Abstract
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease. Involvement of the nervous system, either primary or secondary, is common in SLE, and 19 different neuropsychiatric clinical syndromes have been recognised in association with the disease. Several pathophysiological mechanisms have been implicated in the pathogenesis of neuropsychiatric SLE (NPSLE), including vasculopathy, autoantibodies, and cytokine-mediated tissue injury. Careful evaluation of the patient is required to rule out secondary causes before attributing the neurological symptoms to SLE. Treatment depends on the nature and severity of NPSLE syndrome.
Keywords: Systemic lupus erythematosus (SLE), Nervous system, Neuropsychiatric, Syndrome
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disorder which commonly affects the nervous system. It affects around 130:100,000 people in the United States.1 Females are affected 7 times more than males.2 In the United States, African American, Hispanic, and Asian populations are most commonly affected.1 The overall survival of SLE patients has significantly improved over the last 50 years, from 74.8 to 94.8% and from 63.2 to 91.4% for 5-year and 10-year survival, respectively.3 Recent data has suggested that both renal and neuropsychiatric involvement negatively affects the overall 5-year survival rate, whereas the neuropsychiatric involvement did not change for the 10-year survival rate.3
The involvement of the nervous system is due primarily to the disease process, secondarily, to infection, or metabolic or drug-related effects. The aim of this review is to discuss the clinical picture, pathogenesis, evaluation, and treatment of neuropsychiatric SLE.
Clinical Picture and Epidemiology
The American College of Rheumatology (ACR) diagnostic criteria for SLE includes only psychosis and seizures as nervous system manifestations. In spite of this, different neuropsychiatric manifestations are recognised in patients with SLE. Because of the variable presentation, in 1999 the ACR established 19 different neuropsychiatric SLE syndromes (NPSLE). The classification of NPSLE takes into consideration the part of the nervous system that is affected—the central or the peripheral nervous system [Table 1]. The prevalence of NPSLE varies from 40.3% to 91.0%.4–6 The difference in the reported prevalence depends on the case definition used and the use of formal neuropsychological testing for patient evaluation.
Table 1:
Neuropsychiatric syndromes associated with systemic lupus erythematosus
| NPSLE associated with the central nervous system | NPSLE associated with the peripheral nervous system |
|---|---|
| Aseptic meningitis | Acute inflammatory demyelinating |
| Cerebrovascular disease | Syndromes (Guillain-Barré syndrome) |
| Demyelinating syndromes | Autonomic neuropathy |
| Headaches | Mononeuropathy, single or multiplex |
| Movement disorders (chorea) | Myasthenia gravis |
| Myelopathy | Cranial neuropathy |
| Seizure disorders | Plexopathy |
| Anxiety disorders | Polyneuropathy |
| Cognitive dysfunction | |
| Mood disorders | |
| Psychosis |
NPSLE = neuropsychiatric systemic lupus erythematosus.
Recent data show that NPSLE affects the central nervous system in around 93.1% of patients and the peripheral nervous system in 6.9% of patients.4 Diffuse NPSLE accounted for 79% of the events and only 21% were focal in nature.4 Most patients will have one neuropsychiatric event, while only 17.4% of the patients have two or more events.4 Around 24% of nervous system involvement occurs as an initial presentation.7 In general, around 50–60% of NPSLE occurrs at disease onset or within the first 5 years after SLE onset.8
The reported prevalence of specific NPSLE is as follows: headache (47.1–57%), seizure (7.5–16%), cerebrovascular disease (4.7–17%), and polyneuropathy (2.4–22%).4–7 The prevalence of cognitive impairment ranges from 5.1–81%, depending on the study.4–6 The same observation has been noted for mood disorders, with the prevalence ranging from 16.5–51%.4,6 The major difference that contributes to this finding is the screening modality in each study. In studies where patients were screened with formal neuropsychiatric and sensitive psychiatric testing, the prevalence of mood disorder and cognitive impairment was high. Mild cognitive impairment was the most frequent abnormality among these patients, with only 3–5% exhibiting severe cognitive impairment.8 Other NPSLE syndromes are rarer. For example, the reported prevalence for movement disorders and transverse myelitis is around 1% in each case.
