Abstract
Background
In China, the reference population of genotyped Holstein cattle is relatively small with to date, 80 bulls and 2091 cows genotyped with the Illumina 54 K chip. Including genotyped Holstein cattle from other countries in the reference population could improve the accuracy of genomic prediction of the Chinese Holstein population. This study investigated the consistency of linkage disequilibrium between adjacent markers between the Chinese and Nordic Holstein populations, and compared the reliability of genomic predictions based on the Chinese reference population only or the combined Chinese and Nordic reference populations.
Methods
Genomic estimated breeding values of Chinese Holstein cattle were predicted using a single-trait GBLUP model based on the Chinese reference dataset, and using a two-trait GBLUP model based on a joint reference dataset that included both the Chinese and Nordic Holstein data.
Results
The extent of linkage disequilibrium was similar in the Chinese and Nordic Holstein populations and the consistency of linkage disequilibrium between the two populations was very high, with a correlation of 0.97. Genomic prediction using the joint versus the Chinese reference dataset increased reliabilities of genomic predictions of Chinese Holstein bulls in the test data from 0.22, 0.15 and 0.11 to 0.51, 0.47 and 0.36 for milk yield, fat yield and protein yield, respectively. Using five-fold cross-validation, reliabilities of genomic predictions of Chinese cows increased from 0.15, 0.12 and 0.15 to 0.26, 0.17 and 0.20 for milk yield, fat yield and protein yield, respectively.
Conclusions
The linkage disequilibrium between the two populations was very consistent and using the combined Nordic and Chinese reference dataset substantially increased reliabilities of genomic predictions for Chinese Holstein cattle.
Background
Genomic selection was proposed in 2001 [1] and has since then become a major research topic in animal breeding. Accuracy of genomic prediction depends greatly on the size of the reference population [2,3]. The larger the reference population, the more accurate genomic prediction is. It was reported that reliabilities of genomic prediction of Holstein cattle increased when Holstein cattle of other countries were added to the reference dataset [4-6]. Similarly, pooling genotypes from other countries or populations to form a common reference population helped to increase the reliability of predictions in Brown Swiss cattle [6,7]. In addition, reliabilities of genomic prediction obtained by combining the reference populations of Danish, Swedish and Finnish red cattle were higher than those using single-country reference populations [8]. Holstein dairy cattle in China were originally imported from Europe and North America and were mostly derived from cross-breeding between the local yellow cattle and imported foreign Holstein bulls. It is assumed that the current Chinese Holstein population is genetically close to the other Holstein populations in the world. To date, the reference population of genotyped Holstein cattle in China is relatively small and includes mainly cows. It is expected that a joint reference dataset that combines Chinese Holstein cattle and Holstein cattle from other populations will greatly improve the reliability of genomic predictions of the Chinese Holstein population, assuming linkage disequilibrium between markers and quantitative trait loci (QTL) is consistent between the populations.
The objectives of this study were to: (1) estimate the consistency of linkage disequilibrium between the Chinese and the Nordic Holstein populations and (2) assess the gains in reliability of genomic predictions in Chinese Holstein from using a joint Chinese and Nordic reference dataset, compared with using the Chinese reference dataset alone.
Methods
Data
In this study, both the Chinese Holstein (CH) and Nordic Holstein (NH) cattle were genotyped with the Illumina BovineSNP50 BeadChip (Illumina, San Diego, CA). The single nucleotide polymorphism (SNP) data of each population were edited separately by deleting SNP with minor allele frequencies less than 0.01 or call rates less than 0.10, and excluding individuals with more than 10% missing marker genotypes. After editing, 41 838 SNP on 29 autosomes were retained in both populations. The genotyped CH cattle included 80 bulls born between 1993 and 2002 and 2091 cows born between 2001 and 2006, which were daughters of 13 of the genotyped bulls. The number of daughters per bull ranged from 63 to 358, with a mean of 135. The genotyped NH cattle included 5216 bulls born between 1974 and 2008. All animals of both populations were used in the linkage disequilibrium analysis. Deregressed proofs (DRP) were used as phenotypes for genomic prediction. DRP of CH bulls and cows were derived from the estimated breeding values (EBV) obtained from the Chinese genetic evaluations in April 2012 (Dairy Association of China), and DRP of NH bulls were derived from the EBV of Nordic genetic evaluations in November 2010 (Nordic Genetic Evaluation). Three traits (milk yield, fat yield and protein yield) were analyzed. In total, 4398 NH bulls and all CH animals had phenotypes for the three traits. 512 CH cows with possible incorrect sire information were discarded based on parentage verification with 255 SNP performed in a previous study [9] in which paternity was considered incorrect if five or more SNP were in conflict (i.e., a sire was homozygous for one allele but its daughter was homozygous for the other allele). Consequently, 1572 CH cows and 80 CH bulls with reliable pedigree information were used for genomic prediction.
