Abstract
Background. Selective urinary biomarkers have been considered superior to total proteinuria in predicting response to treatment and outcome in patients with membranous nephropathy (MN). Methods. We prospectively tested whether urinary (U) excretion of retinol-binding protein (RBP), α1-microglobulin (α1M), albumin, immunoglobulinIgG and IgM and/or anti-phospholipase 2 receptor (PLA2R) levels could predict response to rituximab (RTX) therapy better than standard measures in MN. We also correlated changes in antibodies to PLA2R with these urinary biomarkers. Results. Twenty patients with MN and proteinuria (P) >5 g/24 h received RTX (375 mg/m2 × 4) and at 12 months, 1 patient was in complete remission (CR), 9 were in partial remission (PR), 5 had a limited response (LR) and 4 were non-responders (NR). At 24 months, CR occurred in 4, PR in 12, LR in 1, NR in 2 and 1 patient relapsed. By simple linear regression analysis, UIgG at baseline (mg/24 h) was a significant predictor of change in proteinuria at 12 months (Δ urinary protein) (P = 0.04). In addition, fractional excretion (FE) of IgG, urinary alpha 1 microglobulin (Uα1M) (mg/24 h) and URBP (μg/24 h) were also predictors of response (P = 0.05, 0.04, and 0.03, respectively). On the other hand, UIgM, FEIgM, albumin and FE albumin did not predict response (P = 0.10, 0.27, 0.22 and 0.20, respectively). However, when results were analyzed in relation to proteinuria at 24 months, none of the U markers that predicted response at 12 m could predict response at 24 m (P = 0.55, 0.42, 0.29 and 0.20). Decline in anti-PLA2R levels was associated with and often preceded urinary biomarker response but positivity at baseline was not a predictor of proteinuria response. Conclusions. The results suggest that in patients with MN, quantification of low-, medium- and high-molecular-weight urinary proteins may be associated with rate of response to RTX, but do not correlate with longer term outcomes.
Keywords: membranous nephropathy, rituximab, urinary proteins
Introduction
Idiopathic membranous nephropathy (MN) is a common immune-mediated glomerular disease and remains the leading cause of nephrotic syndrome in Caucasian adults [1]. Although in most patients the disease progresses relatively slowly, ∼40% of patients eventually develop end-stage renal disease (ESRD) [2]. Because of its frequency, it remains the second or the third most common type of primary glomerulonephritis resulting in ESRD [3]. Patients with MN who remain nephrotic are at an increased risk of thromboembolic [4] and cardiovascular events [5, 6]. Available immunosuppressive therapies include the use of corticosteroids combined with cytotoxic agents, and calcineurin inhibitors. These therapies are at least partially successful in reducing proteinuria, but their use is controversial, associated with significant adverse effects and carries a high rate of relapse (reviewed in [7]). These are important considerations in a disease where up to 30% of MN patients may achieve spontaneous remission of proteinuria and enjoy long-term renal survival without such therapy [8].
Given the variability in the natural history of the disease, an approach has been to limit immunosuppression treatment to those subjects identified as being at higher risk of progression, and a number of predictors of renal outcome and disease progression have been identified for patients with idiopathic MN [9–11]. Among them, some qualitative aspects of proteinuria such as urinary (U) excretion of α1-microglobulin (α1M), β2-microglobulin (β2M), immunoglobulinIgG and IgM have been reported as strong predictors of renal disease progression [12–21]. However, little is known regarding factors that may predict response to therapy, and only a few studies have evaluated the use of urinary markers for this purpose [22–24].
Bazzi et al. [17] quantified UIgG and Uα1M in 38 patients with nephrotic syndrome and normal renal function. Using an arbitrary cutoff value of 110 mg/g urinary creatinine (uCr) for IgG and 33.5 mg/g uCr for α1M, these investigators showed that 100% of patients with a baseline IgG excretion of <110 mg/g uCr underwent remission of proteinuria versus 20% in those with an IgG excretion of >110 mg/g uCr. Similarly, 77% of the patients with an α1M of <33.5 mg/g uCr went into remission versus 17% of the patients with an α1M of >33.5 mg/g uCr. Conversely, the remission rate was independent of baseline proteinuria. Of the 38 patients, 19 were allocated, in a non-randomized fashion, to receive either corticosteroids and cyclophosphamide (CYC) for 6 months (n = 16), or corticosteroids alone (n = 3), versus continuation of conservative therapy (n = 19). There was no difference in remission of proteinuria between the treated and the untreated patients. A greater percentage of patients with UIgG and Uα1M above the cutoff value went into remission with immunosuppressive therapy versus those on conservative treatment, but the results did not reach statistical significance [17]. The same group also suggested the use of fractional excretion (FE) of IgG as a helpful predictor of response in patients with focal segmental glomerulosclerosis (FSGS) [22].
We recently conducted a study in 20 patients with MN treated with response to rituximab (RTX) [25]. Of the 18 patients who completed a 24-month follow-up, remission of proteinuria was seen in 16 patients. However, baseline proteinuria, pharmacokinetic studies and evaluation of B and T cell subsets could not predict which patient would respond to RTX. On the other hand, depletion of anti-PLA2R autoantibodies, a new biomarker identified in the majority of patients with MN [26], predicted proteinuria response in MN [27]. The present study aimed to evaluate whether in patients with MN urinary excretion of low-, medium- and high-molecular-weight (MW) proteins and their correlations with anti-PLA2R antibodies could identify a priori patients who may respond to RTX and thus benefit from this form of therapy.
Materials and methods
Study details including inclusion/exclusion criteria have been previously reported [25]. Briefly, patients included in the study met the following criteria: (i) biopsy-proven MN; (ii) creatinine clearance (CrCl) ≥30 mL/min/1.73 m2 and (iii) persistent proteinuria >5 g/24 h despite maximal tolerated angiotensin II blockade for at least 4 months. Patients with active infection, diabetes or a secondary cause of MN were excluded. Patients who had been on treatment with prednisone, cyclosporine or mycophenolate mofetil within the last 4 months, or alkylating agents within the last 6 months were also excluded from the study. Patients who fulfilled the inclusion criteria received RTX, 375 mg/m2 (iv) on Days 1, 8, 15 and 22, with retreatment (IRB) at Month 6 regardless of their clinical status. The Institutional Review Boards (IRB) at Mayo Clinic, University of North Carolina Chapel Hill, and the University Health Network, University of Toronto, approved the study and all the patients provided written informed consent, which was registered on www.clinicaltrials.gov, identifier NCT00405340.
