Abstract
An anomalous origin of the right coronary artery from the pulmonary artery (ARCAPA) is a very rare coronary artery anomaly with only 98 cases reported in literature till date. We report the oldest surgically treated patient and the fourth ever septuagenarian with this anomaly diagnosed ante-mortem with an eleven year follow-up. The literature on this anomaly was reviewed and discussed to highlight the clinical implications.
Keywords: Coronary vessel anomalies, Pulmonary artery, Coronary angiography, Coronary circulation, Case report, New Zealand
Anomalous right coronary artery from the pulmonary artery (ARCAPA) is a very rare coronary artery anomaly, with only 98 cases reported in the literature to date.1,2 As most patients are asymptomatic until late adulthood, sudden cardiac death is often misreported as cause of death; hence, surgical management, even in asymptomatic patients, is highly recommended.1
Case Report
A 71-year-old hypertensive male who was a diabetic ex-smoker with a positive family history of coronary artery disease (CAD) was referred by a general practitioner for evaluation of recent onset of episodes of upper abdominal pain on exertion. His past history included treatment for duodenal ulcer, nephrolithiasis, bilateral inguinal hernia, and prostatism, and had undergone a right acromioplasty. In addition to other relevant investigations, he underwent a stress test which was strongly positive for inducible ischaemia.
A coronary angiogram (CAG) showed critical disease involving the left anterior descending artery (LAD) and ARCAPA. The right coronary artery (RCA) was dilated and filling in a retrograde manner by collaterals from the LAD and draining into the pulmonary artery [Figures 1]. The aortic root injection did not show the RCA. The treatment plan was to re-implant the RCA into the ascending aorta and graft the LAD with the left internal mammary artery (LIMA). The patient was reluctant to undergo surgery and instead underwent stenting of the LAD using a bare-metal stent. He was discharged 7 days after the procedure having been prescribed anti-platelet medications, including aspirin and ticlopidine as per the hospital protocol.
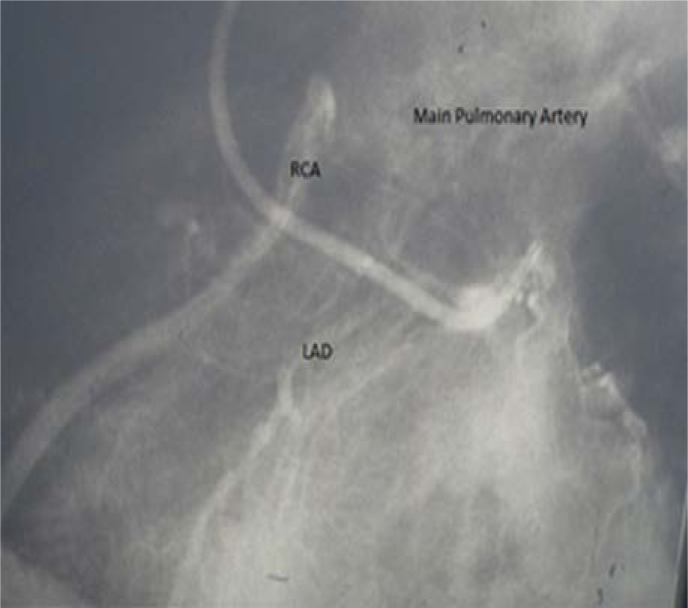
Figure 1:
Pre-operative angiogram showing a normally arising left anterior descending artery (LAD) with the right coronary artery filled in a retrograde fashion by collaterals from the LAD and draining into the pulmonary artery.
He presented the next day with sudden severe chest pain with echocardiogram (ECG) evidence of acute myocardial infarction (MI). A repeat CAG showed a thrombus proximal to the previous stent and a small area of dissection downstream from the previous stent. Two additional stents were inserted in the artery to open up the thrombosed as well as the dissected areas. However, he sustained a large anterior infarct and developed congestive heart failure. An ECG done two days later showed concentric left ventricular hypertrophy (LVH), an akinetic septum, and a hypokinetic inferior wall with an ejection fraction (EF) of 45%.
