Abstract
Ocular ischaemic syndrome (OIS) is a relatively uncommon condition. Simultaneous bilateral involvement is even less common and has been reported in only 22% of all cases of OIS. It has variable clinical presentations, of which visual loss and ocular pain are the most common. It is believed to occur when there is a 90% or greater carotid artery obstruction. This syndrome is often associated with a number of systemic diseases including diabetes mellitus, hypertension, coronary artery disease, and cerebrovascular disease. Only occasionally has it been described as a complication of rhinocerebral mucormycosis. We report an unusual case of bilateral OIS secondary to bilateral internal carotid artery thrombosis as a complication of invasive rhinocerebral mucormycosis. In addition, a review of clinical presentation, diagnostic work-up and treatment options for OIS is provided.
Keywords: Ocular ischemic syndrome, Mucormycosis, Carotid stenosis, Carotid system imaging, Case report, Oman
Mucormycosis is the most invasive and rapidly progressive fungal infection in humans, of which rhino-orbito-cerebral involvement is a common manifestation.1 Affected individuals invariably have underlying disorders like diabetes mellitus, renal failure, liver cirrhosis, leukaemia, lymphoma, or other immunocompromised states.1,2,3 Ocular involvement in mucormycosis is usually in the form of orbital cellulitis. The pathway of spread begins with inoculation of the nasal mucosa with the mucor pathogen followed by invasion of the paranasal sinuses. The pathogen then moves to the inferior orbital fissure leading to thrombosis of regional blood vessels. This results in acute ocular symptoms of the eyelid, including oedema and chemosis. Extension of the infection into the orbital apex causes proptosis. Visual loss may develop secondary to invasion into the optic nerve or thrombosis of the retinal artery.1 Intracranial spread of the infection is believed to occur through the cribriform plate or orbital apex. In addition, the organism spreads along the meninges, nerves, and vessels. Hyphae can erode through the vascular endothelium leading to thrombosis, brain infarction, and necrosis. Vascular thrombosis in this mechanism may involve cavernous sinuses or the carotid artery.2 Ocular ischaemic syndrome results from severe and chronic hypoperfusion to ocular structures commonly due to severe atherosclerosis of the carotid vascular system with insufficient collateral circulation. There are few case reports in the literature of carotid vascular system thrombosis in association with invasive mucormycosis with secondary ocular involvement and severe visual loss. Most of these patients had a direct orbital involvement with signs of orbital cellulitis.2,4 In this article we report a case of rhinocerebral mucormycosis with no evidence of direct spread through the orbit. The patient developed cavernous sinuses and bilateral internal carotid artery thrombosis leading to bilateral OIS, which resulted in severe visual loss.
Case Report
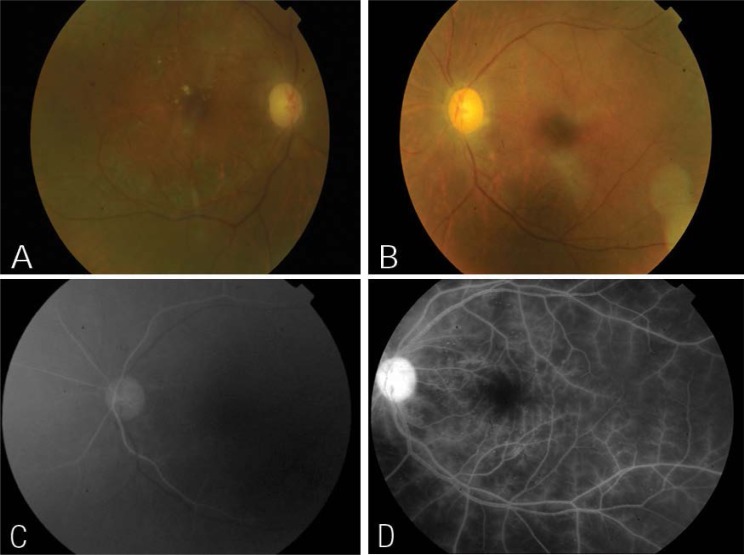