Follow-up
In all patients, clinical and laboratory parameters, including complete blood counts, electrolytes, serum albumin, serum IgG (IgM, IgA) and a lipid panel, were evaluated at study entry, and at Months 3, 6, 9, 12, 18 and 24. CrCl, urinary protein (UP) and uCr were assessed by performing two consecutive 24-h urine collections at baseline, 6, 12 and 24 months, and one collection at the other visits. Data were considered accurate when uCr excretion was consistent with a complete 24 h collection. The mean of the two measurements was considered for the analysis.
Measurement of UPs
Urinary excretion of retinol-binding protein (RBP; MW 22 kd), α1M (MW 31.8 kd), albumin (MW 67 kd), IgG (MW 150 kd) and IgM (MW 850 kd) were measured in the Mayo Renal Function Laboratory at baseline, 3, 6, 9 and 12 months. Quantification of IgG, α1M and RBP assays were performed using a Dade Behring Nephelometer II and manufacturer supplied kits. IgM was measured by enzyme-linked immunosorbent assay using a two-site immunoenzymetric assay (Cygnus Technologies, Inc., Southport, NC). The lower limit of detection, defined as the concentration corresponding to a signal two standard deviations above the mean of the zero standard, was ∼4 mg/dL for IgG and 177 pg/mL for IgM. The lower limit of quantification, defined as the lowest concentration where concentration coefficients of variation are <20%, was 3.6 mg/L for IgG and 250 pg/mL for IgM. The levels of albumin and creatinine were measured on a Hitachi 912 chemistry autoanalyzer using an immunometric method (Tina-quant reagent system) and an enzymatic creatinase assay respectively (Roche Diagnostics, Indianapolis, IN) Total protein was measured on the Hiatchi 912 by pylogallo red (Wako Chemicals USA, Inc., 1600 Bellwood Road, Richmond, VA).
Anti-PLA2R immunoassay
Baseline samples were tested for the presence of anti-PLA2R antibodies by western blot immunoassay against both native PLA2R (present in extracts from human glomeruli) and cell-expressed recombinant human PLA2R, as previously described [26]. Subsequent sera from patients initially found to be positive in both assays were subsequently tested against native PLA2R only. Sera were routinely tested at a titer of 1:25, which has been shown to be both sensitive and specific. In those samples that were initially negative at 1:25, repeat testing was carried out at a serum dilution of 1:10 to detect very weakly positive samples. Samples that did not show anti-PLA2R reactivity at 1:10 dilution were considered negative.
Data handling and statistical considerations
The primary efficacy parameter was defined as change in UP excretion from baseline (Week 0) to 12 months post treatment. With institutional review board permission, the follow-up was extended to 24 months. Significance was assessed using a two-sided paired t-test with P-values < 0.05 accepted as significant. Complete remission (CR) was defined as a UP of <0.3 g/24 h, partial remission (PR) a reduction in UP of >50% plus a final UP of <3.5 g but >0.3 g/24 h, limited response (LR) a reduction in UP of >50% plus a final UP of >3.5 g/24 h and no response (NR) a reduction in UP of <50%. UIgG, UIgM, Uα1M, urinary retinol binding protein (URBP), FE IgG, FE IgM and albumin as well as the change in proteinuria at 12 and 24 months were also evaluated by means of simple linear regression analysis. FE of IgG, IgM and albumin, expressed per 100 mL of CrCl, was calculated according to the following formula:
All values were log-transformed. Data are presented as means (±SD) or medians (range). All statistical calculations were performed using the JMP software, version 8.0 (Cary, NC).
Percentage (%) of reduction in anti-PLA2R levels was calculated as % of reduction of each marker for each individual patient at each time point and then average (% reduction = [(UP baseline – UP mo)/UP baseline] × 100.
Results
Twenty patients (17 men and 3 women), aged 49 ± 13 years (mean ± SD), were enrolled in the study (Table 1). All the patients were severely nephrotic (proteinuria 11.9 ± 4.9 g/24 h). Systolic and diastolic blood pressures averaged 118 ± 14 and 74 ± 11 mmHg, respectively, at baseline, whereas the mean serum creatinine was 1.5 ± 0.5 mg/dL and the mean CrCl was 72.4 ± 33.1 mL/min/1.73 m2.
Table 1.
Main clinical and laboratory characteristics at study entry (baseline) of individual patients with IMN
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender (male/female) | M | M | M | M | M | M | M | M | M | F | M | M | M | F | M | M | M | M | F | M | ||
| Age (years) | 47 | 49 | 29 | 50 | 44 | 54 | 80 | 43 | 42 | 57 | 38 | 29 | 62 | 64 | 30 | 42 | 51 | 42 | 60 | 59 | 48.6 | 12.9 |
| Urinary protein excretion (g per 24 h) | 14.2 | 8.1 | 13.7 | 15.5 | 11.9 | 5.7 | 9.9 | 12.8 | 9.9 | 8.6 | 8.3 | 11.3 | 19.2 | 7 | 12.1 | 7.8 | 16.3 | 26.5 | 8.5 | 9.9 | 11.9 | 4.9 |
| Serum creatinine (mg/dL) | 1.8 | 1.4 | 1.4 | 2 | 1.6 | 2.3 | 2 | 1.9 | 1.3 | 0.8 | 0.8 | 1 | 1.4 | 1 | 2.1 | 1.1 | 2.3 | 1 | 1.4 | 1.1 | 1.5 | 0.5 |
| Creatinine clearance (mL per min per 1.73 m2) | 49 | 79 | 49 | 46 | 80 | 49 | 34 | 59 | 83 | 30 | 156 | 126 | 67 | 89 | 73 | 90 | 36 | 119 | 46 | 87 | 72.4 | 33.2 |
IMN, idiopathic membranous nephropathy.
Patient 7 was discontinued from the study.