Measure of linkage disequilibrium (LD) and consistency of LD
Each chromosome was phased separately using Beagle [10] for each population, and all missing genotypes were simultaneously imputed by Beagle. Linkage disequilibrium between a pair of SNP was measured as [11], and rLD was calculated as follows:
where f(AB), f(A), f(a), f(B) and f(b) are observed frequencies of haplotype AB, alleles A, a, B and b, respectively. Maternal and paternal haplotypes were pooled to calculate LD. The consistency of LD in the two populations was measured by the correlation of rLD of adjacent marker pairs on each autosome between the two populations [12].
Prediction of genomic breeding values
A single-trait GBLUP model was used for genomic prediction based on the Chinese reference dataset, and a two-trait GBLUP was used for genomic prediction based on the joint reference dataset that included both the CH and NH cattle. In the latter, a single biological trait was regarded as a different trait in the two populations. The basic GBLUP model [13,14] was
where y is the vector of phenotypes, μ is the population mean, g is the vector of breeding values, e is the vector of residuals, and Z is a design matrix allocating g to y. It was assumed that and , where G is the genomic relationship matrix, is the additive genetic variance, D is a diagonal matrix with weights of the residual variance [15], and is the residual variance. The G matrix was constructed according to the method (method 1) described by VanRaden [14], i.e. G = MM '/∑2pi(1 - pi), where elements in column i of M are 0 - 2pi, 1 - 2pi and 2 - 2pi for genotypes A1A1, A1A2 and A2A2, respectively, and pi is the allele frequency of A2 at locus i, calculated from the available marker data. Variances and covariances in the GBLUP models were estimated using the “average information” restricted maximum likelihood algorithm, and the GBLUP analyses were conducted using the DMU Package [16].
Validation
Accuracy of genomic predictions using the CH and the joint reference datasets was assessed by two validation procedures. In the first procedure, the reference dataset comprised 13 genotyped CH bulls and their genotyped daughters and the test dataset consisted of 48 CH bulls without genotyped daughters. The remaining 19 CH bulls, which were highly related with the 13 reference bulls, were not used in the test dataset. In the second procedure, a five-fold cross-validation procedure was used for genomic prediction of CH cows. In each fold of cross-validation, two or three half-sib families of cows were used as the test dataset and the remaining cows and genotyped CH bulls were used as reference dataset. The numbers of animals in the reference and test datasets for the two validation procedures are shown in Table 1. Genomic EBV (GEBV) for the animals in a test dataset were predicted using both the CH and the joint reference datasets. The joint reference dataset included all 4398 Nordic bulls.
Table 1.
The size of test and reference datasets used for validating genomic predictions of Chinese Holstein (CH) bulls and cows
| Categories | Test datasets | Reference datasets* | |
|---|---|---|---|
| CH bulls |
48 |
13 bulls and 1572 cows |
|
| CH cows | set 1 |
337 |
80 bulls and 1235 cows |
| set 2 |
309 |
80 bulls and 1263 cows |
|
| set 3 |
323 |
80 bulls and 1249 cows |
|
| set 4 |
307 |
80 bulls and 1265 cows |
|
| set 5 | 296 | 80 bulls and 1276 cows | |
* CH reference datasets; when using joint reference datasets for prediction 4398 Nordic Holstein bulls were included.