In January 2010, a 57-year-old Omani woman who was a known diabetic and had been receiving treatment for hypertension for many years developed right cavernous sinus thrombosis. In May 2010, she was diagnosed with chronic invasive rhinocerebral mucormycosis for which she was started on amphotericin and voriconazole and underwent multiple sinus debridement surgeries. In February 2011, she developed a right middle cerebral artery and anterior cerebral artery stroke and renal failure. She had a partial recovery with residual dysarthria for which she was treated with aspirin and clopidogrel. Amphotericin was substituted for caspofungin and posaconazole due to renal failure. During one of her recent admissions for another sinus debridement surgery, she complained of bilateral sudden visual loss associated with right eye pain. Her past ocular history revealed a bilateral cataract extraction with an intraocular lens implantation, bilateral non-proliferative diabetic retinopathy, and diabetic macular oedema. Her right eye was treated with a focal laser. Her baseline visual acuity was 0.3 in the right eye and 0.8 in the left eye. An ophthalmic examination on the day of referral revealed no light perception in either eye. However, after one hour her perception improved to 0.2 in the right eye and 0.1 in the left eye at a distance of one metre. An anterior segment examination for both eyes (OU) was unremarkable except for mid-dilated poorly reactive pupils and pseudophakia. Her intraocular pressure (IOP) OU was normal. There was no proptosis, and ocular motility was normal for OU. A dilated fundus examination revealed bilateral multiple microaneurysms; dot and blot retinal haemorrhages at the posterior pole and to some extent at the mid-periphery; pale optic discs, and attenuated retinal arteries. There was no neovascularisation of the disc or elsewhere, and there was no macular oedema OU [Figures 1a and b]. Fundus fluorescein angiography (FFA) revealed a bilateral delayed choroidal filling with evidence of widespread retinal ischaemia [Figures 1c and d].
Figure 1:
(A&B) Color fundus photographs of the right and left eyes respectively, shows pale discs, attenuated retinal arteries, microaneurysms, no neovascularisation of discs or elsewhere and no macular oedema in both eyes. Scattered exudates were noted over the macula of the right eye. (C) Fundus fluorescein angiography (FFA) of the left eye at 07 seconds shows delayed choroidal filling. (D) FFA of the left eye at 3:36 seconds shows widespread of retinal ischaemia and prominent staining of retinal arterioles.
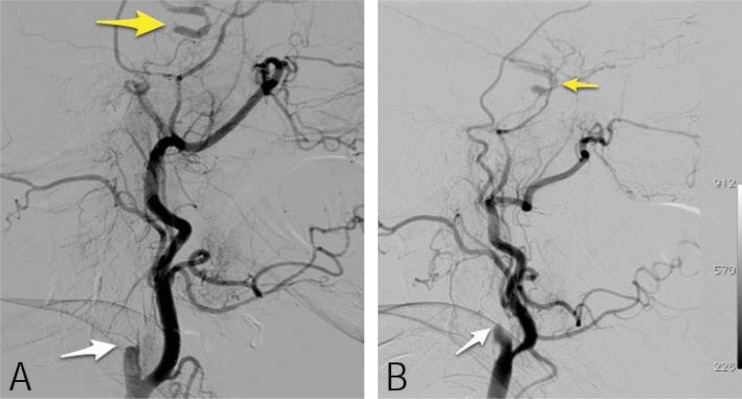
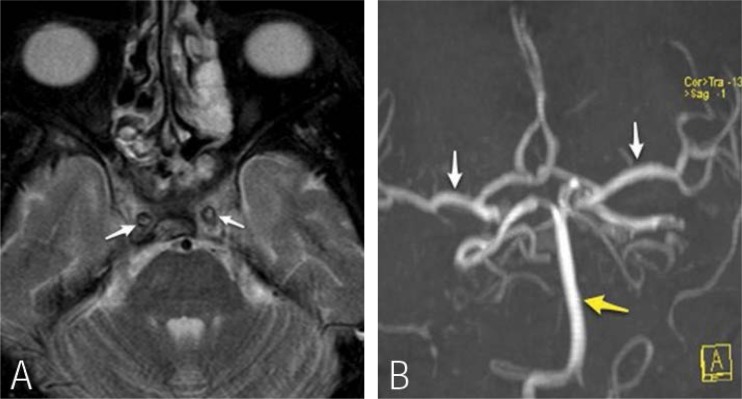
A magnetic resonance imaging (MRI) scan showed diffuse inflammatory changes in the paranasal sinuses. There was a loss of the normal signal void within both internal carotid arteries at the level of the cavernous sinuses in keeping with thrombosis of the internal carotid arteries (ICAs). A MRI angiogram showed complete absence of flow in the ICAs [Figures 3a and b]. This was confirmed by conventional digital subtraction angiography (DSA) of the ICAs as well as by cerebral angiography, which revealed bilateral abrupt occlusion of the internal carotid arteries. However, there were good collaterals with opacification of the internal carotid arteries distal to cavernous sinuses through dural and meningeal collateral arteries [Figures 3a and b]. Electroretinograghy showed a decrease in the amplitude of both A- and B-waves.