Outcome
In the 18 patients who completed 24 months of follow-up, proteinuria decreased from a baseline of 11.9 ± 4.9 g/24 h (mean ± SD) to 4.2 ± 3.8 g/24 h at 12 months and 2.0 ± 1.7 g/24 h at 24 months (both P < 0.001, paired t-test). By the end of 24 months, CR was achieved in 4 patients, PR in 12 patients, 1 patient had a LR and 1 patient relapsed. The reduction in proteinuria was gradual, and in 18 patients who responded to treatment, the delta (Δ) proteinuria at 24 months was −10.1 ± 5.0 g. Using within-patient slopes (log scale), the average monthly drop in proteinuria through 24 months was found to be 8.5 ± 6.6% (P < 0.001). The reduction in proteinuria was paralleled by a progressive and significant increase in serum albumin levels from 2.7 ± 0.6 g/dL at baseline (≤3 g/dL in 12/20 patients) to 3.7 ± 0.4 g/dL at 12 months (≤3 g/dL in only 1/19 patients) and 4.0 ± 0.5 (mean ± SD) at 24 months. CrCl increased from 72.4 ± 33 mL/min/1.73 m2 at baseline to 88.4 ± 31.5 mL/min/1.73 m2 at 24 months (P = 0.02). There were significant changes in serum IgG and IgM levels, with baseline IgG levels increasing toward the normal range and IgM levels decreasing at 12 months and later. Serum IgA levels remained stable over the 2-year time period.
Two patients did not respond to the treatment. One patient (#7) who was immunosuppression-naive prior to entry was removed from the study at 6 months because of worsening proteinuria and progressive decline in kidney function (CrCl = 46 mL/min/ 1.73 m2 at baseline; 25 mL/min/1.73 m2 at Month 6). He was treated with prednisone and CYC and went into CR. A second patient who had failed multiple previous immunosuppressive treatments also did not respond to RTX at 12 months. He was subsequently treated with Tacrolimus and went into PR.
Correlation of UP excretion and response to RTX
There were significant changes in the UP profile, with Uα1M, URBP, Ualbumin, UIgG, FE IgG and FE albumin all showing significant decreases from baseline to 12 months. On the other hand, UIgM and FE IgM did not change significantly between baseline and Month 12 (Table 2).
Table 2.
Urinary proteins in patients with IMN treated with rituximab
| Parameter | Baseline | Month 3 | Month 6 | Month 9a | Month 12a |
|---|---|---|---|---|---|
| Urinary protein g/24 h | 11.853 ± 4.851 | 7.339 ± 4.114* | 6.629 ± 5.277* | 4.131 ± 3.441* | 4.221 ± 3.778* |
| Uα1M mg/24 h | 99.215 ± 63.009 | 70.744 ± 48.091* | 62.659 ± 52.463* | 39.377 ± 26.887* | 43.970 ± 45.139* |
| RBP µg/24 h | 12580.656 ± 12751.142 | 6911.791 ± 7474.773 | 6469.075 ± 9928.350 | 2515.243 ± 2688.234* | 3797.034 ± 6490.790* |
| MALB mg/24 h | 7091.837 ± 3654.818 | 5420.448 ± 3035.985 | 4173.181 ± 3043.012* | 3134.082 ± 2521.974* | 3376.997 ± 2776.854* |
| FE albumin | 0.26152 ± 0.17329 | 0.19418 ± 0.17742* | 0.13416 ± 0.13828* | 0.07405 ± 0.05381* | 0.07106 ± 0.06593* |
| UIgG mg/24 h | 442.890 ± 328.361 | 337.515 ± 346.372 | 206.958 ± 205.535* | 128.266 ± 117.523* | 150.979 ± 248.928* |
| FEIgG | 0.12184 ± 0.11188 | 0.07751 ± 0.11056* | 0.05983 ± 0.10180* | 0.01855 ± 0.01681* | 0.02223 ± 0.04522* |
| UIgM mg/24 h | 3.420 ± 3.449 | 3.002 ± 3.772 | 2.696 ± 4.326 | 1.229 ± 2.110* | 2.511 ± 6.593 |
| FEIgM | 0.00495 ± 0.00615 | 0.00459 ± 0.00670 | 0.00426 ± 0.00940 | 0.00126 ± 0.00110* | 0.00517 ± 0.01292 |
Mean ± SD.
aMean values at 9 and 12 months are presented for 19 patients. Patient #7 was discontinued from the study at Month 6.
*P ≤ 0.05 versus Month 0.
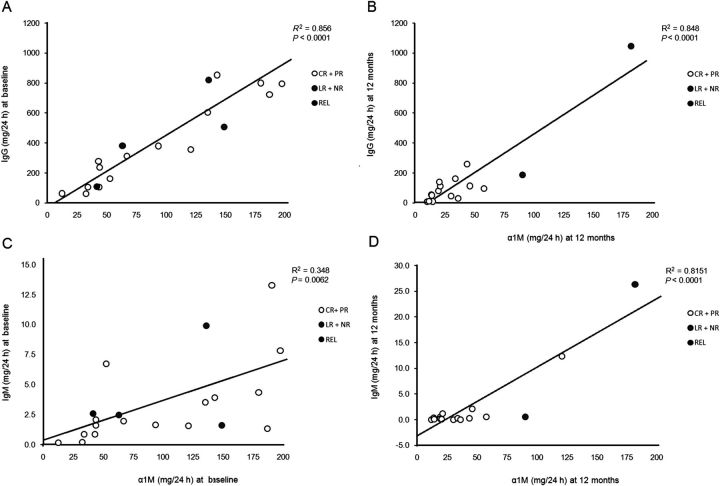
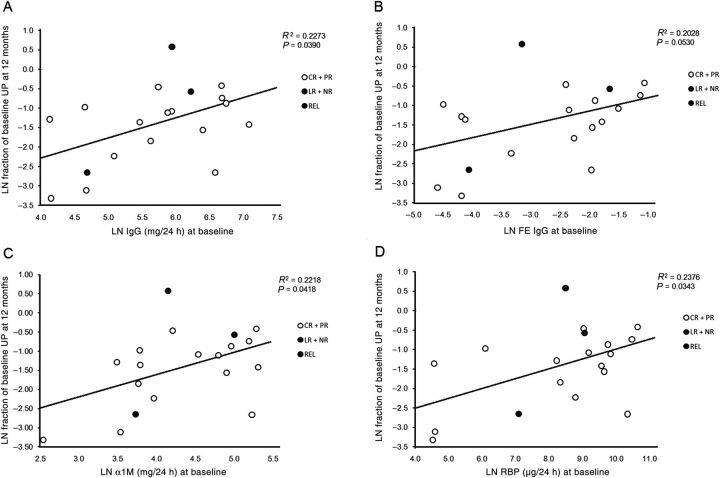
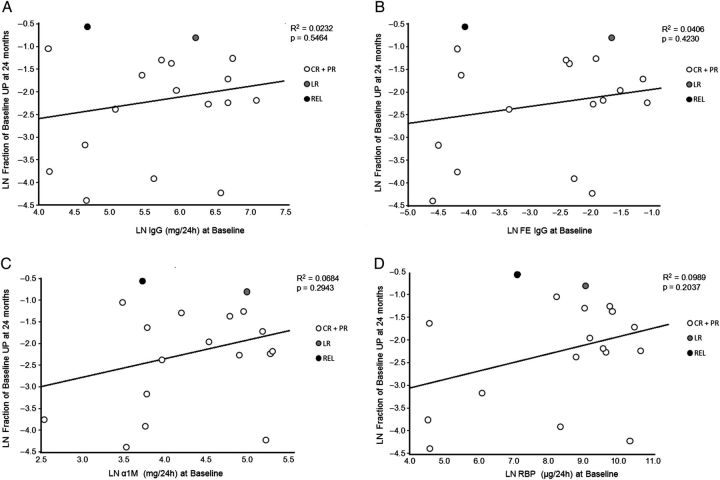
As reported by Bazzi et al. [22], we found a positive correlation between baseline UIgG and Uα1M P < 0.0001 (Figure 1A). A similar correlation was found between baseline UIgM and Uα1M P = 0.006 (Figure 1B). When results were analyzed by simple linear regression analysis in relation to proteinuria at 12 months, baseline UIgG (P = 0.04), FE IgG (P = 0.05), Uα1M (P = 0.04) and URBP (P = 0.03) predicted proteinuria response (Figure 2A–D), while UIgM, FE IgM, Ualbumin and FE albumin did not (P = 0.10, 0.27, 0.22 and 0.20, respectively; data not shown). However, when the same analysis was conducted in relation to proteinuria at 24 months, the correlations were no longer significant: UIgG (P = 0.55), FE IgG (P = 0.42), Uα1M (P = 0.29) and URBP (P = 0.20) (Figures 3A–D and Table 3). Baseline values for low- and high-MW proteins for individual patients and stratified according to proteinuria response at 24 months are presented in Table 4.
