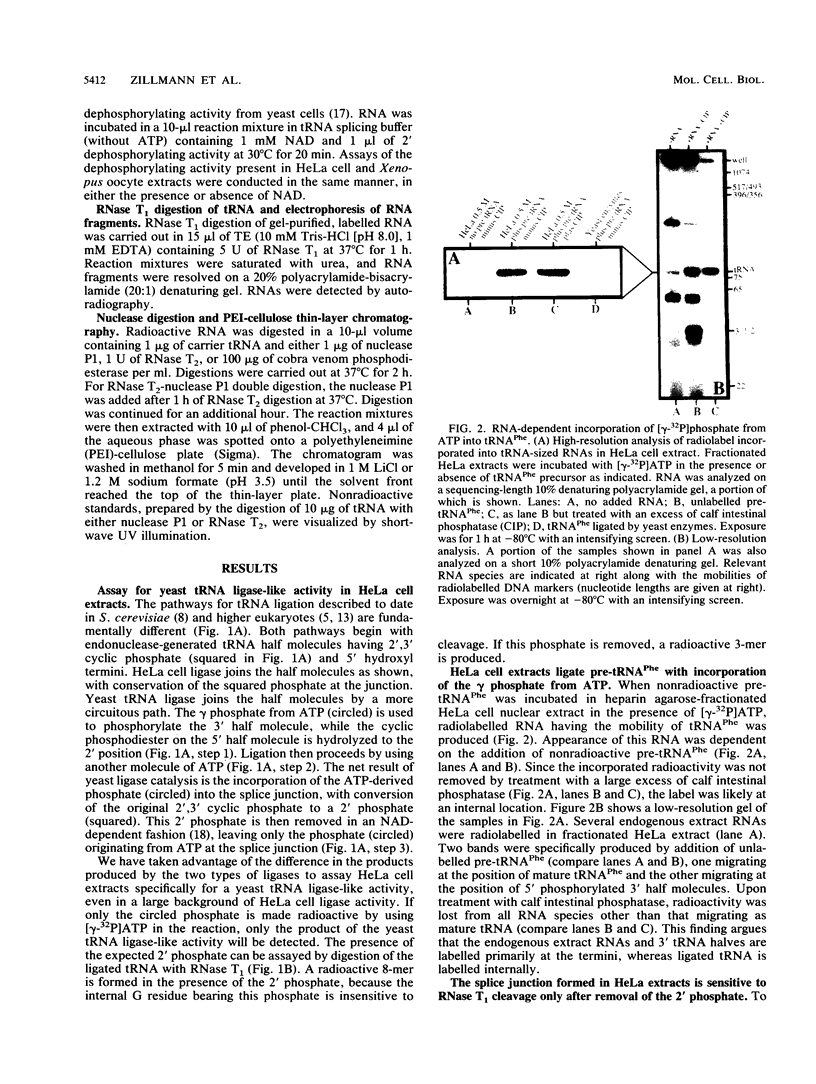
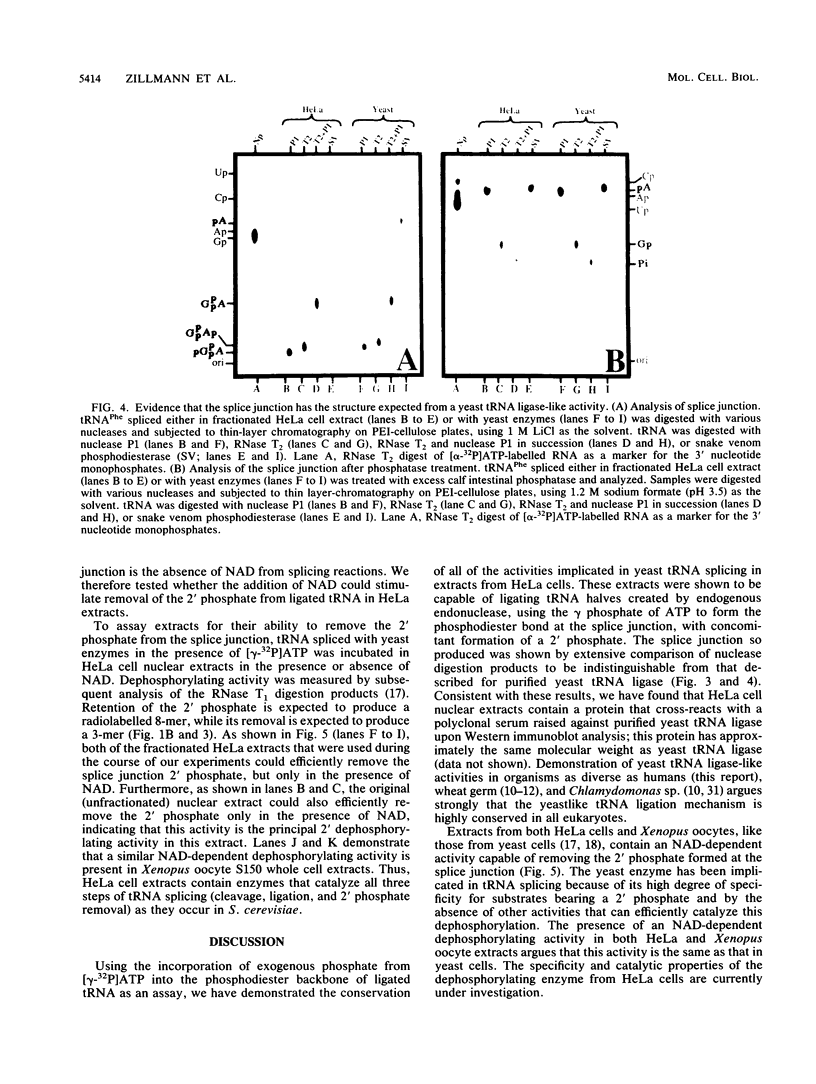
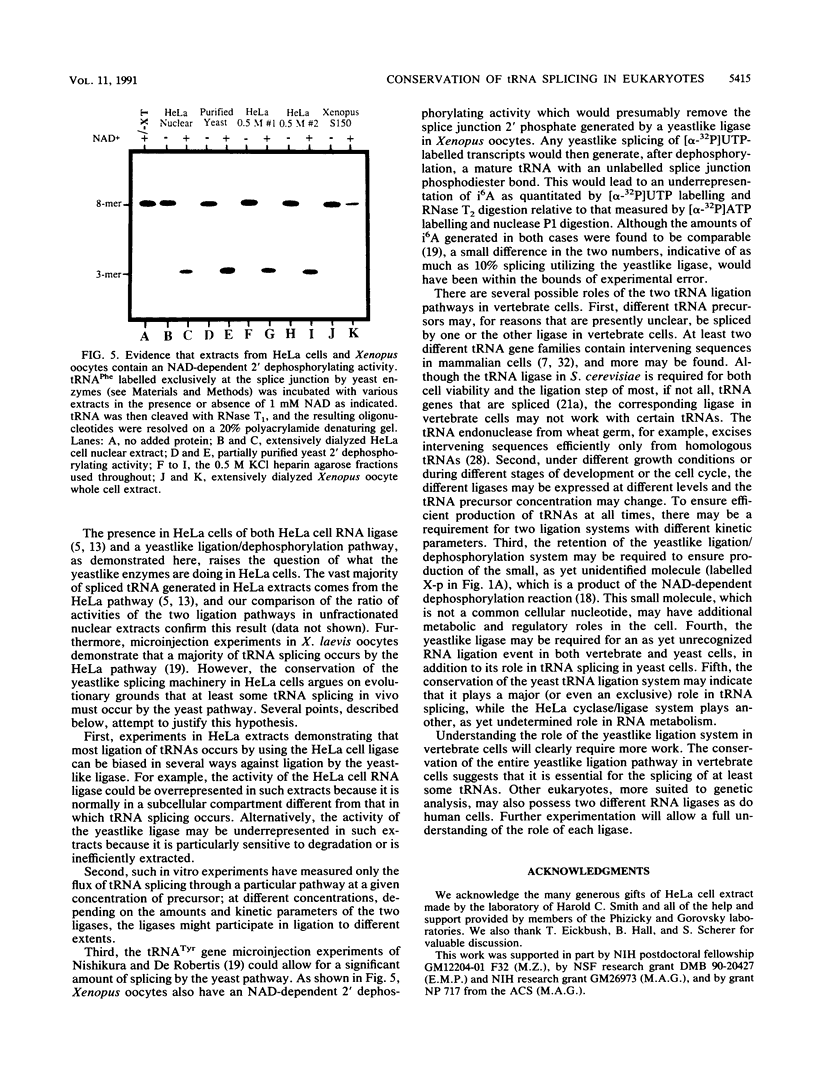
The ligation steps of tRNA splicing in yeast and vertebrate cells have been thought to proceed by fundamentally different mechanisms. Ligation in yeast cells occurs by incorporation of an exogenous phosphate from ATP into the splice junction, with concomitant formation of a 2' phosphate at the 5' junction nucleotide. This phosphate is removed in a subsequent step which, in vitro, is catalyzed by an NAD-dependent dephosphorylating activity. In contrast, tRNA ligation in vertebrates has been reported to occur without incorporation of exogenous phosphate or formation of a 2' phosphate. We demonstrate in this study the existence of a yeast tRNA ligase-like activity in HeLa cells. Furthermore, in extracts from these cells, the entire yeastlike tRNA splicing machinery is intact, including that for cleavage, ligation, and removal of the 2' phosphate in an NAD-dependent fashion to give mature tRNA. These results argue that the mechanism of tRNA splicing is conserved among eukaryotes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostol B. L., Westaway S. K., Abelson J., Greer C. L. Deletion analysis of a multifunctional yeast tRNA ligase polypeptide. Identification of essential and dispensable functional domains. J Biol Chem. 1991 Apr 25;266(12):7445–7455. [PubMed] [Google Scholar]
- Attardi D. G., Margarit I., Tocchini-Valentini G. P. Structural alterations in mutant precursors of the yeast tRNALeu3 gene which behave as defective substrates for a highly purified splicing endoribonuclease. EMBO J. 1985 Dec 1;4(12):3289–3297. doi: 10.1002/j.1460-2075.1985.tb04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi M. I., Mattoccia E., Ciafrè S., Attardi D. G., Tocchini-Valentini G. P. Binding and cleavage of pre-tRNA by the Xenopus splicing endonuclease: two separable steps of the intron excision reaction. Cell. 1986 Dec 26;47(6):965–971. doi: 10.1016/0092-8674(86)90811-1. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Konarska M., Gross H. J., Shatkin A. J. RNA 3'-terminal phosphate cyclase activity and RNA ligation in HeLa cell extract. Nucleic Acids Res. 1983 Mar 11;11(5):1405–1418. doi: 10.1093/nar/11.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Shatkin A. J. Origin of splice junction phosphate in tRNAs processed by HeLa cell extract. Cell. 1983 Feb;32(2):547–557. doi: 10.1016/0092-8674(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Green C. J., Sohel I., Vold B. S. The discovery of new intron-containing human tRNA genes using the polymerase chain reaction. J Biol Chem. 1990 Jul 25;265(21):12139–12142. [PubMed] [Google Scholar]
- Greer C. L., Peebles C. L., Gegenheimer P., Abelson J. Mechanism of action of a yeast RNA ligase in tRNA splicing. Cell. 1983 Feb;32(2):537–546. doi: 10.1016/0092-8674(83)90473-7. [DOI] [PubMed] [Google Scholar]
- Greer C. L., Söll D., Willis I. Substrate recognition and identification of splice sites by the tRNA-splicing endonuclease and ligase from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jan;7(1):76–84. doi: 10.1128/mcb.7.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Gross H. J. RNA ligation via 2'-phosphomonoester, 3'5'-phosphodiester linkage: requirement of 2',3'-cyclic phosphate termini and involvement of a 5'-hydroxyl polynucleotide kinase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1474–1478. doi: 10.1073/pnas.79.5.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Fire A. Z., RajBhandary U. L., Sharp P. A. Characterization of tRNA precursor splicing in mammalian extracts. J Biol Chem. 1983 Oct 10;258(19):11974–11980. [PubMed] [Google Scholar]
- Lee M. C., Knapp G. Transfer RNA splicing in Saccharomyces cerevisiae. Secondary and tertiary structures of the substrates. J Biol Chem. 1985 Mar 10;260(5):3108–3115. [PubMed] [Google Scholar]