Reliabilities of GEBV for bulls and cows were measured as the squared correlation of GEBV and DRP divided by the average reliabilities of the DRP in the test dataset (Cor2(GEBV,DRP)/r2DRP) [15]. Because CH bulls were born between 1993 and 2002, genetic trend due to selection could inflate the correlation between GEBV and DRP. Therefore, in the validation of genomic predictions for CH bulls, genetic trends present in GEBV and DRP were corrected by regressing on birth year, and then the reliabilities were calculated by correlating the corrected GEBV and DRP. In the validation of genomic predictions for cows, reliabilities were calculated based on GEBV pooled over the five test datasets.
Results and discussion
Linkage disequilibrium and consistency of LD
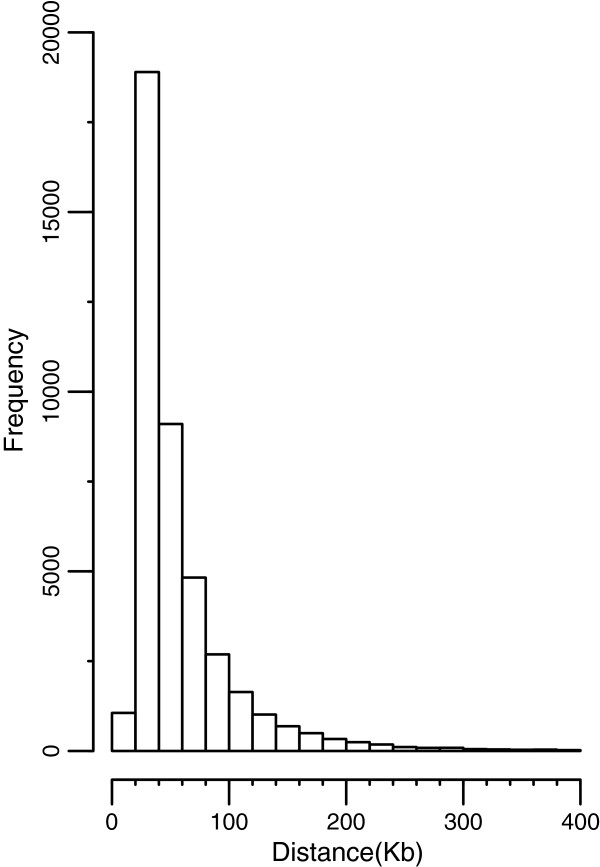
Data on the 41 838 SNP distributed over the 29 bovine autosomes are summarized in Table 2. The average distance between adjacent SNP was 59.59 Kb, and the shortest and longest gaps were 3 bp (on BTA7) and 3820 Kb (on BTA10). As shown in Figure 1, most distances between adjacent markers were less than 100 Kb. However, 2.55% adjacent SNP had large distance gaps (larger than 200 Kb). This indicates that SNP used in the current study were not evenly distributed over the bovine chromosomes.
Table 2.
Distance and linkage disequilibrium () of adjacent SNP for each Bos taurus autosome (BTA)
|
BTA |
Length (Mb) |
Number of SNP |
Mean distance (Kb) |
Mean |
Consistency1 |
||
|---|---|---|---|---|---|---|---|
| Chinese Holstein | Nordic Holstein | ||||||
| 1 |
160.89 |
2729 |
58.98 |
0.22 |
0.23 |
0.97 |
|
| 2 |
138.92 |
2221 |
62.58 |
0.22 |
0.22 |
0.98 |
|
| 3 |
125.41 |
2067 |
60.70 |
0.21 |
0.22 |
0.97 |
|
| 4 |
120.39 |
2031 |
59.30 |
0.20 |
0.21 |
0.97 |
|
| 5 |
124.59 |
1740 |
71.64 |
0.21 |
0.21 |
0.97 |
|
| 6 |
119.01 |
2099 |
56.73 |
0.22 |
0.23 |
0.97 |
|
| 7 |
112.37 |
1809 |
62.15 |
0.22 |
0.24 |
0.98 |
|
| 8 |
115.59 |
1940 |
59.61 |
0.21 |
0.22 |
0.98 |
|
| 9 |
104.64 |
1639 |
63.88 |
0.20 |
0.21 |
0.97 |
|
| 10 |
103.09 |
1752 |
58.87 |
0.22 |
0.22 |
0.97 |
|
| 11 |
106.97 |