In comparison to Western data, NPSLE was uncommon in the data from the Arab Gulf region. The reported prevalence was around 15.6–19%.9–10 In those studies, seizures were reported in 10% of cases, whereas the incidence of stroke ranged from 4–11%.9–10 The other reported NPSLE syndromes were movement disorders, myelitis, neuropathy, and psychosis. The low prevalence of NPSLE in these data is related to the case definition used. In these two studies, there were no reports of cognitive impairment or mood disorders, both of which are considered common NPSLE syndromes in the data where higher prevalence is reported. There has been no specific study done in the region looking specifically for the prevalence of NPSLE syndromes.
Different risk factors are associated with the development of NPSLE. Patients with generalised disease activity, prior history, or concurrent NPSLE, as well as those with antiphospholipid antibodies are at higher risk of developing NPSLE. Skin lesions were the most frequently reported disease activity in association with nervous system involvement.
Pathogenesis
Several pathophysiological mechanisms have been implicated in the pathogenesis of NPSLE. These include vasculopathy, autoantibodies, blood brain barrier and the cytokine effect [Table 2].
Table 2:
Pathogenic mechanisms in neuropsychiatric systemic lupus erythematosus
| Vasculopathy | Small vessel non-inflammatory vasculopathy Vascuilitis (rare) |
| Autoantibodies | Procoagulant effect Direct cytotoxic effect |
| Cytokines | Promotion of antibodies production Recruitment of immune cells Alteration of blood brain barrier |
In post-mortem studies, vascular occlusion is universally found. The most common findings on pathological examinations were multiple infarct, cortical atrophy, microhaemorrhages, and gross infarction. The most commonly reported vasculopathy is small vessel non-inflammatory vasculopathy.11 In several autopsy case reports of patients who died during an acute lupus flare, small vessel occlusions were secondary to leukocyte aggregates in the absence of immune complex deposition. This was most likely secondary to complement activation that resulted in increased leukocyte adhesiveness to the subendothelial surface of the blood vessels.11–13
Multiple autoantibodies have been reported in association with NPSLE. The most frequently reported antibodies are antiphospholipid antibodies, a subset of anti-double-stranded DNA (dsDNA) and anti-ribosomal antibodies. The autoantibodies either obtain access to the nervous system through the permeable blood brain barrier or through direct intrathecal production.14
Antiphospholipid antibodies are a group of antibodies that target the phospholipid binding protein, therefore altering the expression and secretion of procoagulants and promoting thrombosis. They have been associated with different NPSLE syndromes, including stroke, epilepsy. transverse myelitis, and cognitive impairment.15–18 The pattern of cognitive impairment was consistent with subcortical deficit.19
N-methyl-D-aspartate (NMDA) receptors are glutamate receptors, which are present throughout brain tissue. A subset of anti-dsDNA antibodies were found to react with the NR2 subunit of NMDA receptors, which are present in the amygdala and hippocampus. In animal models, anti-NR2 receptors antibodies induced apoptotic cell death of the hippocampus and amygdala in the presence of permeable blood brain barrier.20–22 In human studies, in which both serum and cerebrospinal (CSF) levels of anti-NR2 receptors correlated with NPSLE, no relation between NPSLE and the serum level was found. In contrast, a significant relation was found between diffuse NPSLE and CSF anti-NRS, but none with focal NPSLE or peripheral NPSLE.23
Anti-ribosomal P antibodies cross-react with a protein on neuronal membrane, initiating apoptotic cell death.24 The role of anti-ribosomal P antibodies in the pathogenesis of NPSLE is controversial. A meta-analysis of the utility of anti-ribosomal P antibodies in the diagnosis of NPSLE or for specific NPSLE did not find a significant relation between them.25
Proinflammatory cytokines and chemokines also play a role in the pathogenesis of NPSLE. Elevated levels of several cytokines, including interleukin-6 (IL-6), IL-1, IL-8, IL-10, tumour necrosis factor-alpha (TNF-α)-, interferon (IFN)-ϒ, monocyte chemotactic protein 1 (MCP-1)/CCL2, interferon gamma-inducible protein 10 (IP-10)/CXCL10, and fractalkine (CX3CL1) have been found in the CSF of patients with NPSLE.26 They have different effects, including the promotion of intrathecal antibody production, immune cell recruitment, affecting the blood-brain barrier permeability by modifying the neuroendocrine response, and neurotransmitter release.27
Evaluation
When dealing with SLE patients with neurological presentation, careful assessment is necessary to exclude other potential causes [Table 3]. A detailed medical history, past history and medication review are mandatory. Laboratory evaluation is required to rule out secondary causes, such as infection and metabolic abnormalities. There is no gold standard diagnostic test for NPSLE. In general, the diagnostic approach will be the same as for non-SLE patients. Different diagnostic tests can be helpful when assessing patients with possible NPSLE. The use of different tests should be individualised according to the patient’s presentation. An examination of CSF is indicated when infection is suspected. To date, there is no role for CSF autoantibody testing. It remains mainly of research interest.