Since he was still symptomatic, he underwent a myocardial perfusion scan and CAG after stabilisation of the congestive heart failure (CHF). This was in order to complete an assessment of his condition and assess the reversibility of the ischaemia. A myocardial perfusion scan showed a satisfactory perfusion of the lateral wall. The apical infarcted area was not perfused. The inferior wall and posterior septum supplied by the RCA arising from main pulmonary artery (MPA) was under-perfused at all times, and more so with effort.
Cardiac catheterisation showed moderate pulmonary hypertension (pulmonary artery [PA] pressure = 58/24 mmHg, mean = 36 mmHg). Left ventricular end-diastolic pressure was 28 mmHg. A left ventriculogram showed akinesis of the lower third of the anterolateral wall and apex. The left main coronary artery was normal. The stented segment of the LAD was patent, but segments of irregularity were noted within it, approaching 40%, and there was some downstream irregularity beyond the stented segment of about 50%. The left circumflex coronary artery (LCX) was normal. Only the distal RCA was found to be filling by collaterals from left, and runoff into MPA was not seen, as noted in the previous CAG, probably because of pulmonary hypertension.
Being symptomatic, he was advised to undergo surgery, to which he agreed. He was operated for coronary artery bypass grafting (CABG) to the LAD, and a re-implantation of the RCA into the aorta. Significant findings during surgery included cardiomegaly and a dilated right ventricle. The RCA was a 3 mm vessel arising from right side of the MPA and running in the groove between the aorta and the pulmonary artery. The LAD was a 1.5 mm atherosclerotic vessel. On cardio-pulmonary bypass (CPB) on the beating heart, the MPA was separated from the aorta, the RCA was isolated and dissected free of the aorta, the MPA, and the right ventricle. It was taken off the MPA as a small button and was re-implanted anteriorly into the ascending aorta using a side-biting aortic clamp. The hole in the pulmonary artery was closed using a pericardial patch. The LIMA was then anastomosed to the LAD on the cross-clamped arrested heart using cardioplegia.
The post-operative period was uneventful. An ECG done 3 weeks after the surgery showed a moderately dilated left atrium (5.44 cm), a dilated left ventricle with moderately reduced contractility with the left ventricular, and an EF of 32%.
The patient has been on a regular follow-up schedule since then with his general practitioner, cardiologist, and surgeon. Eleven years later, during a follow-up, he was asymptomatic with no history of angina or any features suggestive of congestive cardiac failure. He had good exercise tolerance. An ECG showed improved left ventricular function with an ejection fraction of 43% and minimal regional wall motion abnormalities. A CT angiogram showed good flow through the re-implanted RCA [Figures 2 and 3] and a functioning LIMA to LAD graft [Figure 4].
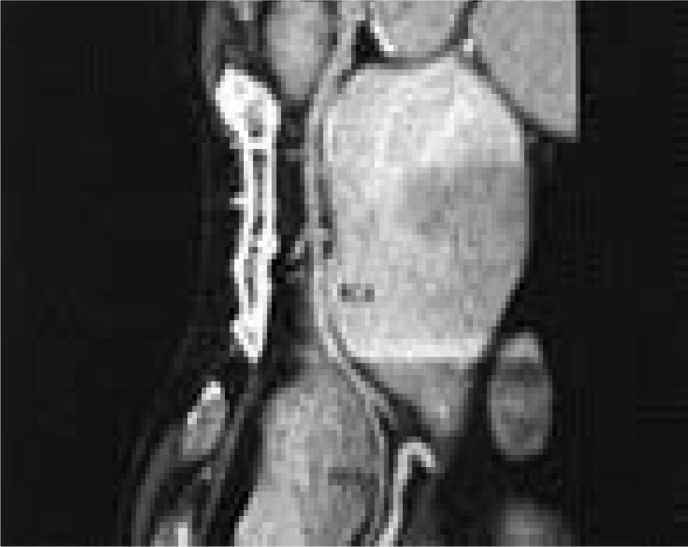
Figure 2:
A reconstructed image of a postoperative follow-up computed tomography angiogram showing the proximal anastomosis of the right coronary artery to the ascending aorta with no evidence of kinking or angulation and the full course of the right coronary artery.