1858 |
57.61 |
0.21 |
0.21 |
0.97 |
|
| 12 |
89.17 |
1338 |
66.70 |
0.19 |
0.20 |
0.97 |
|
| 13 |
83.84 |
1434 |
58.50 |
0.22 |
0.22 |
0.97 |
|
| 14 |
83.15 |
1409 |
59.06 |
0.24 |
0.25 |
0.98 |
|
| 15 |
83.81 |
1371 |
61.17 |
0.18 |
0.19 |
0.96 |
|
| 16 |
80.09 |
1283 |
62.47 |
0.22 |
0.23 |
0.98 |
|
| 17 |
74.89 |
1324 |
56.60 |
0.19 |
0.20 |
0.97 |
|
| 18 |
65.16 |
1101 |
59.24 |
0.19 |
0.19 |
0.96 |
|
| 19 |
62.83 |
1131 |
55.60 |
0.18 |
0.20 |
0.97 |
|
| 20 |
72.00 |
1314 |
54.84 |
0.20 |
0.21 |
0.97 |
|
| 21 |
68.46 |
1110 |
61.73 |
0.21 |
0.22 |
0.97 |
|
| 22 |
60.14 |
1047 |
57.50 |
0.19 |
0.19 |
0.97 |
|
| 23 |
51.73 |
895 |
57.86 |
0.17 |
0.18 |
0.97 |
|
| 24 |
62.77 |
1053 |
59.67 |
0.21 |
0.21 |
0.98 |
|
| 25 |
43.23 |
825 |
52.46 |
0.19 |
0.20 |
0.96 |
|
| 26 |
50.95 |
871 |
58.57 |
0.19 |
0.20 |
0.97 |
|
| 27 |
45.33 |
801 |
56.67 |
0.16 |
0.16 |
0.96 |
|
| 28 |
46.06 |
783 |
58.90 |
0.16 |
0.17 |
0.95 |
|
| 29 |
50.53 |
863 |
58.62 |
0.17 |
0.18 |
0.97 |
|
| Mean | 2506.002 | 41 8382 | 59.59 | 0.20 | 0.21 | 0.97 | |
1The correlation of rLD of adjacent SNP pairs between two populations; 2Sum over 29 autosomes.
Figure 1.
Frequency distribution of distances between adjacent SNP pairs.
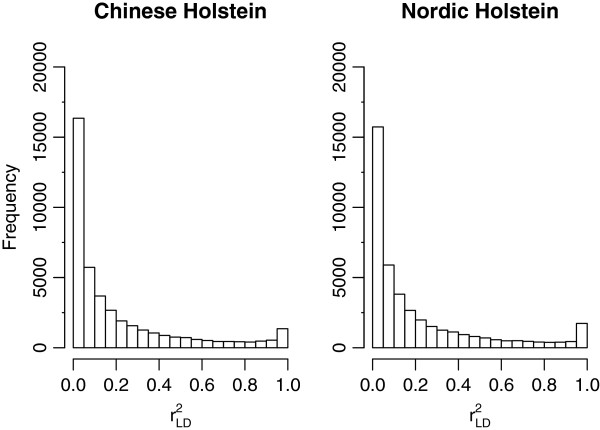
Figure 2 presents the distribution of of adjacent SNP pairs in the CH and NH populations. The LD between adjacent SNP pairs was generally small. The proportion of adjacent SNP pairs which had an < 0.01 was 15.2% for CH and 17.6% for NH; the proportion with an < 0.1 was 52.81% for CH and 51.72% for NH. These results indicate that the SNP markers did not effectively capture all the QTL affecting a trait, since most adjacent SNP pairs were in weak LD. The mean of adjacent SNP pairs within a chromosome ranged from 0.16 to 0.24 in CH and from 0.16 to 0.25 in NH. The mean across all chromosomes was 0.20 in CH and 0.21 in NH. The consistency of LD between the CH and NH populations was high (Table 2). The correlation of rLD of adjacent SNP ranged from 0.95 to 0.98 across all chromosomes, with a mean of 0.97 at an average marker distance of 60 Kb.