Table 3:
Secondary causes of nervous system involvement in systemic lupus erythematosus patients
| Infection |
| Hypertension |
| Metabolic abnormalities e.g. renal failure, electrolyte abnormalities, hypoxia |
| Drugs |
| Thrombotic thrombocytopenic purpura |
| Sleep apnea |
| Thyroid disorders |
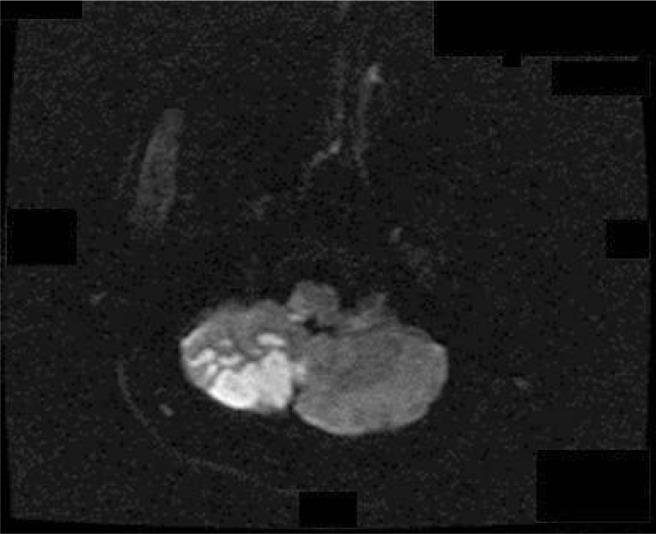
In the presence of focal deficit, neuroimaging is indicated. Magnetic resonance imaging (MRI) can assist with the diagnosis of cerebrovascular disease [Figure 1], transverse myelitis, and demyelinating syndromes. It is also required in patients presenting with seizure, acute confusional states, or movement disorders. An MRI scan will help to exclude other causes of neurological impairment, for example, progressive multifocal leukoencephalopathy.
Figure 1:
Diffusion-weighted image in a 50-year-old male patient with systemic lupus erythematosus and antiphospholipid syndrome showing right cerebellar infarction.
In general, most reported abnormalities on MRI are white matter lesions and cerebral atrophy.28–30 Advanced imaging modalities might be beneficial in patients with normal MRI results. Magnetic resonance spectroscopy (MRS) has shown neurometabolic abnormalities during both active and quiescent NPSLE.29
Different neurophysiological studies can be beneficial in the diagnosis of subsets of NPSLE syndromes. In patients presenting with seizure, an electroencephalogram (EEG) will help to identify those at high risk of seizure recurrence. In patients presenting with acute confusional state, an EEG study is required to rule out the presence of seizure disorder.
Nerve conduction studies (NCS) and electromyography (EMG) are indicated in patients presenting with symptoms of peripheral nervous system involvement. These examinations can help diagnose different types of neuropathy (mononeuropathy versus polyneuropathy/demyelinating versus axonal), plexopathy, and neuromuscular disorders.