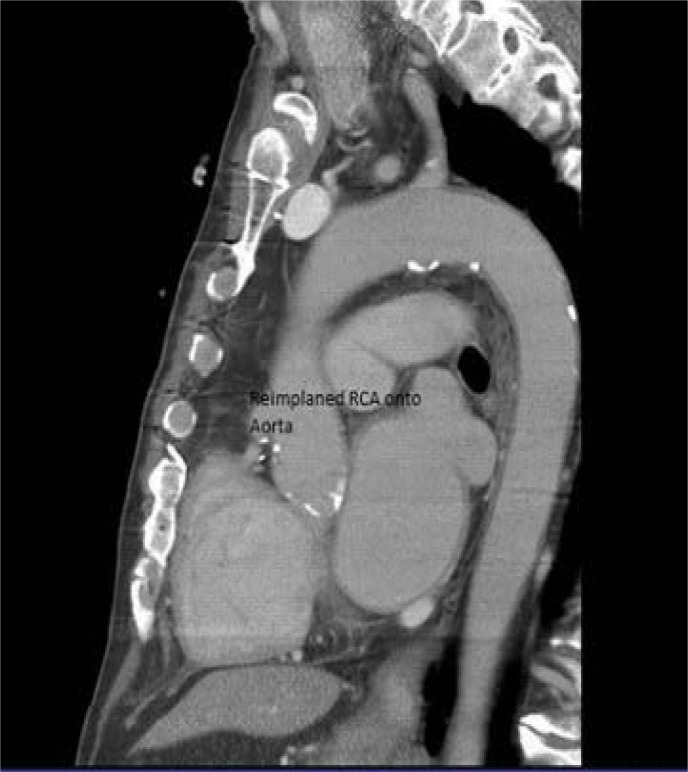
Figure 3:
Proximal anastomosis of the re-implanted right coronary artery to the ascending aorta.
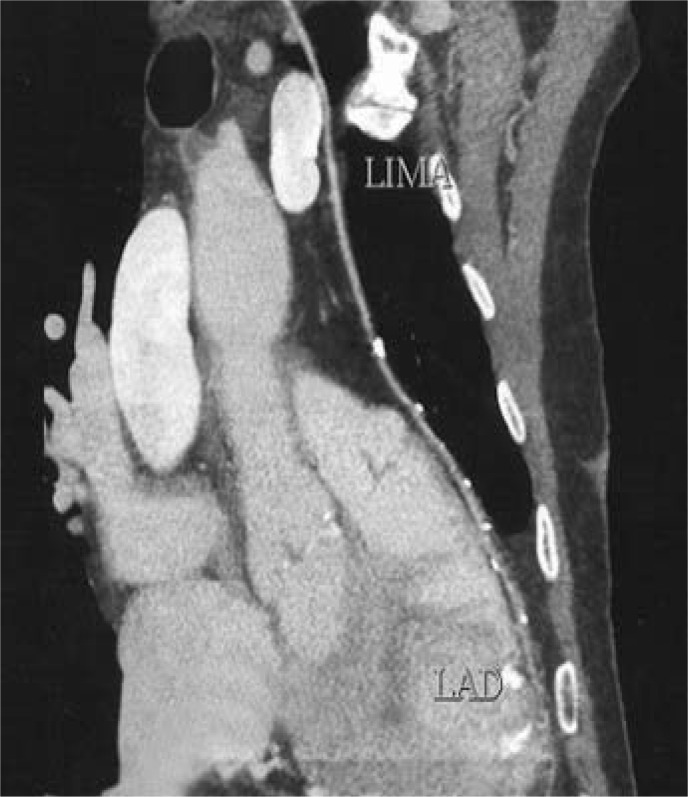
Figure 4:
Reconstructed image of a post-operative computed tomography angiogram showing good flow in the left anterior descending artery through the left internal mammary artery.
Discussion
ARCAPA is a very rare anomaly with only 98 cases having been reported in the literature to date, the first one described in 1885 by Brooks.2 It has been estimated to occur in 0.002% of the population.3 However, since most patients remain asymptomatic or are diagnosed post-mortem, the actual incidence may be higher.