Figure 2.
Frequency distribution of for adjacent SNP pairs in Chinese Holstein and Nordic Holstein populations.
The mean in the CH and NH populations was similar to the degree of LD reported for the Holstein populations in Germany [17], in the Netherlands, Australia and New Zealand [12], and in North America [18]. The consistency of LD between the CH and NH populations agreed with the consistency of LD between the Dutch and Australian Holstein bulls reported in [12] and was in line with the development of the CH population. The first dairy cattle imported in China came from Europe in the 1870’s [19] and since then, Holstein cattle have continuously been imported from Europe, Japan and North America. The imported Holstein bulls were crossed with local yellow cattle, and the crossbred cows were continuously back-crossed with the imported Holstein bulls [20]. The resulting crossed black and white dairy cattle were officially named Chinese Holsteins in 1992. Currently, most of the Holstein bulls found in China were imported from worldwide in the form of embryos or live cattle. Besides, the NH population has also exchanged genetic material with the United States, the Netherlands, Germany and other countries. The genomic relationship matrix showed that some CH bulls could be full-sibs or half-sibs of the NH bulls. Based on these data, it can be inferred that the Chinese Holstein population is genetically close to European and North American Holstein cattle.
Genomic prediction
Reliabilities of GEBV for the 48 CH test bulls that had no genotyped daughters and the 1572 CH cows in the test dataset with the five-fold cross-validation are presented in Table 3. When the CH cattle were used as the reference data, the reliabilities of GEBV were 0.16 for CH test bulls and 0.14 for CH cows, averaged over the three traits. When using the joint reference dataset, the average reliabilities increased to 0.45 for CH bulls and to 0.21 for CH cows. Based on data from the Dairy Association of China, the reliability of a conventional pedigree index (calculated as 0.5*sire EBV + 0.25*maternal grandsire EBV) in the CH population was 0.12 for milk production traits. Thus, the reliabilities of genomic predictions based on the CH reference population were of similar magnitude as the reliabilities of a pedigree index, and the genomic predictions based on the joint reference population gave much higher reliabilities than those based on a pedigree index.
Table 3.
Reliabilities of GEBV of Chinese Holstein (CH) bulls and cows in the test populations when using the CH or the joint reference population
|
Categories |
Traits |
Reliabilities of prediction |
||
|---|---|---|---|---|
| Using CH reference | Using joint reference | Increase | ||
| CH bulls |
Milk yield |
0.22 |
0.51 |
0.29 |
| Fat yield |
0.15 |
0.47 |
0.32 |
|
| Protein yield |
0.11 |
0.36 |
0.25 |
|
| Average |
0.16 |
0.45 |
0.29 |
|
| CH cows | Milk yield |
0.15 |
0.26 |
0.11 |
| Fat yield |
0.12 |
0.17 |
0.05 |
|
| Protein yield |
0.15 |
0.20 |
0.05 |
|
| Average | 0.14 | 0.21 | 0.07 | |
The low reliabilities of genomic predictions using the CH reference dataset alone suggested that the information in the CH reference dataset, which mainly contained cows, was insufficient. According to Goddard [2], the reliability of GEBV for different sizes of reference population and different heritabilities of traits can be predicted as:
where N is the number of individuals in the reference population and a = 1 + 2λ/N. According to Hayes et al. [21], λ = Mek/h2, Me = 2NeL, and k = 1/log(2Ne), where Ne is the effective population size and L is the length of the genome in Morgans. Using the above formula, the reliability of GEBV based on the CH reference dataset was expected to be 0.175, assuming L = 30, Ne = 100, N = 1500 and r2DRP = 0.50. The reliabilities obtained from the validation procedures were consistent with these expected reliabilities. The results indicate that the size of the CH reference population needs to be increased in order to increase the reliability of genomic predictions.
