Abstract
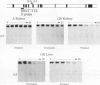
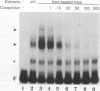
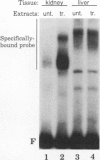
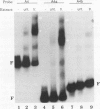
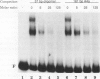
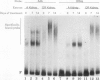
The tissue specificity and genetic variability of the murine beta-glucuronidase (GUS) response to androgen provide useful markers for identifying elements which underlie this responsiveness. While GUS is expressed constitutively in all examined cell types, kidney epithelial cells uniquely exhibit a manyfold yet slow rise in GUS mRNA and enzyme levels when stimulated by androgens. Three major phenotypes of this androgen response have been described among inbred strains of mice: (i) a strong response in strains of the Gusa haplotype, (ii) a reduced response in strains of the Gusb and Gush haplotypes, and (iii) no response, as observed in Gusor mice. These response variants define a cis-active element(s) which is tightly linked to the GUS structural gene. Nuclease hypersensitivity scans of kidney chromatin within and surrounding the structural gene revealed an androgen-inducible hypersensitive site in intron 9 of the gene in Gusa but not in Gusor mice. When a radiolabeled fragment of Gusa DNA containing this hypersensitive site was incubated with kidney nuclear extracts and then subjected to gel electrophoresis, two shifted bands were observed whose levels were dramatically higher in extracts of androgen-treated than in those of untreated Gusa mice. The shifted bands reflect binding of a kidney-specific factor(s) to a 57-bp region of complex dyad symmetry in Gusa and Gusor mice which is partially deleted in Gusb and Gush mice. This binding site is located approximately 130 bp downstream of a glucocorticoid response element sequence motif which is totally deleted in [Gus]or mice. Taken together, our results suggest that the androgen responsiveness of GUS in murine kidney epithelial cells is controlled by elements within the proximal end of intron 9 of the GUS structural gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly A., Le Page C., Rauch M., Milgrom E. Sequence-specific DNA binding of the progesterone receptor to the uteroglobin gene: effects of hormone, antihormone and receptor phosphorylation. EMBO J. 1986 Dec 1;5(12):3235–3241. doi: 10.1002/j.1460-2075.1986.tb04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin C. W., Brown T. R., Mills N. C., Gupta C., Bullock L. P. The regulation of the beta-glucuronidase gene by androgens and progestins. Biol Reprod. 1978 Feb;18(1):74–83. doi: 10.1095/biolreprod18.1.74. [DOI] [PubMed] [Google Scholar]
- Beato M., Chalepakis G., Schauer M., Slater E. P. DNA regulatory elements for steroid hormones. J Steroid Biochem. 1989 May;32(5):737–747. doi: 10.1016/0022-4731(89)90521-9. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Berger F. G., Watson G. Androgen-regulated gene expression. Annu Rev Physiol. 1989;51:51–65. doi: 10.1146/annurev.ph.51.030189.000411. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Hormonal regulation of the conformation of the ovalbumin gene in chick oviduct chromatin. J Biol Chem. 1982 Nov 10;257(21):13018–13027. [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Henderson D., Ponta H. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 1987 Feb;6(2):363–368. doi: 10.1002/j.1460-2075.1987.tb04763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., Kontula K. K., Watson C. S., Seppänen P. J., Funkenstein B., Melanitou E., Hickok N. J., Bardin C. W., Jänne O. A. Regulation of gene expression by androgens in murine kidney. Recent Prog Horm Res. 1986;42:71–109. doi: 10.1016/b978-0-12-571142-5.50006-9. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V. M., Miller D. R., Novak E., Elliott R. W. Alleles of beta-glucuronidase found in wild mice. Curr Top Microbiol Immunol. 1986;127:114–123. doi: 10.1007/978-3-642-71304-0_14. [DOI] [PubMed] [Google Scholar]
- D'Amore M. A., Gallagher P. M., Korfhagen T. R., Ganschow R. E. Complete sequence and organization of the murine beta-glucuronidase gene. Biochemistry. 1988 Sep 6;27(18):7131–7140. doi: 10.1021/bi00418a070. [DOI] [PubMed] [Google Scholar]
- Denison S. H., Sands A., Tindall D. J. A tyrosine aminotransferase glucocorticoid response element also mediates androgen enhancement of gene expression. Endocrinology. 1989 Feb;124(2):1091–1093. doi: 10.1210/endo-124-2-1091. [DOI] [PubMed] [Google Scholar]
- Derman E. Isolation of a cDNA clone for mouse urinary proteins: age- and sex-related expression of mouse urinary protein genes is transcriptionally controlled. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5425–5429. doi: 10.1073/pnas.78.9.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dofuku R., Tettenborn U., Ohno S. Further characterization of Os mutation of mouse beta-glucuronidase locus. Nat New Biol. 1971 Dec 29;234(52):259–261. doi: 10.1038/newbio234259a0. [DOI] [PubMed] [Google Scholar]