Abnormalities in the embryologic development lead to positional anomalies of the coronary arteries. The coronary artery buds appear at about the 12th day of life after the division of the truncus arteriosus that leads to the separation of the aorta and the pulmonary artery.4 Coronary artery anomalies can arise secondary to either malrotation of the spiral septum dividing the truncus or malpositioning of the coronary buds themselves.
The four possible anomalous coronary artery connections were described by Soloff in 1942 and included the following: 1) Anomalous left coronary artery from pulmonary artery (ALCAPA), (this variant is the most common and presents early in life with myocardial ischaemic features); 2) ARCAPA is the next most common variant and generally presents later in life; 3) Origin of both coronaries from the pulmonary artery, (this is a rare variant and is not compatible with life), and 4) Origin of the accessory coronary artery from pulmonary artery, (this is an extremely rare variant).5
In our experience, direct implantation using a button led to a successful repair. To avoid kinking of the right coronary artery, the following precautions need to be taken: 1) A long length of the RCA is mobilised from its origin in the MPA and anastomosed to the anterior ascending aorta such that it lies in a loop in front of the aorta; 2) Insertion of the mobilised RCA into the ascending aorta is done as high as possible in the ascending aorta, and 3) Proximal ligation and re-implantation without a button is potentially technically more challenging with a higher risk of stenosis and kinking at the implantation site.
The pathophysiologic effects of ALCAPA and ARCAPA are related to the direction of blood flow in the coronary artery and to the impact on oxygen delivery to the myocardium. The direction of blood flow in the anomalous coronary artery appears to vary with age.6 In the fetus and neonate, blood flows from the pulmonary artery into the anomalous coronary artery because of the high pulmonary vascular resistance. Although the oxygen content of the blood entering the anomalous coronary artery via the pulmonary artery is lower than that entering the coronary artery from the aorta, it is sufficient to meet the demands of the myocardium, and the neonate remains asymptomatic. Over the first few days of life, the pulmonary vascular resistance falls and three possible scenarios ensue: 1) Insufficient collateralisation between the right and left coronary arteries results in ischaemia and death; 2) Adequate collateralisation produces a steal phenomenon due to relative differences in the diastolic pressures between the pulmonary and systemic arterial beds, or 3) Massive collateralisation may maintain adequate myocardial perfusion even in the presence of the steal phenomenon.
In the adult, the retrograde flow is suggested by several observations: 1)The anomalous vessel is usually thin walled;3,4,7 2) Angiograms have shown blood flow from the left system into the right coronary artery and then into the pulmonary artery,8 and 3) Ligation of the anomalous vessel at its origin in one case caused distention of the distal vessel without electrocardiographic evidence of ischaemia.6
In 70% of reported cases, it is an isolated anomaly.2 However, there are isolated reports of ARCAPA being associated with other cardiac defects like ventricular septal defect, tetralogy of Fallot, aortopulmonary window, atrial septal defect, and a double outlet right ventricle. Most patients are asymptomatic. However, the patients may present with angina pectoris, congestive heart failure, dyspnoea, cyanosis, palpitations, acute myocardial infarction, myocarditis, and bradycardia.9–12
The modality of diagnosis of ARCAPA has changed over time.13 Before 1965, most of the diagnoses were made during autopsy or surgery. Since 1965, angiography has been the most commonly used modality for diagnosis of ARCAPA. In 1985, the first case of ARCAPA was diagnosed by echocardiography.7 Since then, it has been the main diagnostic tool. CAG has been used as a confirmatory method. In two cases, diagnosis was made by multi-slice CT angiography.14,15 Cardiovascular magnetic resonance was used for the first time in 2007 for the diagnosis of ARCAPA.16