Dairy cattle reference populations usually comprise progeny-tested bulls to maximize the information from each genotyped individual. In some countries or in some cattle populations where the number of progeny-tested bulls is small, one solution is to include cows in the reference population. In order to evaluate the value of adding cows to the reference dataset, an additional analysis was performed using a CH reference dataset from which 50% of the cows were deleted. The reliabilities of GEBV for the CH bulls using the reduced CH reference dataset decreased to 0.09, 0.03 and 0.05 for milk yield, fat yield and protein yield, respectively. This indirectly demonstrates that it is feasible to use cows as reference animals for genomic prediction, when the number of available progeny-tested bulls is not sufficient. A simulation study by Mc Hugh et al. [22] also suggested that genomic information from cows could greatly increase genetic gain and accuracy of male selection. To increase the size of the cow reference population at low cost, a good alternative would be to genotype cows using a low density chip like the Bovine LD (7 K) and then impute the genotypes for the 54 K panel.
The joint reference dataset greatly improved the reliability of genomic predictions for the CH cattle. The reliabilities of GEBV for CH bulls based on the joint reference dataset were close to those for NH bulls based on the Nordic reference data [23]. Several studies have reported that the reliability of genomic prediction can be increased by using a joint reference dataset that includes reference animals from other populations. The reliabilities of GEBV increased by 10% on average when four European Holstein populations were combined into a reference dataset, compared to when only one national population was used as the reference population [5]. Reliabilities of genomic prediction for Canadian Holstein bulls increased by 6% on average when about 3000 foreign bulls were included in the reference dataset [4], and by 7% when all North American sires were included [24]. Reliabilities were 2.6% higher for Holstein and 3.2% higher for Brown Swiss cattle when 3593 foreign Holstein and 732 foreign Brown Swiss animals were included in the reference dataset of the USA domestic prediction [6].
With the joint reference dataset, reliabilities of genomic predictions improved more for CH bulls than for CH cows i.e. by 2.3 fold for CH bulls and only by 1.7 fold for CH cows. This is due to the fact that CH bulls have a closer relationship with NH bulls than the CH cows do. Among the 48 CH test bulls, 14 bulls had a genomic relationship with one or more NH bull in the range from 0.45 to 0.56. However, no CH cow had this level of relationship with any NH bull. Moreover, among the 48 CH test bulls, 33 (68.75%) had a genomic relationship greater than 0.2 with at least one NH bull (with 15.5 bulls on average). Among the 1572 CH cows, only 459 (29.2%) had a genomic relationship greater than 0.2 with an NH bull (with 1.3 bulls on average). Many previous studies reported that the existence of a close relationship between test animals and reference animals increased the reliability of genomic predictions for the test animals [25-27].
To avoid overestimation of the reliability of GEBV, 19 CH bulls were excluded from the test dataset because they were highly related to 13 bulls in the reference population. In the five-fold cross-validation for the CH cows, two or three half-sib families were randomly assigned to a single-test dataset, instead of randomly choosing individuals. This was done to avoid overestimation of the reliability of GEBV when animals in the test dataset have a large group of half-sibs in the reference dataset. Moreover, genetic trend can increase the correlation between GEBV and DRP if the birth years of the animals in the test dataset cover a wide range. Therefore, in the validation for the CH bulls, the correlation between GEBV and DRP was calculated after correcting for genetic trend. When ignoring this correction, the validation reliabilities of genomic predictions for CH bulls using the joint reference dataset were unrealistically high at 0.69, 0.54 and 0.60 for milk yield, fat yield and protein yield, respectively.
In the current study, when using the joint reference dataset, genomic predictions were estimated using a two-trait model, in which the same biological trait was considered to be a different trait in the CH and NH populations. The reason for using a two-trait model, instead of a single-trait model, was that the DRP had different scales in the two populations due to the use of a standardization procedure in the NH population. The two-trait model also accounts for the presence of any genotype by environment interactions. When genotypes of bulls from three foreign countries were included in the USA domestic predictions, multi-trait methods were not more accurate than the single-trait model for Holstein cattle, but gave higher reliabilities (1.4% higher on average) for Brown Swiss cattle [6]. The authors suggested that this could be due to lower genetic correlations of traits between Brown Swiss populations. Using the two-trait GBLUP model, the estimated genetic correlations between the CH and NH populations were 0.85, 0.70 and 0.75 for milk yield, fat yield and protein yield respectively, which were much lower than the value of the consistency of LD of neighboring markers, which was 0.97 between the two populations. Assuming that the consistency of LD was appropriate to represent the genetic associations between different populations, its clear difference with the estimated genetic correlations suggests the existence of a large genotype by environment interaction between China and Nordic countries.
