Abstract
Background
Although dialysis after kidney transplant failure (TF) is common, the outcomes of these patients remain unclear. We compared outcomes of TF patients with transplant-naïve (TN) patients wait-listed for kidney transplantation.
Methods
We used data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), including laboratory markers and health-related quality of life (HR-QOL). Mortality and hospitalization of participants with one prior TF versus TN patients were compared using the Cox regression analysis. HR-QOL physical and mental component summary scores (PCS and MCS) were examined using linear mixed models, and clinical practices were compared using logistic regression.
Results
Compared with TN patients (n = 2806), TF patients (n = 1856) were younger (48 versus 51 years, P = 0.003), less likely to be diabetic (18 versus 27%, P < 0.0001) and to use a permanent surgical vascular access {adjusted odds ratio (AOR): 0.85 [95% confidence interval (CI): 0.70–1.03], P = 0.10}, particularly within the first 3 months after TF [AOR 0.45 (0.32–0.62), P < 0.0001]. TF patients also had lower PCS [mean difference −2.56 (−3.36, −1.75), P < 0.0001] but not MCS [−0.42 (−1.34, 0.50), P = 0.37]. All-cause mortality [adjusted hazard ratio (AHR): 1.32 (95% CI: 1.05–1.66), P = 0.02], especially infection-related [AHR 2.45 (95% CI: 1.36–4.41), P = 0.01], was higher among TF patients.
Conclusions
TF patients have reduced QOL and higher mortality, particularly due to infections, than TN patients. Interventions to optimize care before and after starting dialysis remain to be identified and applied in clinical practice.
Keywords: health-related quality of life, hemodialysis, kidney allograft loss, kidney transplantation, survival
Introduction
Compared with chronic dialysis, kidney transplantation offers longer life expectancy and improved quality of life (QOL), physical functioning and vocational abilities [1, 2]. Advances in kidney transplantation have translated into greater improvements in short-term kidney allograft survival relative to long-term graft survival [3]. Therefore, many patients will experience kidney transplant failure (TF) and will require initiation of dialysis. In the USA, return to dialysis after kidney TF represents the cause of dialysis initiation in 4.1% of incident dialysis patients, and 16% of patients wait-listed for kidney transplantation have a history of kidney TF [4]. As the kidney transplantation rates in developed countries increase, and with a fixed duration of graft survival, the absolute numbers of patients returning to dialysis after kidney TF are expected to increase.
A better understanding of the outcomes of TF patients is necessary. In North American registries, high mortality rates have been described in patients returning to dialysis after TF [5–7]. When the survival of these patients is compared with those with ongoing graft function, the annual adjusted death rate is 3-fold greater among TF patients [8].
Less clear are the outcomes of TF patients when compared with transplant-naïve (TN) patients initiating dialysis for the first time. Patients initiating dialysis after TF are a selected group whom at one point were placed on a transplant waiting list and received a kidney transplant. Restriction of the comparator TN group only to those patients wait-listed for kidney transplantation provides the opportunity to minimize selection bias by the restriction of the comparator group to a similar transplant-eligible population. Our primary objective was to evaluate the impact of kidney TF on mortality and hospitalization in an international cohort of patients enrolled in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Secondary objectives were to determine the association between a TF and infection-related adverse outcomes, achievement of performance targets on dialysis therapy and health-related QOL (HR-QOL).
Materials and methods
Data source
This study used data from DOPPS 1 (1996–2001), 2 (2002–04) and 3 (2005–08). Adults (≥18 years of age) receiving long-term in-center hemodialysis (HD) were randomly selected from all participating facilities across all DOPPS phases [308 dialysis facilities in DOPPS 1 (n = 17 034), 322 dialysis facilities in DOPPS 2 (n = 12 839) and 300 dialysis facilities in DOPPS 3 (n = 11 361)]. Patients in DOPPS 2 and 3 were enrolled from the same countries as in DOPPS 1 with the addition of Australia, Belgium, Canada, New Zealand and Sweden (Appendix 1). The DOPPS sampling plan and study methods have been previously published [9]. Institutional review boards approved the DOPPS and informed patient consent was obtained in accordance with local requirements.
Study population
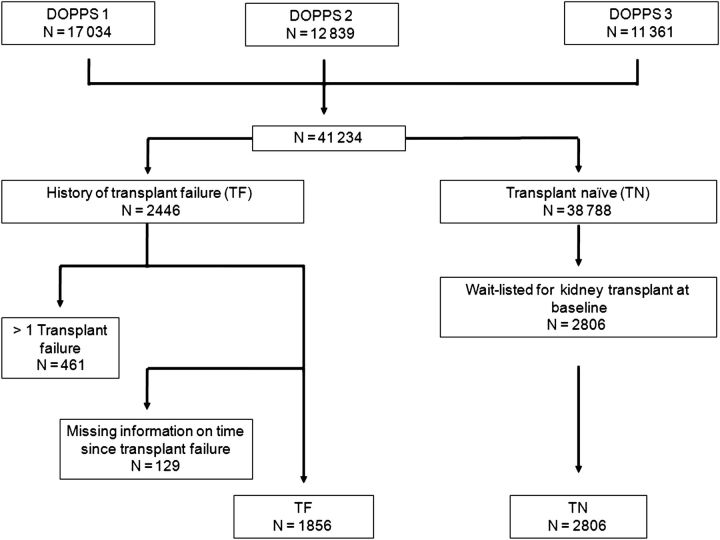
Figure 1 demonstrates the derivation of the study cohort. Patients with a history of greater than one kidney transplant, and those in whom the time from TF to DOPPS enrollment was missing were excluded. The comparator group consisted of TN HD patients who were on a waiting list for kidney transplantation at DOPPS enrollment.