Management
The management of patients with NPSLE consists of correcting the aggravating factors, providing symptomatic treatment, and applying specific measures related to the disease process. One should try to identify and correct possible aggravating factors, which include metabolic or blood pressure abnormalities, and possible offending drugs.
Symptomatic treatment will be required depending on the nature of NPSLE, and it may be initiated before disease-specific therapy. Symptomatic treatment for patients with NPSLE is similar to symptomatic treatment of the same condition in non-SLE patients. This includes antipsychotic treatment for psychosis, antiepileptic treatment for patients with seizure conditions, and antidepressants for patients with depression.
The severity and nature of the underlying neurological manifestation will direct the treatment decision. Some patients with mild forms will only require symptomatic treatment such as in the case of patients with headaches. In 2010, the European League against Rheumatism (EULAR) developed recommendations for the management of NPSLE. The treatment of NPSLE depends on whether the process is more likely secondary to an inflammatory or thrombotic process.8
In inflammatory processes like transverse myelitis, peripheral neuropathy, refractory seizures, or psychosis, treatment with immunosuppressive therapy is indicated. Treatment should include glucocorticoids with or without more potent immunosuppressive treatment for maintenance. The most commonly used immunosuppressive therapy includes azathioprine and cyclophosphamide. In cases of poor response or failure of conventional treatment, other modalities can be used like plasma exchange, intravenous immunoglobulin, or rituximab.
In severe cases like transverse myelitis, early treatment with glucocorticoids in combination with cyclophosphamide can provide a better outcome than glucocorticoids alone.31 The combination of three modalities comprising glucocorticoids, cyclophosphamide, and plasma exchange has been used in severe cases of transverse myelitis.32–35 In the presence of antiphospholipid antibodies, the use of antiplatelets/anticoagulants can be considered in addition to immunosuppressive therapy.32,34
Rituximab is an anti-CD20 antibody that targets the B-cell directly. It induces antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity and apoptosis. Data on the use of rituximab in NPSLE are mostly derived from case reports and open-labelled studies. Tokunaga et al. reported 10 patients with severe NPSLE who failed to achieve improvements with conventional treatment modalities but had favourable outcomes with rituximab treatment.36 In their series, all of the treated patients showed improvement in their neurological statuses. A recent randomised controlled trial evaluated the efficacy of rituximab versus placebo in moderate to severe active SLE. The study included 32 patients with neurological SLE. There was no difference between the treated and placebo groups.37 Further clinical trials are required before making a recommendation regarding the use of rituximab in NPSLE. In spite of this, rituximab should still be considered in patients with severe cases who have failed to improve using other treatments.
The treatment of patients with acute thrombotic events is similar to that in non-SLE patients. Thrombolytic therapy should be considered in the absence of contraindications. Secondary causes of stroke like carotid stenosis and endocarditis should be ruled out before attributing them to SLE. Further treatment should depend on the presence of antiphospholipid antibodies. In patients with positive antiphospholipid antibodies, anticoagulation therapy is indicated.8 In the absence of antiphospholipid antibodies, patients should receive antiplatelet therapy and cardiovascular risk factor modifications.
Outcome
In spite of recent advances in understanding and managing NPSLE, it remains a major cause of morbidity and impaired quality of life (QOL). In a Swedish cohort consisting of patients with NPSLE, it was found that the patients had higher levels of work incapacity compared to the normal population, although there was no increase in mortality.38 In a large cohort of SLE patients, the development of neuropsychiatric events was associated with significant reduction of patients’ self-reported health-related QOL.4
Conclusion
Nervous system involvement with SLE remains poorly understood. There is currently no gold standard test that can diagnose NPSLE, and the diagnosis of the disease remains an exercise of exclusion. Different diagnostic tests can be helpful, but are not specific to the disease itself. The mainstay of treatment includes the correction of possible aggravating factors, symptomatic treatment, immunosuppressive therapy, and anticoagulation/antiplatelet treatment in a subset of patients with thrombotic events. The treatment should be individualised according to the patients’ conditions.