Multislice CT and cardiovascular MRI (CMR) are non-invasive three-dimensional (3D) imaging modalities with high diagnostic accuracy for coronary artery anatomic anomalies. If echocardiography and conventional angiography have been non-conclusive, multislice computed tomography (CT) and CMR can be especially important diagnostic tools.16
Coronary artery imaging with echocardiography may be difficult in some patients due to poor acoustic windows. Cardiac CT is an effective noninvasive test but uses ionising radiation and requires intravenous administration of iodinated contrast agent. Conventional X-ray cineangiography is an invasive test and may also be difficult because of the lack of 3D information that relates the coronary artery to its surrounding structures. However, despite the fact that CT uses contrast and radiation, it has emerged as the cross-sectional imaging of choice for optimal evaluation of the anomalies of the origin of the coronary arteries. As compared to invasive angiography or coronary CT angiography, the spatial resolution of magnetic resonance (MR) angiography is significantly lower.17
The electrocardiographical features are non-specific for ARCAPA. However, the reported features include left or right or biventricular enlargement or hypertrophy, ischaemic changes, bundle branch blocks and atrial fibrillation, or bradycardia. The anomalous RCA has been described in surgical and pathological reports to be thin-walled, dilated, and/or vein-like in character. The origin of the anomalous coronary artery may be from the anterior sinus of valsalva of the pulmonary artery, the right posterior sinus, or the anterior aspect of the pulmonary trunk.1,18–20
Corrective surgery has been recommended for ARCAPA, even in asymptomatic patients and mainly for two reasons. First, with the reimplantation of the aberrant vessel into the aorta, a double ostium coronary system is established with a potentially lower risk for sudden cardiac death. Second, the operation provides relief from coronary steal phenomenon, which may be responsible for myocardial ischaemia.
Three different surgical procedures have been advocated as follows: 1) Ligation of RCA at the origin from the pulmonary trunk to eliminate coronary steal, (it is well-tolerated but the long-term results are unpredictable); 2) Ligation of the RCA at its origin and the CABG to restore blood flow; however, the long term patency of the vein graft is questionable, or 3) Direct re-implantation of the RCA from the pulmonary artery into the anterior wall of ascending aorta to re-establish a double coronary artery system. The latter is the most physiological procedure and has been shown to have good long-term results.21
In a few cases, a post-operative impairment of flow has been observed in the restored right coronary artery and it has been hypothesised to be due to long-term hypokinetic circulation associated with ARCAPA, probably due to persistent small vessel disease.22
Conclusion
The patient reported here was, at the time of surgery, a 71-year-old gentleman who presented with ischaemic symptoms and was diagnosed by coronary angiogram as having ARCAPA. In addition, there was a significant stenotic lesion of the LAD. Although surgery was advised, he opted instead for stenting of the LAD. Unfortunately, he re-presented with recurrent angina and had further stenting of the LAD but sustained acute MI. He remained symptomatic and further investigation revealed ischaemic RCA territory with moderate disease in the LAD. He then agreed to surgery and underwent re-implantation of the RCA and a LIMA graft to the LAD. Eleven years after surgery, during a follow-up, the patient remained asymptomatic with improved EF on echocardiogram. A CT angiogram showed patent LIMA to the LAD and a well-functioning re-implanted RCA. The patient had returned to his normal routine of swimming, playing golf, and enjoying other regular exercise.
References
- 1.Radke PW, Messmer BJ, Haager PK, Klues HG. Anomalous origin of right coronary artery: pre-operative and post-operative hemodynamics. Ann Thorac Surg. 1998;66:1444–9. doi: 10.1016/s0003-4975(98)00716-4. [DOI] [PubMed] [Google Scholar]
- 2.Brooks HSJ. Two cases of an abnormal coronary artery of the heart arising from the pulmonary artery with some remarks upon the effect of this anomaly in producing cirsoid dilatation of the vessels. J Anat Physiol. 1885;20:26–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan RA, Dry TJ, Edwards JE. Anomalous origin of the right coronary artery from pulmonary trunk. Mayo Clin Proc. 1950;25:673–8. [PubMed] [Google Scholar]