Fig. 1.
Assembly of the study cohort.
Outcomes
The primary outcomes were mortality and hospitalization events due to (i) any cause, (ii) cardiovascular disease and (iii) infection. Definitions for cause-specific mortality and hospitalization are shown in Appendix 2. Hospital admissions, diagnoses and major procedures were recorded during study follow-up.
Secondary outcomes were time to first infectious complication defined as time to first of either infection-related hospitalization or infection-related death, achievements of clinical practice recommendations and HR-QOL. Achievements of clinical practice targets were based on accepted practice guidelines over the course of the study and included (i) use of arteriovenous fistula (AVF) or graft (versus catheter), (ii) hemoglobin 11–13 g/dL (versus else), (iii) albumin >4.0 g/dL (versus ≤4.0 g/dL), (iv) Kt/V >1.2 (versus ≤1.2), (v) phosphorus >5.5 mg/dL (versus ≤5.5 mg/dL) and (vi) PTH >500 pg/mL (versus ≤500 pg/mL). HR-QOL was measured at study enrollment with the SF-36 Health Survey using standard scoring procedures [10, 11]. The SF-36 measures eight separate scales of HR-QOL: physical functioning, role physical, bodily pain, general health, mental health, role emotional, social functioning and vitality. The two general summary scales were also computed: the physical component summary (PCS) and mental component summary (MCS) [12]. We evaluated three scales of patient health-related concerns by using the KDQOL-SF [13]: (i) symptoms/problems, (ii) effects of kidney disease on daily life and (iii) burden of kidney disease. Scales were scored from 0 to 100 points, with higher scores representing better HR-QOL. Depressive symptoms were assessed at study enrollment by the short, 10-item version of the Center for Epidemiological Studies Depression Screening Index (CES-D). Each response item is scored from 0 to 3 points. A summary CES-D score (0–30 points) is derived, with higher scores indicating greater depressive symptoms. The cut-off value of ≥10 was used for the summary CES-D score as an indicator of possible clinical depression [14]. Physician-diagnosed depression within the past 12 months was obtained from the baseline medical questionnaire.
Covariates
Demographic, comorbidity, laboratory and vascular access-related data were collected at the time of study entry. Dialysis vintage was the time since TF for TF patients and the time since first ever dialysis for TN patients. Thirteen summarised comorbid conditions are described in Appendix 3. Laboratory values included hemoglobin, serum albumin, calcium, phosphorus, parathyroid hormone (PTH) and ferritin. Three types of vascular access were AVF, arteriovenous graft (AVG) and central venous catheter (CVC). Education was classified as the highest level of education received: less than high school, high school or above high school.
Statistical analysis
Descriptive statistics evaluated differences in baseline characteristics between TN and TF patients. TF patients were stratified into subgroups based on time since kidney TF (defined as <3, 3–12 and >12 months from kidney TF to DOPPS enrollment). For each of the outcomes (mortality, hospitalizations, time to first infection, achievement of clinical practices and HR-QOL), we tested for overall differences between TF and TN patients. For HR-QOL, we also assessed trends across TF patient subgroups.
The associations of transplantation status (TF versus TN) with all-cause, infection-related and cardiovascular-related mortality, time to first infectious complication and time to first hospitalization were examined using the Cox proportional hazards regression. Time at risk began at study entry. For hospitalization, follow-up was censored at the earliest time point: death, departure from study, kidney transplantation or change in dialysis modality. For mortality, follow-up was censored at the earliest time point: 7 days after departure from the study, or change in renal replacement modality. Models were adjusted for demographic information, body mass index (BMI), vintage, 13 summarised comorbid conditions, serum albumin and catheter use; stratified by country and study phase; and used the sandwich covariance estimator to control for clustering by facility. The proportional hazards assumption was checked graphically and using time-by-covariate interactions. When non-proportional hazards were found, stratification by the corresponding covariate was performed as a sensitivity analysis. To better characterize the impact of wait-list status on outcomes after kidney TF, we performed an additional sensitivity analysis in which hazard ratios for all-cause mortality and hospitalization among TF patients were estimated separately by wait-list status for repeat kidney transplantation at study enrollment.
Logistic regression, using generalized estimating equations to adjust for clustering by facility, was used to identify associations between transplantation status and achievement of clinical practice guidelines [15–17]. Models were adjusted for demographic information, 13 summarised comorbid conditions, serum albumin (except among analyses pertaining to serum albumin as the outcome variable), vascular access (except among analyses pertaining to vascular access type as the outcome variable), study phase and country. Linear mixed models with the same adjustments, with random effects for facility, were used to (i) assess the differences in HR-QOL and depression measures between TN and TF patients, and (ii) test for trends across three TF patient subgroups.
Statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). The STROBE Statement guidelines were followed for reporting observational studies [18].
Results
Baseline patient characteristics
Among 41 234 DOPPS participants, 4.5% (n = 1856) had a history of a first kidney TF, while 6.8% (n = 2806) had no prior TF and were wait-listed for kidney transplantation at the time of DOPPS enrollment, 25% (n = 466) of TF patients were wait-listed for repeat transplantation, while 37.5% (n = 696) did not have information on waiting list status. Table 1 lists the distribution of patient characteristics for TN wait-listed patients, all TF patients and TF patients stratified by the time since TF. TF patients tended to be younger, have a lower BMI and of greater dialysis vintage (all P < 0.05). TF patients had a lower prevalence of diabetes, but a higher prevalence of congestive heart failure, cancer, psychiatric disorders and recurrent cellulitis/gastrointestinal bleed (all P < 0.05). TF patients were less likely to use an AVF (P = 0.06) or AVG (P = 0.01) as HD vascular access and more likely to use a CVC (P = 0.0003). There was a lower mean hemoglobin and serum albumin among TF patients, but higher PTH levels and serum ferritin relative to TN patients (all P < 0.05).