- Mattoccia E., Baldi I. M., Gandini-Attardi D., Ciafrè S., Tocchini-Valentini G. P. Site selection by the tRNA splicing endonuclease of Xenopus laevis. Cell. 1988 Nov 18;55(4):731–738. doi: 10.1016/0092-8674(88)90231-0. [DOI] [PubMed] [Google Scholar]
- McCraith S. M., Phizicky E. M. A highly specific phosphatase from Saccharomyces cerevisiae implicated in tRNA splicing. Mol Cell Biol. 1990 Mar;10(3):1049–1055. doi: 10.1128/mcb.10.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCraith S. M., Phizicky E. M. An enzyme from Saccharomyces cerevisiae uses NAD+ to transfer the splice junction 2'-phosphate from ligated tRNA to an acceptor molecule. J Biol Chem. 1991 Jun 25;266(18):11986–11992. [PubMed] [Google Scholar]
- Nishikura K., De Robertis E. M. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J Mol Biol. 1981 Jan 15;145(2):405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- Peebles C. L., Gegenheimer P., Abelson J. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell. 1983 Feb;32(2):525–536. doi: 10.1016/0092-8674(83)90472-5. [DOI] [PubMed] [Google Scholar]
- Perkins K. K., Furneaux H., Hurwitz J. Isolation and characterization of an RNA ligase from HeLa cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):684–688. doi: 10.1073/pnas.82.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E. M., Schwartz R. C., Abelson J. Saccharomyces cerevisiae tRNA ligase. Purification of the protein and isolation of the structural gene. J Biol Chem. 1986 Feb 25;261(6):2978–2986. [PubMed] [Google Scholar]
- Pick L., Furneaux H., Hurwitz J. Purification of wheat germ RNA ligase. II. Mechanism of action of wheat germ RNA ligase. J Biol Chem. 1986 May 25;261(15):6694–6704. [PubMed] [Google Scholar]
- Pick L., Hurwitz J. Purification of wheat germ RNA ligase. I. Characterization of a ligase-associated 5'-hydroxyl polynucleotide kinase activity. J Biol Chem. 1986 May 25;261(15):6684–6693. [PubMed] [Google Scholar]