- Dofuku R., Tettenborn U., Ohno S. Testosterone-"regulon" in the mouse kidney. Nat New Biol. 1971 Jul 7;232(27):5–7. doi: 10.1038/newbio232005a0. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H. Beta-glucuronidase and the action of steroid hormones. Ann N Y Acad Sci. 1951 Dec;54(4):548–557. doi: 10.1111/j.1749-6632.1951.tb46612.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Folger K., Anderson J. N., Hayward M. A., Shapiro D. J. Nuclease sensitivity and DNA methylation in estrogen regulation of Xenopus laevis vitellogenin gene expression. J Biol Chem. 1983 Jul 25;258(14):8908–8914. [PubMed] [Google Scholar]
- Gerber-Huber S., Felber B. K., Weber R., Ryffel G. U. Estrogen induces tissue specific changes in the chromatin conformation of the vitellogenin genes in Xenopus. Nucleic Acids Res. 1981 Jun 11;9(11):2475–2494. doi: 10.1093/nar/9.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowland P. L., Buetti E. Mutations in the hormone regulatory element of mouse mammary tumor virus differentially affect the response to progestins, androgens, and glucocorticoids. Mol Cell Biol. 1989 Sep;9(9):3999–4008. doi: 10.1128/mcb.9.9.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J., Thomson A., Needham M., Webb P., Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res. 1988 Jun 24;16(12):5263–5276. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hodo H. G., 3rd, Blatti S. P. Purification using polyethylenimine precipitation and low molecular weight subunit analyses of calf thymus and wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1977 May 31;16(11):2334–2343. doi: 10.1021/bi00630a005. [DOI] [PubMed] [Google Scholar]
- Kandala J. C., Kistler M. K., Kistler W. S. Androgen regulated genes from prostate and seminal vesicle share upstream sequence homologies. Biochem Biophys Res Commun. 1985 Jan 31;126(2):948–952. doi: 10.1016/0006-291x(85)90277-3. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240(4850):327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Lund S. D., Miller D., Chapman V., Ganschow R. E. Androgen regulation of murine beta-glucuronidase expression: identification and characterization of a nonresponse variant. Genetics. 1988 May;119(1):151–156. doi: 10.1093/genetics/119.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Selective binding of the estradiol receptor to a region at least one kilobase upstream from the rat prolactin gene. DNA. 1985 Feb;4(1):1–9. doi: 10.1089/dna.1985.4.1. [DOI] [PubMed] [Google Scholar]
- Nitsch D., Stewart A. F., Boshart M., Mestril R., Weih F., Schütz G. Chromatin structures of the rat tyrosine aminotransferase gene relate to the function of its cis-acting elements. Mol Cell Biol. 1990 Jul;10(7):3334–3342. doi: 10.1128/mcb.10.7.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K. Mammalian beta-glucuronidase: genetics, molecular biology, and cell biology. Prog Nucleic Acid Res Mol Biol. 1989;37:155–205. doi: 10.1016/s0079-6603(08)60698-4. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Rundlett S. E., Wu X. P., Miesfeld R. L. Functional characterizations of the androgen receptor confirm that the molecular basis of androgen action is transcriptional regulation. Mol Endocrinol. 1990 May;4(5):708–714. doi: 10.1210/mend-4-5-708. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Paigen K., Davey R., Chapman V., Labarca C., Watson G., Ganschow R., Brandt E. J., Novak E. Genetic regulation of mammalian glucuronidase. Recent Prog Horm Res. 1978;34:401–436. doi: 10.1016/b978-0-12-571134-0.50015-6. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Paigen K., Ganschow R. E. Genetic control of glucuronidase induction in mice. J Mol Biol. 1973 Dec 5;81(2):225–243. doi: 10.1016/0022-2836(73)90191-5. [DOI] [PubMed] [Google Scholar]
- Watson G., Davey R. A., Labarca C., Paigen K. Genetic determination of kinetic parameters in beta-glucuronidase induction by androgen. J Biol Chem. 1981 Mar 25;256(6):3005–3011. [PubMed] [Google Scholar]
- Watson G., Paigen K. mRNA synthesis rates in vivo for androgen-inducible sequences in mouse kidney. Mol Cell Biol. 1988 May;8(5):2117–2124. doi: 10.1128/mcb.8.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L., McDonald C., Higgins S. Sequence organisation of rat seminal vesicle F gene: location of transcriptional start point and sequence comparison with six other androgen-regulated genes. Nucleic Acids Res. 1985 Feb 11;13(3):659–672. doi: 10.1093/nar/13.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Yamamoto K. R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984 Aug;38(1):29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]