Table 1.
Patient characteristics at study enrollment
| TN patients (n = 2806) | TF patients (n = 1856) | P-valuea | TF patients |
P-valueb | |||
|---|---|---|---|---|---|---|---|
| <3 months (n = 313) | 3–12 months (n = 299) | >12 months (n = 1244) | |||||
| Age (years, mean ± SD) | 51.1 | 48.3 | 0.0032 | 47.4 | 49.9 | 48.1 | 0.22 |
| Male (%) | 61.6 | 61 | 0.66 | 64.2 | 59.2 | 60.7 | 0.58 |
| Black (%) | 15.1 | 10.6 | 0.04 | 15.3 | 9.7 | 9.6 | 0.90 |
| Weight (kg) | 73.5 | 66.5 | <0.0001 | 71.2 | 68.3 | 65.0 | <0.0001 |
| BMI (kg/m2, mean ± SD) | 25.4 | 23.5 | <0.0001 | 24.2 | 23.7 | 23.3 | 0.0030 |
| Vintage (years, mean ± SD)c | 3.6 | 4.2 | <0.0001 | – | – | – | – |
| Comorbidities (%) | |||||||
| Coronary artery disease | 39.5 | 33.1 | 0.69 | 27.9 | 36.2 | 33.7 | 0.55 |
| Congestive heart failure | 21.5 | 28.6 | <0.0001 | 28.9 | 32.9 | 27.5 | 0.64 |
| Other cardiac disease | 21.9 | 33.1 | <0.0001 | 26.6 | 35.7 | 34.0 | 0.11 |
| Diabetes | 26.9 | 17.5 | <0.0001 | 26.0 | 24.2 | 13.7 | <0.0001 |
| Hypertension | 80.6 | 79.5 | 0.51 | 83.2 | 84.8 | 77.4 | 0.03 |
| Cerebrovascular disease | 7.8 | 8.4 | 0.71 | 9.4 | 9.5 | 8.0 | 0.51 |
| Peripheral vascular disease | 15.0 | 15.8 | 0.06 | 15.9 | 15.9 | 15.7 | 0.95 |
| Cancer | 6.0 | 7.7 | 0.0035 | 5.5 | 7.1 | 8.4 | 0.19 |
| Lung disease | 6.2 | 6.7 | 0.35 | 5.2 | 6.7 | 7.1 | 0.17 |
| Gastrointestinal bleed | 3.9 | 4.7 | 0.08 | 6.2 | 6.1 | 4.0 | 0.07 |
| Neurological disease | 7.4 | 8.9 | 0.004 | 5.8 | 8.1 | 9.8 | 0.02 |
| Psychiatric disorder | 14.9 | 21.5 | <0.0001 | 22.4 | 22.6 | 21.1 | 0.61 |
| Recurrent cellulitis/gangrene | 4.1 | 7.0 | 0.0002 | 7.8 | 5.1 | 7.3 | 0.44 |
| Vascular access (%) | |||||||
| Arteriovenous fistula | 70.1 | 63.9 | 0.06 | 52.0 | 60.9 | 67.7 | 0.0030 |
| Graft | 15.2 | 18.5 | 0.01 | 14.8 | 15.8 | 20.1 | <0.0001 |
| Catheter | 14.7 | 17.6 | 0.0003 | 33.2 | 23.2 | 12.2 | <0.0001 |
| Laboratory values (mean ± SD) | |||||||
| Hemoglobin (g/dL) | 11.6 | 11.1 | <0.0001 | 9.8 | 11.2 | 11.3 | <0.0001 |
| Albumin (g/dL) | 3.9 | 3.7 | <0.0001 | 3.4 | 3.7 | 3.8 | <0.0001 |
| Calcium (mg/dL) | 9.4 | 9.5 | 0.35 | 9.3 | 9.4 | 9.6 | 0.0001 |
| Phosphorus (mg/dL) | 5.8 | 5.8 | 0.25 | 5.7 | 5.9 | 5.7 | 0.33 |
| PTH (pg/mL) | 325 | 371 | 0.03 | 478 | 333 | 360 | 0.04 |
| Ferritin (ng/mL) | 423 | 426 | 0.01 | 325 | 428 | 445 | 0.01 |
Model adjusted for country and study phase and accounted for facility clustering.
aTest for difference between TF and TN patients in adjusted models.
bTest for trend across TF patient subgroups in adjusted models.
cTime since first ever dialysis from TN patients, and time since TF for TF patients.
BMI, body mass index; SD, standard deviation; PTH, parathyroid hormone.
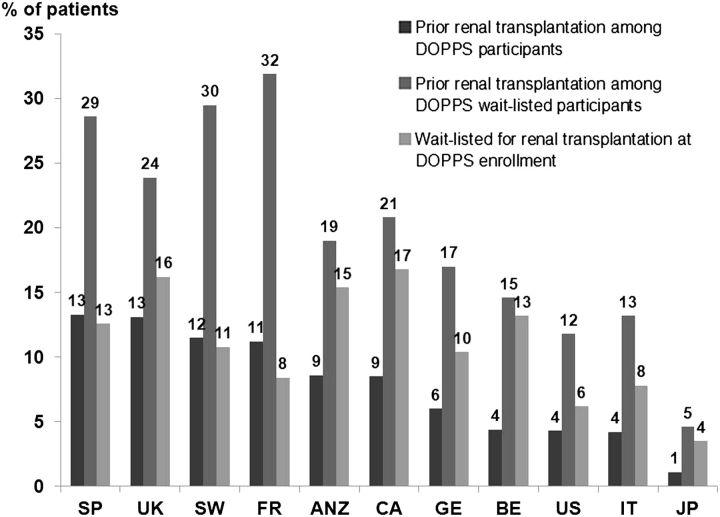
Prevalence of TF and TN patients by country
Figure 2 shows the prevalence of patients with a history of TF among (i) all patients and (ii) patients wait-listed for kidney transplant. The prevalence of prior TF ranges from 13.3% in Spain to 1.1% in Japan, with 4.3% in the USA. Among wait-listed patients, the prevalence of prior TF ranges from 31.9% in France to 4.6% in Japan, with 11.8% in the USA.