Conclusions
The consistency of LD is very high between the CH and NH populations, indicating a high level of genetic similarity between the two populations. Genomic prediction for CH cattle can be greatly improved using a joint reference dataset that includes CH and NH cattle. In order to obtain satisfactory reliabilities of genomic predictions for CH cattle, it is necessary to increase the size of the CH reference population or to include foreign Holstein cattle in the reference population.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LZ performed the analysis and drafted the manuscript with help from GS. XD prepared Chinese Holstein data and helped in analyzing the data. GS, MSL, QZ and YW conceived and designed the study. MSL and GS helped in interpreting results. All the authors read, revised and approved the final manuscript.
Contributor Information
Lei Zhou, Email: lei.zhou@agrsci.dk.
Xiangdong Ding, Email: xding@cau.edu.cn.
Qin Zhang, Email: qzhang@cau.edu.cn.
Yachun Wang, Email: wangyachun@cau.edu.cn.
Mogens S Lund, Email: mogens.lund@agrsci.dk.
Guosheng Su, Email: guosheng.su@agrsci.dk.
Acknowledgments
This work was performed in the projects “Genomic Selection—From function to efficient utilization in cattle breeding (grant no. 3405-10-0137)”, funded under Green Development and Demonstration Programme (Denmark); “Combining Nordic and Chinese reference populations for genomic selection”, funded by VikingGenetics (Denmark); the Chinese National ‘948’ Project (2011-G2A); The National Natural Science Foundation of China (31272418); Ph.D. Programs Foundation of Ministry of Education of China (20110008110001); and China earmarks fund for CARS-37.
References
- Meuwissen THE, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard M. Genomic selection: prediction of accuracy and maximisation of long term response. Genetica. 2009;136:245–257. doi: 10.1007/s10709-008-9308-0. [DOI] [PubMed] [Google Scholar]
- Daetwyler HD, Villanueva B, Woolliams JA. Accuracy of predicting the genetic risk of disease using a genome-wide approach. PLoS One. 2008;3:e3395. doi: 10.1371/journal.pone.0003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel FS, Sargolzaei M, Kistemaker G, Jansen GB, Sullivan P, Van Doormaal BJ, VanRaden PM, Wiggans GR. Reliability of genomic evaluation of Holstein cattle in Canada. Interbull Bull. 2009;39:51–57. [Google Scholar]
- Lund MS, de Roos APW, de Vries AG, Druet T, Ducrocq V, Fritz S, Guillaume F, Guldbrandtsen B, Liu ZT, Reents R, Schrooten C, Seefried F, Su GS. A common reference population from four European Holstein populations increases reliability of genomic predictions. Genet Sel Evol. 2011;43:43. doi: 10.1186/1297-9686-43-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRaden PM, Olson KM, Null DJ, Sargolzaei MMW, van Kaam JBCHM. Reliability increases from combining 50,000- and 777,000-marker genotypes from four countries. Interbull Bull. 2012;46:75–79. [Google Scholar]
- Jorjani H, Jakobsen J, Nilforooshan MA, Hjerpe E, Zumbach B, Palucci V, Dürr J. Genomic evaluation of BSW populations interGenomics: results and deliverables. Interbull Bull. 2011;43:5–8. [Google Scholar]
- Brondum RF, Rius-Vilarrasa E, Stranden I, Su G, Guldbrandtsen B, Fikse WF, Lund MS. Reliabilities of genomic prediction using combined reference data of the Nordic Red dairy cattle populations. J Dairy Sci. 2011;94:4700–4707. doi: 10.3168/jds.2010-3765. [DOI] [PubMed] [Google Scholar]
- Guo G, Zhou L, Liu L, Li D, Zhang SL, Liu JF, Ding XD, Zhang Y, Wang YC, Zhang Q, Zhang Y. Parentage inference with single nucleotide polymorphism markers in the Chinese Holstein in Beijing. Xu Mu Shou Yi Xue Bao. 2011;43:44–49. [Google Scholar]
- Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WG, Robertson A. Linkage disequilibrium in finite populations. Theor Appl Genet. 1968;38:226–231. doi: 10.1007/BF01245622. [DOI] [PubMed] [Google Scholar]
- de Roos APW, Hayes BJ, Spelman RJ, Goddard ME. Linkage disequilibrium and persistence of phase in Holstein-Friesian, Jersey and Angus cattle. Genetics. 2008;179:1503–1512. doi: 10.1534/genetics.107.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes BJ, Goddard ME. Technical note: Prediction of breeding values using marker-derived relationship matrices. J Anim Sci. 2008;86:2089–2092. doi: 10.2527/jas.2007-0733. [DOI] [PubMed] [Google Scholar]
- VanRaden PM. Efficient methods to compute genomic predictions. J Dairy Sci. 2008;91:4414–4423. doi: 10.3168/jds.2007-0980. [DOI] [PubMed] [Google Scholar]
- Su G, Madsen P, Nielsen US, Mantysaari EA, Aamand GP, Christensen OF, Lund MS. Genomic prediction for Nordic Red Cattle using one-step and selection index blending. J Dairy Sci. 2012;95:909–917. doi: 10.3168/jds.2011-4804. [DOI] [PubMed] [Google Scholar]
- Madsen P, Jensen J. A users guide to DMU, version 6, release 5. Tjele: University of Aarhus, Faculty of Agricultural Science; 2010. [Google Scholar]
- Qanbari S, Pimentel ECG, Tetens J, Thaller G, Lichtner P, Sharifi AR, Simianer H. The pattern of linkage disequilibrium in German Holstein cattle. Anim Genet. 2010;41:346–356. doi: 10.1111/j.1365-2052.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- Sargolzaei M, Schenkel FS, Jansen GB, Schaeffer LR. Extent of linkage disequilibrium in Holstein cattle in North America. J Dairy Sci. 2008;91:2106–2117. doi: 10.3168/jds.2007-0553. [DOI] [PubMed] [Google Scholar]
- Qiu H, Qin Z, Chen Y, Wang A. Bovine breeds in China. Shanghai: Shanghai Scientific & Technical Publishers; 1988. [Google Scholar]
- Qin ZY. The breeding of Chinese Holstein. China Dairy. 2001;10:26–27. [Google Scholar]
- Hayes BJ, Visscher PM, Goddard ME. Increased accuracy of artificial selection by using the realized relationship matrix. Genet Res. 2009;91:47–60. doi: 10.1017/S0016672308009981. [DOI] [PubMed] [Google Scholar]
- Mc Hugh N, Meuwissen THE, Cromie AR, Sonesson AK. Use of female information in dairy cattle genomic breeding programs. J Dairy Sci. 2011;94:4109–4118. doi: 10.3168/jds.2010-4016. [DOI] [PubMed] [Google Scholar]
- Gao HD, Christensen OF, Madsen P, Nielsen US, Zhang Y, Lund MS, Su GS. Comparison on genomic predictions using three GBLUP methods and two single-step blending methods in the Nordic Holstein population. Genet Sel Evol. 2012;44:8. doi: 10.1186/1297-9686-44-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir B, Van Doormaal B, Kistemaker G. International genomic cooperation – North American perspective. Interbull Bull. 2010;41:71–76. [Google Scholar]
- Clark SA, Hickey JM, Daetwyler HD, van der Werf JHJ. The importance of information on relatives for the prediction of genomic breeding values and the implications for the makeup of reference data sets in livestock breeding schemes. Genet Sel Evol. 2012;44:4. doi: 10.1186/1297-9686-44-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habier D, Fernando RL, Dekkers JCM. The impact of genetic relationship information on genome-assisted breeding values. Genetics. 2007;177:2389–2397. doi: 10.1534/genetics.107.081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pszczola M, Strabel T, Mulder HA, Calus MPL. Reliability of direct genomic values for animals with different relationships within and to the reference population. J Dairy Sci. 2012;95:389–400. doi: 10.3168/jds.2011-4338. [DOI] [PubMed] [Google Scholar]