Figure 3:
(A) Lateral left carotid conventional angiogram demonstrating complete occlusion of the proximal left internal carotid artery (ICA) (white arrow). There is opacification of the supra-clinoid ICA (yellow arrow). (B) Lateral right carotid angiogram demonstrating similar findings as the left side.
No neovascularisation of anterior or posterior segments were detected throughout the follow-up period. However, the patient developed open angle glaucoma with poorly controlled IOP, optic nerve cupping with further deterioration in vision to light perception in the right eye, and no light perception in the left eye. In April 2012, she was still attending hospital follow-up visits to monitor her rhinocerebral mucormycosis for which oral posaconazole was prescribed.
Discussion
The incidence of OIS is estimated to be 7.5 cases per million persons per year.5 This figure could be underestimating the true incidence, probably due to the overlap between OIS in its clinical presentation and other ocular vascular diseases such as retinal vein occlusions and diabetic retinopathy.5,6 The fact that atherosclerotic vascular disease is the major cause of OIS explains why it is twice as common in males and why it typically occurs at a mean age of 65 years.5 This also contributes to the close association of OIS with vasculopathic conditions such as diabetes, hypertension, and hypercholesterolaemia, and the increased risk for OIS among patients with a previous history of stroke and myocardial infarction. Severe carotid artery occlusion by atherosclerosis with poor collateral circulation is evident in the majority of patients with OIS who present with the above risk factors. On the other hand, in a large randomised prospective study, around 26% of affected eyes with OIS were found to have no or mild ipsilateral carotid stenosis. This was explained by a possible vascular occlusion at the level of the aortic arch, and at the ophthalmic, central retinal, or ciliary arteries.7
Other aetiological factors leading to OIS include giant cell arteritis, aortic arch syndrome, Takayasu arteritis, and hypercysteinemia.5–8,9 Moreover, there have been several reported cases in the literature of carotid artery thrombosis secondary to invasion with mucormycosis, making mucormycosis another risk factor for OIS.1–3
Our patient had multiple atherosclerotic risk factors in addition to mucormycosis, which might together have contributed to the ICA occlusion. Her initial visual presentation was probably due to transient embolisation of the central retinal artery, or its branches or vasospasm.5,4 Visual loss in OIS is the most common symptom and it could be transient, sudden, or, most commonly, gradual.10,11 Ocular pain is the second most common symptom and it is a result of either an increase in intraocular pressure or ischaemia to the globe or ipsilateral dura.6 Our patient had ocular pain at presentation in the absence of neovascular glaucoma which might suggest ischaemia was the likely cause.
Anterior segment signs of ocular ischaemia include corneal oedema with Descemet’s folds, scleral melting, iris atrophy and neovascularisation, fixed semi-dilated or poorly reactive pupils, peripheral anterior synechia with angle closure, mild iritis, and cataracts. Among these signs, neovascularisation of the iris (NVI) is the most common (90%) and when seen at presentation it is an indicator of a poor prognosis.10 Our patient did not have iris atrophy or NVI; however, she had poorly reactive pupils, probably due to retinal ischaemia and/or ischaemic optic neuropathy or iris ischaemia.
Generally, in OIS, intraocular pressure may be raised if fibrovascular tissue develops in the angle causing angle closure, or it may be normal or even low if aqueous production is reduced due to ciliary body ischaemia. Also, normal tension glaucoma is described as occurring in conjunction with OIS due to a chronic reduction of the retrobulbar blood flow.5 Our patient developed limited peripheral anterior synechiae (PAS) probably secondary to undetected fine angle neovascularisation, but the angles remained open. During subsequent follow-up visits, our patient was found to have high intraocular pressure OU with optic nerve cupping due to uncontrolled open angle glaucoma probably not directly related to OIS.