Fig. 2.
Percentage of patients by country with a history of transplant failure and wait-listed for kidney transplantation at DOPPS enrollment (Phases 1–3). SP, Spain; UK, United Kingdom; SW, Sweden; FR, France; ANZ, Australia–New Zealand; CA, Canada; GE, Germany; BE, Belgium; USA, United States; IT, Italy; JP, Japan.
Mortality and hospitalizations
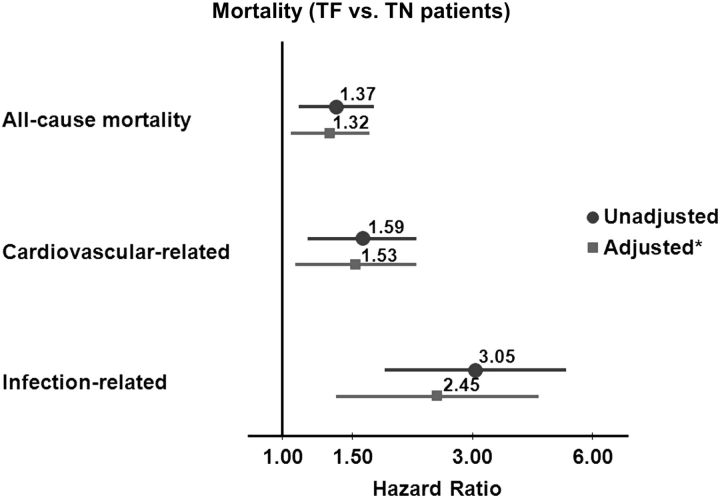
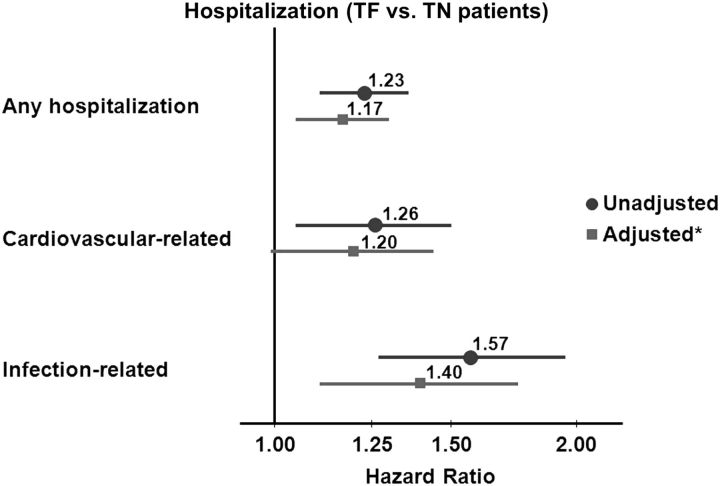
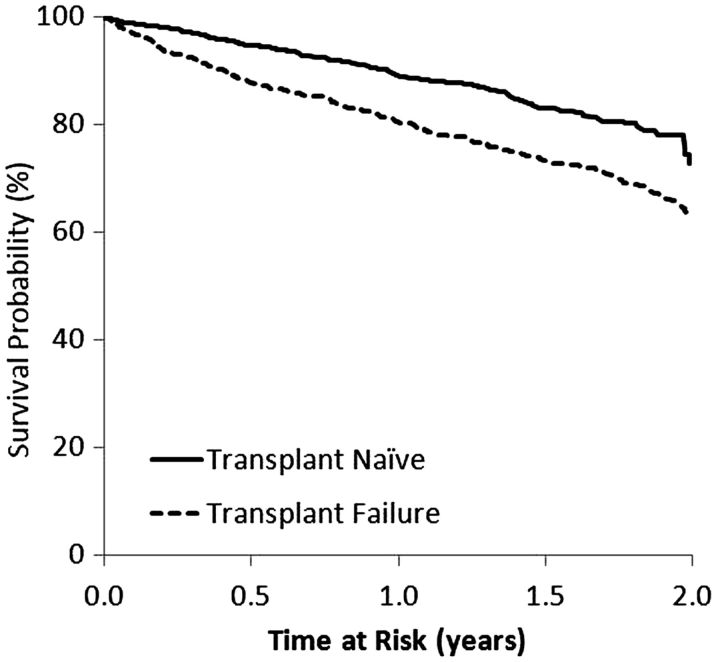
Among TN patients, 225 deaths occurred over a median of 1.4 years. After a median of 1.69 years, 246 TF patients died. Among TN patients, 1139 had a hospitalization after a median of 0.66 years; among TF patients, there were 1004 hospitalizations after a median of 0.58 years of follow-up. After a median of 1.2 years, 833 TN patients had a kidney transplant, while 233 TF patients had a repeat kidney transplant after a median of 1.5 years. Elevated mortality hazards were found for TF patients (versus TN patients) with and without adjusting for key covariates (Figure 3). Compared with TN patients, the adjusted hazards for TF patients were 32% higher for all-cause mortality, 53% higher for cardiovascular-related mortality and 145% higher for infection-related mortality (all P < 0.05). In exploring differences in infection-related mortality between TF and TN patients, using a forward-selection process in the multivariable model, albumin had the highest impact on the unadjusted or crude hazards ratio (HR) followed by dialysis vintage. In contrast, adjustment for vascular access type did not significantly alter the crude HR. Yet, within the fully adjusted final model, compared with AVF/AVG use, CVC use was independently associated with a 131% increased risk of infection-related mortality. We found elevated hazards of hospitalization events for TF versus TN patients (Figure 4). In sensitivity analyses comparing wait-listed TF and TN patients, we observed an increased trend in all-cause hospitalization in the wait-listed TF group [adjusted hazard ratio (AHR) 1.14, 95% confidence interval (CI) = 0.99–1.33, P = 0.07], but no difference in all-cause mortality (AHR 0.93, 95% CI = 0.62–1.39, P = 0.7).