- 4.Danias PG, Stuber M, McConnell MV, Manning WJ. The diagnosis of congenital coronary anomalies with magnetic resonance imaging. Coron Artery Dis. 2001;12:621–6. doi: 10.1097/00019501-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Soloff LA. Anomalous coronary arteries arising from the pulmonary artery. Am Heart J. 1942;24:118–27. [Google Scholar]
- 6.Eugster GS, Oliva PB. Anomalous origin of the right coronary artery from the pulmonary artery. Chest. 1973;63:294–6. doi: 10.1378/chest.63.2.294. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Yokochi K, Yoshioka F, Kato H. Anomalous origin of the right coronary artery from the pulmonary artery: Report of a case. J Cardiogr. 1985;15:241–8. [PubMed] [Google Scholar]
- 8.Ogden JA. Congenital anomalies of the coronary arteries. Am J Cardiol. 1970;25:474–9. doi: 10.1016/0002-9149(70)90016-0. (Additional information also received through personal communication from Ogden JA). [DOI] [PubMed] [Google Scholar]
- 9.Di Luozzo, Berni A, Nigri A. Origine anomala della coronaria destra dalla arteria polmonare: Descrizione di un caso clinico. G Ital Cardiol. 1998;28:57–60. [PubMed] [Google Scholar]
- 10.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 11.Ross TG, Lantham RD, Craig WE. Anomalous origin of the right coronary artery from the main pulmonary artery. South Med J. 1987;80:783–6. doi: 10.1097/00007611-198706000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Nakano M, Emoto H, Koyanagi K, Okuyama H, Saitoh F, Kurosawa H. Report of a case of the anomalous origin of the right coronary artery from the pulmonary artery with atrial fibrillation and bradycardia. J Jpn Assoc Thor Surg. 1993;41:479–85. [PubMed] [Google Scholar]
- 13.Modi H, Ariyachaipanich A, Dia M. Anomalous origin of right coronary artery from pulmonary artery and severe mitral regurgitation. J Invasive Cardiol. 2010;22:49–55. [PubMed] [Google Scholar]
- 14.Waite S, Ng T, Afari A, Gohari A, Lowery R. CT diagnosis of isoloated anomalous origin of the RCA arising from the main pulmonary artery. J Thorac Imag. 2008;23:145–7. doi: 10.1097/RTI.0b013e3181653c5a. [DOI] [PubMed] [Google Scholar]
- 15.Tedeschi C, Briguori C, De Rosa R, Ratti G, Cademartiri F, Sacco M, et al. Right coronary artery arising from pulmonary trunk: Assessment with conventional coronary angiography and multislice computed tomography coronary angiography. J Cardiovasc Med (Hagerstown) 2009;10:178–82. doi: 10.2459/JCM.0b013e32831de545. [DOI] [PubMed] [Google Scholar]
- 16.Gilmour J, Hafka H, Ropchan G, Johri A M. Case report, Anomalous Right Coronary Artery : A multimodality hunt for the origin. Cardiol. 2011 doi: 10.1155/2011/286598. Article ID 286598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleumke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, Hundley WG, et al. Noninvasive Coronary Artery Imaging: Magnetic resonance angiography and multidetector computed tomography angiography: A scientific statement from The American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118:586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 18.Huang TY, Hsueh Y, Tsung SH. Endocardial fibroelastosis and myocardial calcification secondary to an anomalous right coronary artery arising from the pulmonary trunk. Hum Pathol. 1985;16:959–60. doi: 10.1016/s0046-8177(85)80138-6. [DOI] [PubMed] [Google Scholar]
- 19.Achtel RA, Zaret BL, Iben AB, Hurley EJ. Surgical correction of left coronary artery-main pulmonary artery fistula in association with anomalous right coronary artery. J Thorac Cardiovasc Surg. 1975;70:46–51. [PubMed] [Google Scholar]
- 20.Mintz GS, Iskandrian AS, Bemis CE, Mundth ED, Owens JS. Myocardial ischemia in anomalous origin of the right coronary artery from the pulmonary trunk. Proof of a coronary steal. Am J Cardiol. 1983;51:610–12. doi: 10.1016/s0002-9149(83)80108-8. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson RM, Raphael M, Radley-Smith R, Yacoub M. Angiographic diagnosis of anomalous origin of the right coronary artery from the pulmonary artery. Br J Radiol. 1983;56:17–19. doi: 10.1259/0007-1285-56-661-17. [DOI] [PubMed] [Google Scholar]
- 22.Schley J. Abnormer Ursprung der rechten Kranzarterie and der Pulmonalis bei einem 61 jahrigen Mann. Frankfurt Z Path. 1925;32:1–7. [Google Scholar]