Posterior segment signs in OIS include attenuated retinal arteries; dilated but not tortuous retinal veins; mid-peripheral and posterior pole dot and blot retinal haemorrhages; microaneurysms; macular oedema; cherry red spots; cotton wool spots; anterior ischaemic optic neuropathy; neovascularisation of the disc or less commonly of the retina, and vitreous haemorrhage.5 Our patient had attenuated retinal arteries and pale optic discs, possibly due to anterior ischaemic optic neuropathy. This latter sign is seen in up to 18% of eyes affected by OIS.5 Anterior ischaemic optic neuropathy can result from chronic IOP elevation in the presence of compromised ocular perfusion.10
Diagnosis of OIS is made based on high clinical suspicion and confirmed by ancillary tests including FFA, indocyanine green angiography (ICG), electroretinograghy, carotid system studies. FFA is useful in OIS not only for diagnosis but also for planning treatment. If it reveals significant retinal ischaemia with the presence of retinal neovascularisation, then panretinal photocoagulation (PRP) laser treatment is indicated.10 The most specific angiographic sign in OIS is delayed or patchy choroidal filling, which is found in 60% of cases. A highly sensitive sign is prolonged retinal arteriovenous time (95%).5 Other angiographic signs are prolonged retinal circulation time, staining of retinal vessels (85%), well-demarcated fluoresce in the dye edge, and retinal capillary non-perfusion.5
Computed tomography (CT) angiography and MRI angiography have gradually replaced the digital subtraction angiography (DSA) method for studying carotid occlusive disease with high sensitivity and specificity reaching 97% and 99%, respectively.5
ICG typically shows prolonged choroidal filling.5 Moreover, electroretinograghy in OIS will typically show a decrease in the amplitude of both A- and B-waves reflecting ischaemia of both the outer and inner retinas.5,6 The aim of OIS treatment is to control anterior segment inflammation, ablate retinal ischaemia with PRP, and control neovascular glaucoma.5,10 Anterior segment inflammation is treated with regular topical steroids and long-acting cycloplegic agents to stabilise the blood-aqueous barrier. Our patient had no anterior segment inflammation.
PRP is generally recommended in cases of established retina ischaemia with presence of iris or angle neovascularisation.10 PRP can result in regression of anterior segment neovascularisation in only 36% of eyes.12 There is no role for PRP if no retinal ischaemia is found with anterior segment neovascularisation as the latter may result from uveal ischaemia alone.13 Our patient did not develop iris or angle neovascularisation; therefore, PRP was not required. Intravitreal injection of antivascular endothelial growth factor and steroids have been used in some eyes with OIS with NVI or macular oedema which resulted in reasonable regression of neovascularisation and improvement in macular oedema but no change in visual acuity.5,14
In eyes with useful vision or that have potential for visual recovery and uncontrolled IOP, trabeculectomy with antimetabolites, or aqueous shunt implants may be indicated. In painful blind eyes, cycloablation or even enucleation or evisceration are considered.10
The management of OIS requires a multidisciplinary approach to assess and optimise treatment of underlying systemic conditions. The 5-year mortality rate in OIS is around 40%, mostly due to cardiac disease.10 Risk of stroke is also significantly increased (4% per year in patients with OIS as compared to 0.49% per year in the control group).5
Carotid artery surgery, either endarterectomy or bypass surgery, may relieve OIS and also reduce the risk of stroke.10 Surgery has to be considered in cases of carotid artery stenosis and occlusion with atherosclerosis.
In cases of carotid artery thrombosis, thrombolytic therapy may be considered along with treatment of the underlying cause. Gelston et al. and Simmons et al. reported successful treatment of a diabetic child with rhino-orbital mucormycosis with orbital compromise and evidence of ipsilateral carotid artery and cavernous sinus thrombosis. The child was treated with systemic posaconazole, amphotericin B, granulocyte colony-stimulating factor, interferon gamma, and low-molecular weight heparin. She showed clinical improvement with partial recovery of her vision.2,4
Conclusion
We presented a case of bilateral OIS which is one of the rare ocular complications of rhinocerebral mucormycosis. OIS has variable clinical presentations and can be masked by other ocular vascular conditions; therefore, it is probably underdiagnosed. Various ocular tests and carotid system studies are useful in diagnosis and planning treatment. The mainstay of treatment is IOP control, PRP if indicated, and medical treatment of underlying conditions.