Fig. 3.
Mortality: TF versus TN. All models stratified by country and study phase, and accounted for facility clustering. *Model adjusted for age, sex, race, BMI, time since initiation of HD or TX failure, 13 summary comorbid conditions, albumin and catheter use.
Fig. 4.
Time to first hospitalization: TF versus TN. All models stratified by country and study phase, and accounted for facility clustering. *Model adjusted for age, sex, race, BMI, time since initiation of HD or TX failure, 13 summary comorbid conditions, albumin and catheter use.
Restricted to TF patients, we evaluated the impact of the duration of renal allograft function and the duration of dialysis prior to renal allograft on mortality and hospitalization due to any causes (model adjusted for key covariates). Neither measure was associated with mortality or hospitalization due to any causes (all P > 0.15).
Infection-related complications
The most common causes of infection-related hospitalizations included septicemia (n = 114) and pneumonia (n = 79), while for infection-related mortality, septicemia (n = 25) and infections related to gangrene (n = 16) were most common. TF patients experienced a greater rate of infection-related complications (either hospitalization or death; Figure 5), a difference that was apparent soon after dialysis initiation and persisted after multivariable adjustment (AHR 1.45, 95% CI = [1.16–1.80], P = 0.001).
Fig. 5.
Infection-related hospitalization or death: TF versus TN. All models stratified by country and study phase, and accounted for facility clustering. *Model adjusted for age, sex, race, BMI, time since initiation of HD or TX failure, 13 summary comorbid conditions, albumin and catheter use.
HR-QOL scores
HR-QOL scores of TN and TF patients are presented in Table 2. The adjusted difference (AD) was greater than 3 points (positive AD for lower score in TF patients) for physical functioning, role physical, general health and bodily pain scales (P < 0.05 for all), and considered clinically significant [19]. The AD was 2.5 points for PCS (P < 0.0001). For mental scales, social functioning (AD = 5.2, P = 0.02) and vitality (AD = 6.7, P = 0.0002) were lower among TF patients. TF patients had lower scores for health-related symptoms/problems (AD = 3.0, P = 0.02) and higher prevalence of physician-diagnosed depression [adjusted odds ratio (AOR) = 1.42, P = 0.003].
Table 2.
QOL score and depression symptoms
| TN patients (n = 2806) | TF patients (n = 1856) | P-valuea | TF patients |
P-valueb | |||
|---|---|---|---|---|---|---|---|
| <3 months (n = 313) | 3–12 months (n = 299) | >12 months (n = 1244) | |||||
| Physical component summary | 39.6 | 37.1 | <0.0001 | 36.4 | 36.5 | 37.4 | 0.81 |
| Physical functioning | 54.2 | 47.3 | <0.0001 | 46.2 | 47.7 | 47.4 | 0.22 |
| Role physical | 42.3 | 34.7 | 0.0007 | 25.9 | 30.8 | 37.5 | 0.03 |
| General health | 46.9 | 42.4 | 0.0005 | 41.0 | 40.4 | 43.1 | 0.88 |
| Bodily pain | 66.3 | 60.8 | 0.0001 | 59.1 | 59.4 | 61.4 | 0.93 |
| Mental component summary | 46.5 | 44.8 | 0.51 | 43.5 | 43.9 | 45.3 | 0.34 |
| Mental health | 65.1 | 61.5 | 0.36 | 59.2 | 61.5 | 62.0 | 0.38 |
| Role emotional | 59.6 | 55.8 | 0.74 | 49.5 | 54.2 | 57.6 | 0.49 |
| Social functioning | 65.8 | 60.6 | 0.02 | 60.6 | 56.1 | 61.6 | 0.50 |
| Vitality | 45.5 | 38.8 | 0.0002 | 33.7 | 38.5 | 40.0 | 0.20 |
| Burden | 40.4 | 38.2 | 0.68 | 40.4 | 35.9 | 38.3 | 0.0022 |
| Effects | 61.5 | 57.3 | 0.06 | 60.0 | 55.8 | 57.1 | 0.0004 |
| Symptoms | 75.3 | 72.3 | 0.02 | 71.0 | 70.8 | 72.9 | 0.77 |
| CES-D ≥ 10 (%) | 36.7 | 41.7 | 0.11 | 38.5 | 49.5 | 40.6 | 0.80 |
| Depression (%) | 9.1 | 14.0 | 0.0027 | 13.1 | 15.5 | 13.9 | 0.12 |
Models adjusted for age, sex, race, BMI, 13 comorbidities, albumin, country and study phase and accounted for facility level clustering.
aTest for difference between TF and TN patients in adjusted models.
bTest for trend across TF patient subgroups in adjusted models.
QOL, quality of life; TN, transplant naïve; TF, transplant failure; CES-D, Centers for Epidemiologic Studies Depression scale.