Fig. 1.
Correlation between urinary (U) excretion of IgG or IgM and α1M at baseline and 12 months. Correlation between UIgG (mg/24 h) and Uα1M (mg/24 h) at baseline (A, R2 = 0.8675) and at 12 months (B, R2 = 0.8480). Correlation between UIgM (mg/24 h) and Uα1M (mg/24 h) at baseline (C, R2 = 0.3544) and at 12 months (D, R2 = 0.810).
Fig. 2.
Correlation of UP at 12 months with excretion of high- and low-MW proteins at baseline. By simple linear regression analysis UIgG at baseline was found to be a significant predictor of UP excretion at 12 months (A, P = 0.04). FE IgG (B), Uα1M (C) and URBP (D) were also significant predictors of UP excretion at 12 months (P = 0.05, 0.04 and 0.03, respectively). The fraction of baseline proteinuria at 12 or 24 m is calculated as: Logarithmic number (LN) (UP 12 or 24 m/UP baseline), which is the equivalent to LN (UP 12 or 24 m) – LN (UP baseline).
Fig. 3.
Correlation of UP at 24 months with excretion of UIgG at baseline (A), FE IgG (B), Uα1M (C) and URBP (D). Baseline UIgG, FE IgG, Uα1M and URBP failed to predict change in proteinuria at 2 years.
Table 3.
Correlation between urinary markers at baseline and patients response at 12 and 24 months
| Urinary marker | Patients response at 12 months |
Patients response at 24 months |
||
|---|---|---|---|---|
| Fraction of Baseline UP |
Fraction of Baseline UP |
|||
| R2 | p value | R2 | p value | |
| α1M (mg/24h) | 0.2218 | 0.0418 | 0.0684 | 0.2943 |
| RBP (μg/24h) | 0.2376 | 0.0343 | 0.0989 | 0.2037 |
| MALB (mg/24) | 0.0877 | 0.2189 | 0.0054 | 0.7724 |
| FE Alb. | 0.0938 | 0.2021 | 0.0025 | 0.8428 |
| IgG (mg/24h) | 0.2273 | 0.0390 | 0.0231 | 0.5464 |
| FE IgG | 0.2028 | 0.0530 | 0.0406 | 0.4230 |
| IgM (mg/24h) | 0.1489 | 0.1028 | 0.0732 | 0.2776 |
| FE IgM | 0.0766 | 0.2662 | 0.0341 | 0.4779 |
Significant values are given in bold.
Table 4.
Urinary markers at baseline and patient response at 12 and 24 months
| UP at Baseline (g/24 h) | UP at 24 months (g/24 h) | α1M (mg/24h) | α1M/UCr (mg/g) | RBP (μg/24h) | RBP/UCr (μg/g) | MALB (mg/24) | FE Alb. | MALB/UCr (mg/g) | IgG (mg/24h) | FE IgG | IgG/UCr (mg/g) | IgM (mg/24h) | FE IgM | IgM/UCr (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 8.59 | 0.20 | 12.7 | 29.7 | 93.1 | 217.1 | 1541.1 | 0.087 | 3594.3 | 63.5 | 0.01467 | 148.0 | 0.152 | 0.00101 | 0.354 |
| 12 | 11.33 | 0.14 | 34.4 | 13.1 | 98.8 | 37.8 | 6118.0 | 0.071 | 2339.2 | 107.2 | 0.01009 | 41.0 | 0.860 | 0.00046 | 0.329 |
| 13 | 19.17 | 0.28 | 187.3 | 94.2 | 31314.4 | 15757.6 | 16578.6 | 0.531 | 8342.4 | 722.6 | 0.13685 | 363.6 | 1.328 | 0.00132 | 0.668 |
| 19 | 8.49 | 0.17 | 43.3 | 42.8 | 4226.2 | 4177.8 | 6457.4 | 0.308 | 6383.3 | 278.8 | 0.10179 | 275.6 | 0.877 | 0.00225 | 0.867 |
| 1 | 14.23 | 2.56 | 179.9 | 112.4 | 35800.1 | 22359.6 | 7744.7 | 0.435 | 4837.1 | 800.6 | 0.31579 | 500.0 | 4.336 | 0.00840 | 2.708 |
| 3 | 13.69 | 1.93 | 93.9 | 83.5 | 9867.7 | 8780.2 | 5119.1 | 0.336 | 4554.9 | 380.4 | 0.21736 | 338.5 | 1.651 | 0.00527 | 1.469 |
| 4 | 15.46 | 1.60 | 135.4 | 65.5 | 15704.0 | 7591.2 | 9905.6 | 0.383 | 4788.3 | 604.0 | 0.13870 | 292.0 | 3.524 | 0.00181 | 1.704 |
| 5 | 11.87 | 3.01 | 121.2 | 56.4 | 18873.2 | 8787.9 | 7866.5 | 0.189 | 3662.9 | 357.9 | 0.09390 | 166.7 | 1.568 | 0.00238 | 0.730 |
| 6 | 5.73 | 2.01 | 32.9 | 19.2 | 3794.7 | 2215.9 | 1383.6 | 0.050 | 808.0 | 62.5 | 0.01485 | 36.5 | 0.200 | 0.00100 | 0.117 |
| 9 | 9.88 | 2.70 | 67.3 | 38.1 | 8482.5 | 4801.5 | 7332.9 | 0.216 | 4150.7 | 311.8 | 0.08858 | 176.5 | 1.974 | 0.00096 | 1.118 |
| 11 | 8.33 | 0.35 | 44.0 | 17.2 | 444.7 | 174.0 | 6960.0 | 0.068 | 2723.0 | 105.1 | 0.01061 | 41.1 | 1.618 | 0.00083 | 0.633 |