- Reyes V. M., Abelson J. Substrate recognition and splice site determination in yeast tRNA splicing. Cell. 1988 Nov 18;55(4):719–730. doi: 10.1016/0092-8674(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Schwartz R. C., Greer C. L., Gegenheimer P., Abelson J. Enzymatic mechanism of an RNA ligase from wheat germ. J Biol Chem. 1983 Jul 10;258(13):8374–8383. [PubMed] [Google Scholar]
- Shimamura A., Tremethick D., Worcel A. Characterization of the repressed 5S DNA minichromosomes assembled in vitro with a high-speed supernatant of Xenopus laevis oocytes. Mol Cell Biol. 1988 Oct;8(10):4257–4269. doi: 10.1128/mcb.8.10.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Gross H. J., Beier H. Wheat germ splicing endonuclease is highly specific for plant pre-tRNAs. EMBO J. 1988 Dec 1;7(12):3823–3828. doi: 10.1002/j.1460-2075.1988.tb03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow H., Guthrie C. Structure of intron-containing tRNA precursors. Analysis of solution conformation using chemical and enzymatic probes. J Biol Chem. 1984 Apr 25;259(8):5197–5207. [PubMed] [Google Scholar]
- Szekely E., Belford H. G., Greer C. L. Intron sequence and structure requirements for tRNA splicing in Saccharomyces cerevisiae. J Biol Chem. 1988 Sep 25;263(27):13839–13847. [PubMed] [Google Scholar]
- Tyc K., Kikuchi Y., Konarska M., Filipowicz W., Gross H. J. Ligation of endogenous tRNA 3' half molecules to their corresponding 5' halves via 2'-phosphomonoester,3',5'-phosphodiester bonds in extracts of Chlamydomonas. EMBO J. 1983;2(4):605–610. doi: 10.1002/j.1460-2075.1983.tb01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicov I., Button J. D. Nuclear ligation of RNA 5'-OH kinase products in tRNA. Mol Cell Biol. 1982 Mar;2(3):241–249. doi: 10.1128/mcb.2.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol H., Beier H. All human tRNATyr genes contain introns as a prerequisite for pseudouridine biosynthesis in the anticodon. Nucleic Acids Res. 1988 Mar 25;16(5):1951–1966. doi: 10.1093/nar/16.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]