We examined differences in HR-QOL scores across TF patient subgroups stratified by the time since TF (<3, 3–12 and >12 months). Our results revealed a trend toward the lowest scores among patients enrolled into DOPPS within 3 months from TF, with scores improving over time among those enrolled into DOPPS within 3–12 months after TF and further improving among those with a history of TF but enrolled within DOPPS at least 12 months after TF. The trend was attenuated after adjusting for patient characteristics, country and study phase (P < 0.05 for role physical, burden and effects).
Achievement of clinical practice guidelines
Compared with TN patients, TF patients were less likely to have serum albumin >4.0 g/dL (AOR = 0.67, 95% CI = 0.56–0.80, P = 0.0001) and more likely to have Kt/V >1.2 (AOR = 1.39, 95% CI = 1.10–1.75, P = 0.01) and PTH >500 pg/mL (AOR = 1.45, 95% CI = 1.20–1.74, P = 0.0001). TF patients were less likely to use an AVG or AVF (versus CVC) as vascular access (AOR = 0.85, 95% CI = 0.70–1.03, P = 0.10). TF patients enrolled in DOPPS within 3 months of TF (n = 313), had lower AVG and AVF use (AOR = 0.45, 95% CI = 0.32–0.62, P < 0.0001), were less likely to have hemoglobin of 10–12 g/dL (AOR = 0.54, 95% CI = 0.40–0.73, P < 0.0001), serum albumin >4.0 g/dL (AOR = 0.28, 95% CI = 0.19–0.43, P < 0.0001) and Kt/V >1.2 (AOR = 0.34, 95% CI = 0.22–0.53, P < 0.0001), and more likely to have PTH >500 pg/mL (AOR = 2.32, 95% CI = 1.59–3.38, P < 0.0001).
Discussion
The extensive DOPPS data set allowed us to examine the impact of a prior kidney TF on clinical outcomes and QOL of patients on chronic HD in a large, international cohort. Despite younger age and a lower prevalence of diabetes, patients with a history of TF had reduced survival and reduced QOL compared with wait-listed TN patients. We also found that QOL differences were less apparent in those with a longer time since TF and that survival differences between TF and TN patients were greatest for infection-related mortality.
Previous research examining the mortality of TF patients has yielded conflicting results [6, 7]. Several registry-based observational studies have examined the outcomes of patients with a history of TF; however, these studies were restricted to North American cohorts [5–7]. Data from The Canadian Organ Replacement Register (CORR) demonstrated no survival differences between TF and TN dialysis patients [6]. In this study, the comparator group consisted of all incident dialysis patients, as waiting list data were unavailable. It has been well documented that wait-listed dialysis patients have a lower risk of death than those not yet listed [1]. Moreover, case-mix adjustment was performed using comorbidities obtained at the time of kidney transplantation, not at the time of kidney TF, underestimating accrued comorbidities over the course of renal transplantation.
Using US data from the Scientific Registry of Transplant Recipients, Rao et al. [7] demonstrated a greater mortality risk among TF patients compared with TN patients who were wait-listed for kidney transplantation. While Rao et al. did employ the use of a wait-listed TN comparator group in the second study, other factors, such as the baseline differences in survival between Canadian and US dialysis patients, as well as the limited comorbidity adjustment in the US study may also account in part for the different results obtained. Similar to the study by Rao et al., we performed additional sensitivity analyses restricting survival and hospitalization comparisons between both wait-listed TF and TN patients. In doing so, we saw a trend of a 17% increased risk of hospitalization which persisted among TF wait-listed patients compared with TN wait-listed patients. Unlike the study by Rao et al., we did not see an increased risk of death among TF wait-listed patients compared with TN wait-listed patients. The increased risk of death among TF patients was largely seen among TF patients not wait-listed for kidney TF at study enrollment compared with TN wait-listed patients. These findings may relate to the limited power in our analysis to detect such differences owing to the low mortality rate of wait-listed patients coupled with fewer (<25%) TF patients wait-listed for repeat kidney transplantation. The low wait-list rate may relate to differences in timing and eligibility of wait-listing across DOPPS countries. Alternatively the paper by Rao et al. characterized comorbidities among TF patients at the time of transplantation, while in the present analysis, they were characterized at the time of TF. Therefore, it is possible that accounting for the accrual of comorbidities over the transplant duration may have attenuated survival differences between TF and TN wait-listed patients.
There are several reasons why CVC use may be higher among TF patients. TF patients have experienced a period of HD prior to receiving a kidney transplant. For some patients, options for a surgical vascular access may have been exhausted during the pretransplant HD period, with limited opportunities for a repeat AVF or AVG. The higher rates of CVC use among TF patients may be a proxy for suboptimal chronic kidney disease management prior to starting dialysis. Despite being managed by transplant nephrologists in the predialysis period, TF patients may be referred late for dialysis evaluation as a result of: (i) fragmentation of care between the kidney transplant and dialysis centers, (ii) an overemphasis on preservation of renal allograft function, and an underemphasis of predialysis care, (iii) patient-induced delays including reluctance to accept the need for dialysis and (iv) unanticipated and rapid loss of kidney allograft function [20]. Among patients with native kidney function decline, multidisciplinary predialysis care has been demonstrated to improve the use of surgical HD vascular access, and improve bone mineral metabolism parameters at dialysis initiation [21], while reducing morbidity and mortality upon dialysis initiation [22]. Consistent with previous observations, despite younger age, TF patients within the first 3 months of dialysis initiation were less likely to achieve clinical practice guidelines on HD and had lower serum albumin, lower hemoglobin and greater PTH compared with TN patients [20]. These parameters improved among patients with a longer period of dialysis after TF. The provision of more comprehensive predialysis care among patients with failing renal allografts may improve achievement of practice guidelines and clinical outcomes as well.