| 14 | 7.02 | 0.65 | 52.9 | 41.5 | 6628.6 | 5195.1 | 3034.2 | 0.082 | 2378.0 | 162.1 | 0.03530 | 127.1 | 6.753 | 0.00687 | 5.293 |
| 15 | 12.12 | 3.44 | 143.1 | 73.9 | 17483.8 | 9036.7 | 7463.9 | 0.386 | 3857.8 | 853.8 | 0.14571 | 441.3 | 3.924 | 0.00804 | 2.028 |
| 16 | 7.81 | 1.53 | 44.3 | 20.3 | 96.9 | 44.3 | 6040.6 | 0.101 | 2762.4 | 236.4 | 0.01642 | 108.1 | 2.069 | 0.00226 | 0.946 |
| 17 | 16.27 | 1.74 | 197.9 | 129.5 | 42477.6 | 27789.5 | 8270.3 | 0.479 | 5410.5 | 796.5 | 0.33854 | 521.1 | 7.831 | 0.02507 | 5.123 |
| 18 | 26.46 | 2.98 | 203.2 | 90.9 | 14478.1 | 6475.0 | 13080.6 | 0.325 | 5850.0 | 1196.3 | 0.16411 | 535.0 | 13.176 | 0.00746 | 5.893 |
| 8 | 12.76 | 5.70 | 149.1 | 82.8 | 8732.5 | 4851.9 | 11310.0 | 0.442 | 6284.0 | 506.6 | 0.18765 | 281.5 | 1.604 | 0.00470 | 0.891 |
| 2a | 8.05 | 63.5 | 32.5 | 4930.6 | 2522.7 | 5705.7 | 0.114 | 2919.3 | 382.0 | 0.04190 | 195.5 | 2.456 | 0.00145 | 1.257 | |
| 7a | 9.95 | 136.2 | 122.1 | 26874.2 | 24076.9 | 6056.0 | 0.543 | 5425.6 | 821.4 | 0.34728 | 735.9 | 9.911 | 0.01621 | 8.879 | |
| 20 | 9.88 | 5.63 | 41.8 | 25.9 | 1211.6 | 750.8 | 3868.1 | 0.085 | 2397.1 | 108.6 | 0.01678 | 67.3 | 2.585 | 0.00117 | 1.602 |
| Mean | 11.85 | 2.03 | 99.2 | 59.6 | 12580.7 | 7782.2 | 7091.8 | 0.262 | 4173.4 | 442.9 | 0.12184 | 269.6 | 3.420 | 0.00495 | 2.130 |
| SD | 4.85 | 1.71 | 63.0 | 37.0 | 12751.1 | 8358.6 | 3654.8 | 0.173 | 1792.2 | 328.4 | 0.11188 | 196.5 | 3.449 | 0.00615 | 2.329 |
aPatients 2 and 7 were discontinued from the study at 12 and 6 months respectively. Pt #2 went into PR after treatment with Tacrolimus; Pt #7 went into CR after Corticosteroids
+ Cyclophosphamide therapy.
UP = Urinary Protein; α1M = alpha 1 microglobulin; IgG = Immunoglubilin G; FE =Fractional excretion; RBP = Retinol binding protein; MALB = microalbuminuria; IgM = Immunoglobulin M; UCr= Urinary creatinine.
▭, CR; ▭, PR; ▭, LR; ▭, NR; ▭, REL.
The mean baseline UIgG was 293.03 ± 301.08 and 488.95 ± 356.57 mg/24 h, in patients who reached CR (n = 4) or PR (n = 12) at 24 months, respectively. In patients who were NR or had LR, baseline mean UIgG excretion were 601.7 ± 310.7 and 506.6 mg/24, respectively, and although these results tended to be higher than those found in the group with CR or PR, they did not reach statistical significance maybe due to the small number of patients (P = 0.26). Similar results were found for Uα1M, RBP, Ualbumin, FE IgG, FE albumin and IgM (Table 4).
When, as suggested by Bazzi et al. [28], baseline cutoff values of 110 mg/g uCr for IgG and 33.5 mg/g uCr for α1M were considered predictors of response at 24 months, only 1/4 in the CR and 2/12 in the PR group had IgG values that fulfilled this criteria, while 2/4 and 3/12 in the CR and the PR groups had α1M below the proposed threshold (Table 4).
None of the urinary markers could predict responses to other forms of immunosuppressive therapy. Patient #7 had one of the highest levels of UIgG and UIgM at baseline, but treatment with corticosteroids and CYC resulted in CR at 12 months. Similarly, Patient #2 failed therapy with RTX but went into PR following therapy with Tacrolimus, while all of his baseline urinary markers were well within mean values for the entire group (Table 4).
There was also no difference in the trend of total proteinuria versus the other U markers at 3, 6, 9 and 12 months, suggesting that the improvement in membrane selectivity occurred equally across all urinary markers. These results suggest that quantification of baseline low- and high-MW proteins do not predict ultimate response when using immunosuppressive therapies associated with high rates of response.