Acknowledgments
This work was sponsored by Al Zaidi’s Chair for Research in Rheumatic Diseases at Umm Al-Qura University.
References
- 1.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: A comparison of worldwide disease burden. Lupus. 2006;15:308–18. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: Estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:209. doi: 10.1002/art.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: Meta-analysis and meta-regression of observational studies from 1950s to 2000s. Semin Arthritis Rheum. 2012;41:830–9. doi: 10.1016/j.semarthrit.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Wallace DJ, et al. Retrospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:529–35. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 6.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: Prevalence using standardized definitions. Neurology. 2002;58:1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 7.Joseph FG, Lammie GA, Scolding NJ. CNS lupus: A study of 41 patients. Neurology. 2007;69:644–54. doi: 10.1212/01.wnl.0000267320.48939.d0. [DOI] [PubMed] [Google Scholar]
- 8.Bertsias GK, Ioannidis JP, Aringer M, Bolen E, Bombardieri S, Bruce IN, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: Report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69:2074–82. doi: 10.1136/ard.2010.130476. [DOI] [PubMed] [Google Scholar]
- 9.Heller T, Ahmed M, Siddiqqi A, Wallrauch C, Bahlas S. Systemic lupus erythematosus in Saudi Arabia: Morbidity and mortality in multiethnic population. Lupus. 2007;16:908–914. doi: 10.1177/0961203307081112. [DOI] [PubMed] [Google Scholar]
- 10.AlSaleh J, Jassim V, ElSayed M, Saleh N, Harb D. Clinical and immunological manifestations in 151 SLE patients living in Dubai. Lupus. 2008;17:62–6. doi: 10.1177/0961203307084297. [DOI] [PubMed] [Google Scholar]
- 11.Ellison D, Gatter K, Heryet A, Esiri M. Intramural platelet deposition in cerebral vasculopathy of systemic lupus erythematosus. J Clin Pathol. 1993;46:37–40. doi: 10.1136/jcp.46.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammad A, Tsukada Y, Torre N. Cerebral occlusive vasculopathy in systemic lupus erythematosus and speculation on the part played by complement. Ann Rheum Dis. 1992;51:550–2. doi: 10.1136/ard.51.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis SG, Verity MA. Central nervous system involvement in systemic lupus erythematosus: A review of neuropathologic findings in 57 cases, 1956–1977. Semin Arthritis Rheum. 1979;8:212–21. doi: 10.1016/s0049-0172(79)80009-8. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins P, Belmont HM, Buyon J, Philips M, Weissmann G, Abramson SB. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis Rheum. 1988;31:632–41. doi: 10.1002/art.1780310508. [DOI] [PubMed] [Google Scholar]
- 15.Afeltra A, Garzia P, Mitterhofer AP, Vadacca M, Galluzzo S, Del Porto F, et al. Neuropsychiatric lupus syndromes: Relationship with antiphospholipid antibodies. Neurology. 2003;61:108–10. doi: 10.1212/01.wnl.0000058904.94330.a7. [DOI] [PubMed] [Google Scholar]
- 16.Syuto T, Shimizu A, Takeuchi Y, Tanaka S, Hasegawa M, Nagai Y, et al. Association of antiphosphatidylserine/prothrombin antibodies with neuropsychiatric systemic lupus erythematosus. Clin Rheumatol. 2009;28:841–5. doi: 10.1007/s10067-009-1123-1. [DOI] [PubMed] [Google Scholar]
- 17.Herranz MT, Rivier G, Khamashta MA, Blaser KU, Hughes GR. Association between antiphospholipid antibodies and epilepsy in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:568–71. doi: 10.1002/art.1780370418. [DOI] [PubMed] [Google Scholar]
- 18.Liou HH, Wang CR, Chen CJ, Chen RC, Chuang CY, Chiang IP, et al. Elevated levels of anticardiolipin antibodies and epilepsy in lupus patients. Lupus. 1996;5:307–12. doi: 10.1177/096120339600500412. [DOI] [PubMed] [Google Scholar]