The adverse outcomes of TF patients relative to TN patients may also reflect the deleterious effects of prolonged and chronic immunosuppression exposure over the duration of graft function. Maintenance immunosuppression regimens carry short- and long-term increased risks of cancer, infection and cardiovascular complications [23, 24]. Whether or not immunosuppression exposure after TF may further increase the risks of adverse events has not been well studied.
The presence of a failed renal allograft may be an ongoing source of chronic inflammation, an established risk factor for adverse events among end-stage renal disease patients [25, 26]. We observed several features of chronic inflammatory syndrome including a lower BMI, anemia and elevated ferritin and hypoalbuminemia, features that are often accompanied by elevated systemic inflammatory markers as well as erythropoietin resistance [27]. However, preliminary observational data suggested that transplant nephrectomy may improve erythropoietin sensitivity, correct hypoalbuminemia and is associated with improved survival [27–29]. Further prospective data are required to confirm this finding before definitive conclusions can be drawn.
Depression and reduced QOL are prevalent among HD patients compared with the general population [30]. Kidney transplantation is associated with significant improvements in QOL [2]. Not surprisingly, patients returning to dialysis after kidney TF reported inferior QOL and greater physician-diagnosed depression compared with wait-listed TN patients. The reduction in QOL among TF patients was largest for patients initiating dialysis <3 months after TF. This represents a challenging transitional period as TF patients adapt to the loss of autonomy and significant QOL improvements associated with kidney transplantation. While the QOL deficit lessened over time relative to TN patients, it is possible that the improvements in QOL may reflect the impact of survivor bias; namely, QOL may be greater in TF patients healthy enough to survive to 1 year or more post-failure. Greater differences between PCS relative to MCS between TF and TN patients may relate to functional limitations not only due to the adverse effects of uremia, but may be compounded by the observed elevations in inflammatory markers among TF patients, indicating chronic systemic inflammation and the potential deleterious effects of immunosuppression exposure on muscle strength and exercise tolerance.
There are limitations worth noting in the present study. As with all analyses of observational data, a threat to validity is confounding based on unmeasured facility- and patient-level characteristics. Variables including exposure to multidisciplinary predialysis care and the acuity of dialysis initiation and levels of kidney function at dialysis initiation would have been of interest in understanding the mortality differences between TF and TN patients. Vascular access attempts prior to the start of dialysis were not recorded. Information regarding cause of renal TF, immunosuppression exposure and the use of transplant nephrectomy was limited but may have impacted the survival of TF patients. Furthermore, QOL data were available for a subset of patients and limited to one point in time. Assessment of the impact of the time since TF on HR-QOL would have ideally been addressed using serial assessments of QOL.
Notwithstanding these limitations, we have demonstrated that compared with TN patients, TF patients have reduced survival and QOL. Kidney care practitioners need to familiarize themselves with the medical and psychosocial challenges of this unique and growing patient population. Interventions aimed at reducing the morbidity of TF patients are needed. The role of modifiable practices to improve the outcomes of TF patients including the use of multidisciplinary predialysis care, the method and rapidity of immunosuppression reduction after TF and the use of transplant nephrectomy are questions which need to be answered.
Acknowledgements
F.T. is supported in part by NIDDK grant 1K01DK087762-01A1. The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbott (since 2009), Baxter (since 2011) and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications. The results presented in this paper have not been published previously in whole or part, except in the abstract format.
Conflict of interest statement. J.P. has received speaking honoraria from Amgen Canada and Baxter Healthcare Canada and holds an unrestricted educational fellowship from Baxter Healthcare Canada.
Appendix 1.
International composition of the DOPPS
| DOPPS 1 (1996–2001) | DOPPS 2 (2002–04) | DOPPS 3 (2005–08) |
|---|---|---|
| France | Australia and New Zealand | Australia and New Zealand |
| Germany | Belgium | Belgium |
| Italy | Canada | Canada |
| Japan | France | France |
| Spain | Germany | Germany |
| UK | Italy | Italy |
| USA | Japan | Japan |
| Spain | Spain | |
| Sweden | Sweden | |
| UK | UK | |
| USA | USA |
Appendix 2. Definitions of cause-specific mortality and hospitalization
| Causes of death | Hospitalization |
||
|---|---|---|---|
| Diagnoses | Procedures | ||
| Cardiovascular-related | Myocardial infarction (acute) | Hypertension | Cardiac catheterization |
| Pericarditis (including cardiac tamponade) | Angina | Coronary angioplasty | |
| Atherosclerotic heart disease | Chest pain (MI ruled out) | Coronary bypass graft (CABG) | |
| Cardiomyopathy | Acute myocardial infarction (MI) | Valve repair or replacement | |
| Cardiac arrhythmia | Cardiac arrest/sudden death | Cardioversion | |
| Cardiac arrest | Congestive heart failure | AICD (defibrillator) placement | |
| Valvular heart disease | Cardiomyopathy | Pacemaker placed | |
| Pulmonary edema due to exogenous fluid | Valvular heart disease | Pericardial procedure | |
| Congestive heart failure | Atrial fibrillation | Other cardiac procedures | |
| Cerebrovascular accident (including intracranial hemorrhage) | Other arrythmia | Carotid endarterectomy | |
| Pericarditis and/or tamponade | Evacuation of hematoma | ||
| Hypotension | Angiogram | ||
| Other cardiac diagnosis | Arterial bypass surgery | ||
| TIA | Amputation | ||
| Stroke (CVA) | Aortic aneurysm repair | ||
| Subdural hematoma | Wound debridement | ||
| Claudication/rest pain | Other vascular procedures | ||