Correlation of anti-PLA2R antibodies and excretion of UPs
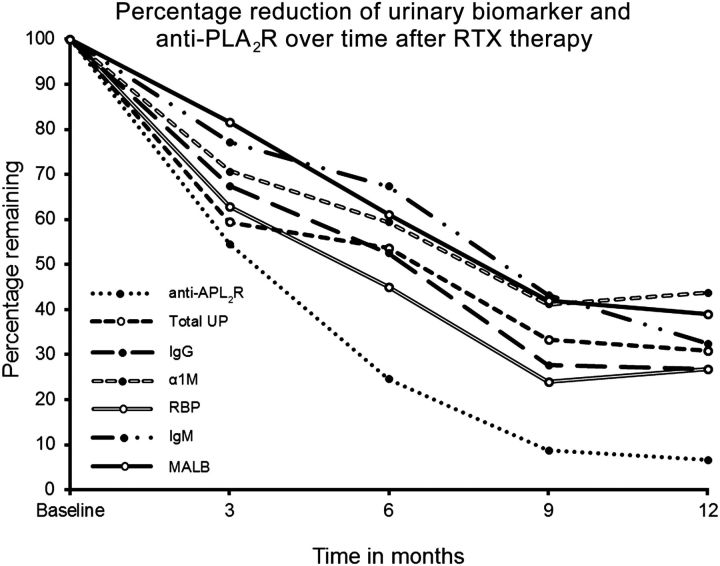
As previously reported, baseline anti-PLA2R levels did not correlate with response to therapy. However, a decreased in anti-PLA2R almost always preceded the decline in proteinuria [27]. When the percentage in reduction of U proteins is compared with reduction in anti-PLA2R, it can be seen that initial decline in anti-PLA2R levels, although a bit faster, paralleled declines in U proteins up to Months 3, while after that time, decline in anti-PLA2R in patients who responded to RTX was greater and at a much higher rate than the decline in U markers (Figure 4).
Fig. 4.
Percentage of reduction of UPs and on anti-PLA2R antibody levels over time in patient who responded to RTX therapy. % reduction = [(UP baseline – UP 3, 6, 9, 12 months)/UP baseline] × 100.
We attempted to compare the rate of decline in anti-PLA2R levels between patients who were in PR and those in CR at 24 months. There are 4 CR at 24 m, but only 2 of those patients were anti-PLA2R positive at baseline so we compared 11 PR at 24 m (1 was anti-PLA2R negative) and 2 CR at 24 m, but the results were not significantly different at any time point.
Discussion
In a number of studies, the use of RTX has induced a CR or PR of proteinuria in 60–80% of patients with idiopathic MN [2, 25, 29]. However, considering the cost of RTX therapy, the fact that not all patients respond to this therapy and the uncertainty regarding the long-term risk of RTX side-effects, it would be desirable to have a marker that could, a priori, identify patients who would respond to RTX and thus spare patients unlikely to respond from receiving RTX.
In a number of studies, quantification of the urinary excretion of low- and high-MW proteins has been reported as accurately predicting renal outcome in patients with glomerular diseases, including MN with variable results, but predicting response to therapy has been less certain [12–23].
In the study by Bazzi et al. [17] quantification of UIgG and Uα1M could not predict response to therapy. Nevertheless, these authors suggested that UIgG and Uα1M could be used to identify patients who are at risk of progression and for whom treatment with immunosuppressive therapy is indicated soon after diagnosis [17]. However, a correlation with baseline FE IgG and response to therapy was reported in patients with FSGS [22]. Twenty-seven patients with FSGS and nephrotic syndrome were treated with corticosteroids and CYC (n = 19) or corticosteroids alone (n =8 ). Seventy percent of the patients with FE IgG <0.025 (n = 0) responded to corticosteroids alone, 20% responded to corticosteroids combined with CYC and 10% were unresponsive. In patients with FE IgG >0.025 but <0.140 (n = 10), 20% were responsive to corticosteroids alone and 80% to corticosteroids and CYC. However, none of the patients with FE IgG >0.140 (n = 7) responded to therapy, suggesting that FE IgG may be used to predict response to immunosuppressive therapy in patients with FSGS [22].
On the other hand, du Buf-Vereijken and Wetzels studied UIgG, Ualb, Uβ2M and Uα1M in 25 patients with MN and renal insufficiency during and after treatment with corticosteroids and CYC and found that neither absolute levels at baseline or at 12 months nor the percentage reduction between baseline and 12 months in any of the studied markers predicted development of remission or relapse of proteinuria [23].
The present study represents the only prospective analysis to date evaluating the use of urinary excretion of low- and high-MW proteins as predictors of response to RTX therapy, and is the one with the largest number of urinary markers evaluated in a single cohort. As previously reported, following treatment with RTX 375 mg/m2 × 4, CR or PR occurred in 10 of 20 patients or 50% at 12 months and in 16/20 or 80% at 24 months [25].
Although the degree of proteinuria is an important prognostic indicator in most glomerular diseases, proteinuria at baseline did not predict response to RTX in this study. Similarly, quantification of Uα1M, URBP, Ualb and UIgG at baseline correlated with proteinuria response at 12 but not at 24 months. This apparent discrepancy could be explained by the fact that patients with MN who respond to treatment with RTX or cytotoxic agents exhibit a gradual reduction in proteinuria and it may take several months for proteinuria to reach its nadir [25, 30]. Thus, in patients with MN, an inaccurate assessment regarding response to therapy may occur if the primary end-point (e.g. proteinuria) is set too soon after initiation of immunosuppressive therapy. Our results are in agreement with those obtained by du Buf-Vereijken and Wetzels, who found that baseline quantification of IgG, α1M and β2M lacked accuracy in ultimate predicting response to cytotoxic therapy in patients with MN and impaired renal function [baseline CrCl 53 (25–68)mL/min)] followed by >24 months [23].
It is interesting that there was a very tight relationship between urinary excretion of IgG and α1M (Figure 3A and B). Given its relatively large size, IgG (MW ∼150 000) should have a significant barrier for filtration, whereas α1M (MW ∼25 000) should be freely filtered. Urinary levels of α1M are likely to reflect proximal tubular function, whereas urinary IgG should reflect both permselectivity defects and tubular reabsorptive capacity. A similar but less compelling relationship existed for urinary IgM and α1M. This tight relationship between urinary level of α1M and IgG and IgM persisted 12 months after therapy, but the correlation was better for IgG than IgM. It is unclear whether tubular cell injury by IgG promotes urinary excretion of multiple proteins due to abnormal proximal tubular reabsorption processes (albumin, α1m, RBP) or whether increased urinary levels of these proteins simply reflect tubular injury by other mechanisms.