Figure 2:
(A) Axial T2W magnetic resonance (MR) image through the cavernous sinus showing loss of normal signal void in the internal carotid arteries (arrow), in keeping with thrombus within the ICA. (B) A time-of-flight MR angiography reformat image shows absence of both internal carotid arteries. The middle cerebral arteries (white arrows), basilar artery are with in normal limits (yellow arrow).
References
- 1.Hosseini S, Borghei P. Rhinocerebral mucormycosis: Pathways of spread. Eur Arch Otorhinolaryngol. 2005;262:932–83. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 2.Simmons J, Zeitler P, Fenton L, Abzug M, Fiallo-Scharer R, Klingensmith G. Rhinocerebral mucormycosis complicated by internal carotid artery thrombosis in a pediatric patient with type 1 diabetes mellitus: A case report and review of the literature. Pediatr Diabetes. 2005;6:234–8. doi: 10.1111/j.1399-543X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 3.Sundaram C. Rhinocerebral zygomycosis—A clinicopathological study. Neurol India. 1998;46:126–9. [PubMed] [Google Scholar]
- 4.Gelston C, Durairaj V, Simoes E. Rhino-orbital mucormycosis causing cavernous and internal carotid thrombosis treated with posaconazole: Case report. Arch Ophthalmol. 2007;125:848–9. doi: 10.1001/archopht.125.6.848. [DOI] [PubMed] [Google Scholar]
- 5.Mendrinos E, Machinis T, Pournaras C. Ocular ischemic syndrome: Major review. Surv Ophthalmol. 2010;55:1. doi: 10.1016/j.survophthal.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 6.de Graeve C, van de Sompel W, Claes C. Ocular ischemic syndrome: Two case reports of bilateral involvement. Bull Soc Belge Ophthalmol. 1999;273:69–74. [PubMed] [Google Scholar]
- 7.Imrie FR, Hammer HM, Jay JL. Bilateral ocular ischemic syndrome in association with hyperhomocysteinaemia: Case report. Eye. 2002;16:497–500. doi: 10.1038/sj.eye.6700010. [DOI] [PubMed] [Google Scholar]
- 8.Koz O, Ates A, Alp M, Gultan E, Karaasaln Y, Kural G. Bilateral ocular ischemic syndrome as an initial manifestation of Takayasu’s arteritis associated with carotid steal syndrome: Case report. Rheumatol Internat Clin Experiment Investig. 2006;27:299–302. doi: 10.1007/s00296-006-0194-4. [DOI] [PubMed] [Google Scholar]
- 9.Hwang J, Girkin CA, Perry JD, Lai JC, Miller NR, Hellmann DB. Bilateral ocular ischemic syndrome secondary to giant cell arteritis progressing despite corticosteroid treatment. Am J Ophthalmol. 1999;127:102–4. doi: 10.1016/s0002-9394(98)00313-4. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra R. Management of ocular ischemic syndrome: Perspective. Br J Ophthalmol. 2000;84:1428–31. doi: 10.1136/bjo.84.12.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen R, Padilla J, Light D, Diller R. Carotid artery occlusive disease and ocular manifestations: Importance of identifying patients at risk. Optometry. 2010;81:359–63. doi: 10.1016/j.optm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome, III. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15:15–20. doi: 10.1007/BF00150974. [DOI] [PubMed] [Google Scholar]
- 13.Hayreh SS, Baines JA. Occlusion of the vortex veins. An experimental study. Br J Ophthalmol. 1973;57:217–38. doi: 10.1136/bjo.57.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luis A, Javier M, Manuel DL, Jose SP, Sophie JB, Paula P, et al. Intravitreal bevacizumab (avastin) injection in ocular ischemic syndrome. Am J Ophthalmol. 2007;144:122–4. doi: 10.1016/j.ajo.2007.02.037. [DOI] [PubMed] [Google Scholar]