| Ulcer of extremity | |||
| Gangrene | |||
| Aortic aneurysm | |||
| Deep vein thrombosis | |||
| Other vascular access diagnosis | |||
| Infection-related | Septicemia due to vascular access | Pneumonia | Abscess drainage |
| Septicemia due to peritonitis | Septicemia | Antibiotic therapy | |
| Septicemia due to peripheral vascular disease (gangrene) | Endocarditis | Other infection procedures | |
| Septicemia (other) | AIDS/HIV | ||
| Pulmonary infection (bacterial) | Urinary tract infection | ||
| Pulmonary infection (fungal) | Wound infection | ||
| Pulmonary infection (other) | Abscess | ||
| Viral infection (CMV) | Meningitis | ||
| Viral infection (other) | Cellulitis/soft tissue infection | ||
| Tuberculosis | Osteomyelitis | ||
| AIDS | Viral infection | ||
| Infections (other) | Fungal infection | ||
| Hepatitis B | Fever or chills (source unknown) | ||
| Other viral hepatitis | Other infection diagnosis | ||
| Fungal peritonitis | |||
Appendix 3. Summary comorbid conditions collected at DOPPS study entry
| Summary comorbid condition |
|---|
| Coronary artery disease (CAD) |
| Congestive heart failure (CHF) |
| Other cardiovascular disease |
| Cancer (other than skin) |
| Cerebrovascular disease |
| Diabetes |
| Gastrointestinal bleeding in prior 12 months |
| Hypertension |
| Lung disease |
| Neurological disease |
| Peripheral artery disease (PAD) |
| Psychiatric disorder |
| Recurrent cellulitis or gangrene |
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. doi:10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235–242. doi: 10.1038/ki.1996.307. doi:10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 3.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. doi: 10.1111/j.1600-6143.2010.03283.x. doi:10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System: USRDS 2007 Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 5.Gill JS, Abichandani R, Kausz AT, et al. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62:1875–1883. doi: 10.1046/j.1523-1755.2002.00640.x. doi:10.1046/j.1523-1755.2002.00640.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao PS, Schaubel DE, Saran R. Impact of graft failure on patient survival on dialysis: a comparison of transplant-naive and post-graft failure mortality rates. Nephrol Dial Transplant. 2005;20:387–391. doi: 10.1093/ndt/gfh595. doi:10.1093/ndt/gfh595. [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Schaubel DE, Jia X, et al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294–300. doi: 10.1053/j.ajkd.2006.11.022. doi:10.1053/j.ajkd.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Ojo A, Wolfe RA, Agodoa LY, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66:1651–1659. doi: 10.1097/00007890-199812270-00014. doi:10.1097/00007890-199812270-00014. [DOI] [PubMed] [Google Scholar]
- 9.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5 Suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE. SF-36 Health Survey: Manual & Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1997. [Google Scholar]
- 11.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi:10.1097/00005650-199206000-00002. [PubMed] [Google Scholar]
- 12.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston: The Health Institute: 1994. [Google Scholar]
- 13.Hays RD, Kallich JD, Mapes DL, et al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. doi:10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 14.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 15.III. NKF-K/DOQI clinical practice guidelines for vascular access: update 2000. Am J Kidney Dis. 2001;37(1 Suppl 1):S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 16.KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. doi:10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S66–S70. doi: 10.1053/ajkd.2001.20748. doi:10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. doi:10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 19.Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–155. doi: 10.2165/00019053-199915020-00003. doi:10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 20.Gill JS, Abichandani R, Khan S, et al. Opportunities to improve the care of patients with kidney transplant failure. Kidney Int. 2002;61:2193–2200. doi: 10.1046/j.1523-1755.2002.00373.x. doi:10.1046/j.1523-1755.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 21.Friedman O, Wald R, Goldstein MB. The impact of prior multidisciplinary predialysis care on mineral metabolic control among chronic hemodialysis patients. Nephron Clin Pract. 2008;110:c229–c234. doi: 10.1159/000167870. doi:10.1159/000167870. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein M, Yassa T, Dacouris N, et al. Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis. 2004;44:706–714. [PubMed] [Google Scholar]
- 23.Gallagher MP, Kelly PJ, Jardine M, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21:852–858. doi: 10.1681/ASN.2009101043. doi:10.1681/ASN.2009101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasiske BL. Cardiovascular disease after renal transplantation. Semin Nephrol. 2000;20:176–187. [PubMed] [Google Scholar]
- 25.Wanner C, Zimmermann J, Schwedler S, et al. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl. 2002:99–102. doi: 10.1046/j.1523-1755.61.s80.18.x. doi:10.1046/j.1523-1755.61.s80.18.x. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. doi:10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Gomez JM, Perez-Flores I, Jofre R, et al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15:2494–2501. doi: 10.1097/01.ASN.0000137879.97445.6E. doi:10.1097/01.ASN.0000137879.97445.6E. [DOI] [PubMed] [Google Scholar]
- 28.Ayus JC, Achinger SG, Lee S, et al. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. 2010;21:374–380. doi: 10.1681/ASN.2009050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston O, Rose C, Landsberg D, et al. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant. 2007;7:1961–1967. doi: 10.1111/j.1600-6143.2007.01884.x. doi:10.1111/j.1600-6143.2007.01884.x. [DOI] [PubMed] [Google Scholar]
- 30.Tentori F, Mapes DL. Health-related quality of life and depression among participants in the DOPPS: predictors and associations with clinical outcomes. Semin Dial. 2010;23:14–16. doi: 10.1111/j.1525-139X.2009.00677.x. doi:10.1111/j.1525-139X.2009.00677.x. [DOI] [PubMed] [Google Scholar]