It should be taken into account that at least 95% of the proteins filtered through the glomeruli undergo proximal tubular reabsorption. Thus, all the proteins measured in the urine represent a small fraction of the glomeruli filtrate, which makes the interpretation difficult [31]. For example, the glomerular fractional clearances (sieving coefficient) of both RBP and α1M are quite high (0.5–1), but their FE in the final urine is almost zero because of a 99.99% proximal tubular reabsorption. Second, albumin and IgG fractional clearances to the Bowman's space are normally not very different. According to, for example, the two-pore model, they may both be filtered through extremely infrequent ∼110Å (radius) pores, allowing the passage of a fraction of only 10−4 of the total glomerular filtration rate (GFR) [32]. Third, IgM seems to be totally rejected in the normal glomerular filtration barrier [31]. The mere presence of IgM in the urine may signal a major size-selective defect, if a post-renal source of IgM can be excluded, although a major problem with using IgM as a biomarker is its low concentration and the relatively high determination errors involved [31].
Having an accurate predictor of response to treatment with RTX would allow individualized targeting of therapy. Unfortunately, none of the following have helped to predict response to RTX: serum RTX levels, quantification of B and T cell subpopulations at baseline, testing for the presence of anti-chimeric antibodies, nor quantification of B and T cell numbers in renal biopsies [25, 29]. Ruggenenti et al. [33] correlated the degree of tubulointerstitial changes (tubulointerstitial atrophy and tubulointerstitial fibrosis) with response to RTX therapy. However, in our previous study, the degree of interstitial fibrosis in the initial biopsy did not predict response to RTX [29, 33]. Since the majority of patients in the current study had little tubulointerstitial fibrosis, we cannot disprove that significant fibrosis could render a patient unresponsive to RTX therapy. However, our results are consistent with recent data suggesting that chronic changes observed at presentation relate more closely to pre-existing patient factors such as age and hypertension rather than the effects of the MN disease itself, and do not preclude a remission of proteinuria among patients treated with immunosuppressive therapy [34]. These results are also compatible with the notion that IgG, α1M and β2M lack accuracy in predicting the response to cytotoxic therapy in patients with MN [23].
More recently, we sought to investigate the response of anti-PLA2R antibodies to treatment with RTX in our two study cohorts [25, 29]. Anti-PLA2R was found in pretreatment samples from 25 of the 35 (71%) patients (16 patients from the present study) [27]. Seventeen of the 25 autoantibody-positive patients (68%) showed a decline and disappearance of anti-PLA2R within 12 months of RTX treatment. These changes in autoantibody almost always preceded changes in proteinuria. Those who demonstrated such an immunologic response fared better clinically than those with persistent anti-PLA2R levels, with 59% attaining complete or PR by 12 months and 88% by 24 months, versus 0 and 33%, respectively.
In the present study, decline in U proteins was already noticeable during the first 3 months. However, as presented in Figure 4, the initial decline in all U markers occurred when significant levels of anti-PLA2R were still present. How can this be reconciled? It is possible that apart from suppression of pathogenic antibodies, RTX may also exert anti-proteinuric effects by modulating podocyte function. Fornoni et al. [35] recently studied 41 patients with ESRD secondary to FSGS and high risk of recurrence post kidney transplant. These investigators studied the effects of sera from patients with recurrent FSGS on normal human podocyte sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b) protein, acid sphingomyelinase (ASMase) activity and cytoskeleton remodeling. These sphingolipid-related proteins are crucial for the organization of receptors and signaling molecules in specialized cells, such as the podocytes [36]. Sera from patients who developed recurrent FSGS caused a decrease in both SMPDL-3b and ASM. Treatment with RTX partially prevented downregulation of SMPDL-3b and ASMase, the disruption of the actin cytoskeleton and podocyte apoptosis induced by podocyte exposure to sera of patients with recurrent FSGS [35]. These results suggest that RTX may have important direct modulatory effects on podocyte function, similar to what has been reported for cyclosporine, and may help explaining the initial decline in urinary proteins seen in the current study [37]. Thus, response to RTX may be characterized by an initial immunological-independent effect on podocyte cytoskeleton that is followed by an immunological-dependent effect on the cause of glomerular injury (i.e. anti-PLA2R antibodies).
Conclusion
In patients with idiopathic MN, treatment with RTX not only appears to be effective in inducing CR or PR in a significant number of patients, but also significantly improves the urinary excretion of a number of selective proteins, namely α1M, RBP, IgG and albumin. However, pre-treatment quantification of these urinary proteins did not predict the long-term response to RTX therapy. Our data should be interpreted narrowly in view that the number of patients involved in the study was small and the proteinuria response to RTX was high. In particular, we did not study the relationship between these proteins and disease progression and did not vary the therapy. Therefore, our data do not contradict previous studies indicating that these proteins can predict disease progression, nor does it preclude the possibility that they could be used to identify patients who should receive more-aggressive therapies. Whether these markers can identify patients who are likely to go into spontaneous remission requires further study. Reliable markers capable of predicting response to RTX in patients with MN remain to be defined.
Acknowledgments
The authors thank Mrs Lori Riess, for her outstanding role as research coordinator for the study. The study was supported by an unrestricted research grant from Genentech Inc., South San Francisco, CA, Biogen Idec, San Diego CA, the Department of Laboratory Medicine and Pathology, Mayo Clinic, the Fulk Family Foundation (FCF) and by a UL1-RR24150 grant to the Center for Translational Science Activities, Mayo Clinic. The results presented in this paper have not been published previously in whole or part, except in abstract format at the American Society of Nephrology meeting in Philadelphia, PA, November 2011.
Conflict of interest statement. None declared.