- 19.Denburg SD, Carbotte RM, Ginsberg JS, Denburg JA. The relationship of antiphospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. J Int Neuropsychol Soc. 1997;3:377–86. [PubMed] [Google Scholar]
- 20.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: Antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103:678–83. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity: Antibody impairs memory. Immunity. 2004;21:179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Aranow C, Diamond B, Mackay M. Glutamate receptor biology and its clinical significance in neuropsychiatric systemic lupus erythematosus. Rheum Dis Clin North Am. 2010;36:187–201. doi: 10.1016/j.rdc.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;58:1130–5. doi: 10.1002/art.23399. [DOI] [PubMed] [Google Scholar]
- 24.Matus S, Burgos PV, Bravo-Zehnder M, Kraft R, Porras OH, Farías P, et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J Exp Med. 2007;204:3221–34. doi: 10.1084/jem.20071285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karassa FB, Afeltra A, Ambrozic A, Chang DM, De Keyser F, Doria A, et al. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum. 2006;54:312–24. doi: 10.1002/art.21539. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto H, Kobayashi A, Yamanaka H. Cytokines and chemokines in neuropsychiatric syndromes of systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:268436. doi: 10.1155/2010/268436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhiannon J. Systemic lupus erthematosus involving the nervous system: Presentation, pathogenesis, and management. Clinic Rev Allergy Immunol. 2008;34:356–60. doi: 10.1007/s12016-007-8052-z. [DOI] [PubMed] [Google Scholar]
- 28.Bosma GP, Huizinga TW, Mooijaart SP, Van Buchem MA. Abnormal brain diffusivity in patients with neuropsychiatric systemic lupus erythematosus. AJNR Am J Neuroradiol. 2003;24:850–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Brey RL. Neuropsychiatric lupus: Clinical and imaging aspects. Bull NYU Hosp J Dis. 2007;65:194–9. [PubMed] [Google Scholar]
- 30.Appenzeller S, Pike GB, Clarke AE. Magnetic resonance imaging in the evaluation of central nervous system manifestations in systemic lupus erythematosus. Clin Rev Allergy Immunol. 2008;34:361–6. doi: 10.1007/s12016-007-8060-z. [DOI] [PubMed] [Google Scholar]
- 31.Barile-Fabris L, Ariza-Andraca R, Olguín-Ortega L, Jara LJ, Fraga-Mouret A, Miranda-Limón JM, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:620–5. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Cruz DP, Mellor-Pita S, Joven B, Sanna G, Allanson J, Taylor J, et al. Transverse myelitis as the first manifestation of systemic lupus erythematosus or lupus-like disease: Good functional outcome and relevance of antiphospholipid antibodies. J Rheumatol. 2004;31:280–5. [PubMed] [Google Scholar]
- 33.Łukjanowicz M, Brzosko M. Myelitis in the course of systemic lupuserythematosus: Review. Pol Arch Med Wewn. 2009;119:67–72. [PubMed] [Google Scholar]
- 34.Kovacs B, Lafferty TL, Brent LH, DeHoratius RJ. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis. 2000;59:120–4. doi: 10.1136/ard.59.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura KY, Seino Y, Hirayama Y, Aramaki T, Yamaguchi H, Amano H, et al. Systemic lupus erythematosus related transverse myelitis presenting longitudinal involvement of the spinal cord. Intern Med. 2002;41:156–60. doi: 10.2169/internalmedicine.41.156. [DOI] [PubMed] [Google Scholar]
- 36.Tokunaga M, Saito K, Kawabata D, Imura Y, Fujii T, Nakayamada S, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66:470–5. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jönsen A, Bengtsson AA, Nived O, Ryberg B, Sturfelt G. Outcome of neuropsychiatric systemic lupus erythematosus within a defined Swedish population: Increased morbidity but low mortality. Rheumatology (Oxford) 2002;41:1308–12. doi: 10.1093/rheumatology/41.11.1308. [DOI] [PubMed] [Google Scholar]