References
- 1.Medawar W, Green A, Campbell E, et al. Clinical and histopathologic findings in adults with the nephrotic syndrome. Ir J Med Sci. 1990;159:137–140. doi: 10.1007/BF02937405. [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Chiurchiu C, Brusegan V, et al. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol. 2003;14:1851–1857. doi: 10.1097/01.asn.0000071511.35221.b3. [DOI] [PubMed] [Google Scholar]
- 3.Cattran DC. Membranous nephropathy: quo vadis? [comment] Kidney Int. 2002;61:349–350. doi: 10.1046/j.1523-1755.2002.00125.x. [DOI] [PubMed] [Google Scholar]
- 4.Wagoner RD, Stanson AW, Holley KE, et al. Renal vein thrombosis in idiopathic membranous glomerulopathy and nephrotic syndrome: incidence and significance. Kidney Int. 1983;23:368–374. doi: 10.1038/ki.1983.28. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez JD, Hiatt RA, Killebrew EJ, et al. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 1993;44:638–642. doi: 10.1038/ki.1993.292. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DC, Bernard DB. Lipid abnormalities in the nephrotic syndrome: causes, consequences, and treatment. Am J Kidney Dis. 1994;23:331–346. doi: 10.1016/s0272-6386(12)80994-2. [DOI] [PubMed] [Google Scholar]
- 7.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3:905–919. doi: 10.2215/CJN.04321007. [DOI] [PubMed] [Google Scholar]
- 8.Polanco N, Gutierrez E, Covarsi A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattran DC, Pei Y, Greenwood C. Predicting progression in membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7(Suppl 1):48–52. [PubMed] [Google Scholar]
- 10.Pei Y, Cattran D, Greenwood C. Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int. 1992;42:960–966. doi: 10.1038/ki.1992.374. [DOI] [PubMed] [Google Scholar]
- 11.Cattran DC, Pei Y, Greenwood CM, et al. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 1997;51:901–907. doi: 10.1038/ki.1997.127. [DOI] [PubMed] [Google Scholar]
- 12.Reichert LJ, Koene RA, Wetzels JF. Urinary excretion of beta 2-microglobulin predicts renal outcome in patients with idiopathic membranous nephropathy. J Am Soc Nephrol. 1995;6:1666–1669. doi: 10.1681/ASN.V661666. [DOI] [PubMed] [Google Scholar]
- 13.Reichert LJ, Koene RA, Wetzels JF. Urinary IgG excretion as a prognostic factor in idiopathic membranous nephropathy. Clin Nephrol. 1997;48:79–84. [PubMed] [Google Scholar]
- 14.Bazzi C, Petrini C, Rizza V, et al. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17:1890–1896. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- 15.Branten AJ, du Buf-Vereijken PW, Klasen IS, et al. Urinary excretion of beta2-microglobulin and IgG predict prognosis in idiopathic membranous nephropathy: a validation study. J Am Soc Nephrol. 2005;16:169–174. doi: 10.1681/ASN.2004040287. [DOI] [PubMed] [Google Scholar]
- 16.Hofstra JM, Deegens JK, Willems HL, et al. Beta-2-microglobulin is superior to N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2008;23:2546–2551. doi: 10.1093/ndt/gfn007. [DOI] [PubMed] [Google Scholar]
- 17.Bazzi C, Petrini C, Rizza V, et al. Urinary excretion of IgG and alpha(1)-microglobulin predicts clinical course better than extent of proteinuria in membranous nephropathy. Am J Kidney Dis. 2001;38:240–248. doi: 10.1053/ajkd.2001.26080. [DOI] [PubMed] [Google Scholar]
- 18.Bakoush O, Torffvit O, Rippe B, et al. High proteinuria selectivity index based upon IgM is a strong predictor of poor renal survival in glomerular diseases. Nephrol Dial Transplant. 2001;16:1357–1363. doi: 10.1093/ndt/16.7.1357. [DOI] [PubMed] [Google Scholar]
- 19.Bakoush O, Torffvit O, Rippe B, et al. Renal function in proteinuric glomerular diseases correlates to the changes in urine IgM excretion but not to the changes in the degree of albuminuria. Clin Nephrol. 2003;59:345–352. doi: 10.5414/cnp59345. [DOI] [PubMed] [Google Scholar]
- 20.Deegens JK, Wetzels JF. Fractional excretion of high- and low-molecular weight proteins and outcome in primary focal segmental glomerulosclerosis. Clin Nephrol. 2007;68:201–208. doi: 10.5414/cnp68201. [DOI] [PubMed] [Google Scholar]
- 21.McQuarrie EP, Shakerdi L, Jardine AG, et al. Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol Dial Transplant. 2011;26:1563–1569. doi: 10.1093/ndt/gfq605. [DOI] [PubMed] [Google Scholar]
- 22.Bazzi C, Petrini C, Rizza V, et al. Fractional excretion of IgG predicts renal outcome and response to therapy in primary focal segmental glomerulosclerosis: a pilot study. Am J Kidney Dis. 2003;41:328–335. doi: 10.1053/ajkd.2003.50040. [DOI] [PubMed] [Google Scholar]
- 23.du Buf-Vereijken PW, Wetzels JF. Treatment-related changes in urinary excretion of high and low molecular weight proteins in patients with idiopathic membranous nephropathy and renal insufficiency. Nephrol Dial Transplant. 2006;21:389–396. doi: 10.1093/ndt/gfi219. [DOI] [PubMed] [Google Scholar]
- 24.Bazzi C, Rizza V, Paparella M, et al. Fractional urinary excretion of IgG is the most powerful predictor of renoprotection by ACE inhibitors in IgA nephropathy. J Nephrol. 2009;22:387–396. [PubMed] [Google Scholar]
- 25.Fervenza FC, Abraham RS, Erickson SB, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck LH, Jr, Fervenza FC, Beck DM, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzi C, D'Amico G. The urinary excretion of IgG and alpha1-microglobulin predicts renal outcome and identifies patients deserving treatment in membranous nephropathy. Kidney Int. 2002;61:2276. doi: 10.1046/j.1523-1755.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 29.Fervenza FC, Cosio FG, Erickson SB, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 30.Ponticelli C, Zucchelli P, Passerini P, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–1604. doi: 10.1038/ki.1995.453. [DOI] [PubMed] [Google Scholar]
- 31.D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63:809–825. doi: 10.1046/j.1523-1755.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 32.Blouch K, Deen WM, Fauvel JP, et al. Molecular configuration and glomerular size selectivity in healthy and nephrotic humans. Am J Physiol. 1997;273:F430–F437. doi: 10.1152/ajprenal.1997.273.3.F430. [DOI] [PubMed] [Google Scholar]
- 33.Ruggenenti P, Chiurchiu C, Abbate M, et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol. 2006;1:738–748. doi: 10.2215/CJN.01080905. [DOI] [PubMed] [Google Scholar]
- 34.Troyanov S, Roasio L, Pandes M, et al. Renal pathology in idiopathic membranous nephropathy: a new perspective. Kidney Int. 2006;69:1641–1648. doi: 10.1038/sj.ki.5000289. [DOI] [PubMed] [Google Scholar]
- 35.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perosa F, Favoino E, Caragnano MA, et al. Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood. 2006;107:1070–1077. doi: 10.1182/blood-2005-04-1769. [DOI] [PubMed] [Google Scholar]
- 37.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]