Abstract
Osmotic stress is a potent regulator of the normal function of cells that are exposed to osmotically active environments under physiologic or pathologic conditions. The ability of cells to alter gene expression and metabolic activity in response to changes in the osmotic environment provides an additional regulatory mechanism for a diverse array of tissues and organs in the human body. In addition to the activation of various osmotically- or volume-activated ion channels, osmotic stress may also act on the genome via a direct biophysical pathway. Changes in extracellular osmolarity alter cell volume, and therefore, the concentration of intracellular macromolecules. In turn, intracellular macromolecule concentration is a key physical parameter affecting the spatial organization and pressurization of the nucleus. Hyper-osmotic stress shrinks the nucleus and causes it to assume a convoluted shape, whereas hypo-osmotic stress swells the nucleus to a size that is limited by stretch of the nuclear lamina and induces a smooth, round shape of the nucleus. These behaviors are consistent with a model of the nucleus as a charged core/shell structure pressurized by uneven partition of macromolecules between the nucleoplasm and the cytoplasm. These osmotically-induced alterations in the internal structure and arrangement of chromatin, as well as potential changes in the nuclear membrane and pores are hypothesized to influence gene transcription and/or nucleocytoplasmic transport. A further understanding of the biophysical and biochemical mechanisms involved in these processes would have important ramifications for a range of fields including differentiation, migration, mechanotransduction, DNA repair and tumorigenesis.
Keywords: cell mechanics, chromatin, nucleolus, cartilage, osteoarthritis, transient receptor potential, ion channel
Osmotic Stress and Cell Physiology
The equilibrium osmolarity of the body is one of the most tightly controlled physiological parameters, regulated by a balance of hydration and solute concentrations [Bourque, 2008]. In addition to this system-level response, osmotic stress is a potent regulator of the normal function of cells that are exposed to osmotically active environments under physiologic or pathologic conditions. The ability at the cellular level to alter gene expression and metabolic activity in response to changes in the osmotic environment provides an additional regulatory mechanism for a diverse array of tissues and organs in the musculoskeletal system [Urban et al., 1993], kidney [Miyakawa et al., 1998], cardiovascular system [Zhou et al., 1997], and lung [Fedan et al., 1999], as well as in the protective response of cells subject to osmotic insult [Yancey et al., 1982], potentially following mechanical injury [Jayakumar et al., 2008].
For example, soft connective tissues such as articular cartilage and intervertebral disc can bear loads of several times body weight because they are osmotically pressurized and hydrated by the presence of large, negatively charged proteoglycans that attract a large number of counter-ions (e.g., Na+, K+, Ca++) that significantly increase the local osmolarity [Maroudas, 1976]. Furthermore, mechanical compression can increase the osmotic pressure acting on cells in the tissue due to exudation of in the interstitial water and subsequent consolidation of the negatively charged matrix [Lai et al., 1991], subsequently changing cell and nuclear volume [Guilak, 1995]. Cartilage homeostasis requires normal loading [Palmoski et al., 1979], indicating that the tissue responds to physical signals. Osmotic stress has been shown to alter gene expression [Chao et al., 2006], actin organization [Erickson et al., 2003] and calcium signaling [Erickson et al., 2001] in articular chondrocytes and may drive the transduction of mechanical loading into biological activity. For these reasons, there has been significant interest in studying the response of cells in cartilaginous tissues to alterations in osmolarity.
In most other tissues, osmolality is maintained in a narrow range by the kidneys and the blood supply. As a consequence of this, cells in the kidneys exist in a very hyper-osmotic environment relative to the rest of the body [Marsh and Azen, 1975]. Kidney dysfunction disrupts osmotic regulation with profound consequences, most notably for the central nervous system [Arieff and Guisado, 1976]. However, in response to injury, local tissue swelling and hydration may be altered dramatically, exposing cells to dynamic changes in osmolarity [Kawamata et al., 2007].
The mechanisms by which cells sense their osmotic environment are not fully understood and appear to involve a number of ion-channel based transduction pathways [Furst et al., 2002]. In particular, recent studies have provided evidence for the important role of the transient receptor potential (TRP) ion channels, as cellular osmosensors [Owsianik et al., 2006]. However, in addition to such membrane-mediated regulation of cell signaling, osmotic stress can have physical effects on cells that may extend to the cell nucleus, altering nuclear morphology and genome architecture directly [Albiez et al., 2006; Chalut et al., 2008; Delpire et al., 1985; Finan et al., 2008; Oswald et al., 2006]. Important nuclear processes such as repair, replication and transcription are all spatially organized, thus providing an alternative mechanism by which osmotic stress may influence cell biological function by rearranging the genome without the involvement of a conventional cell signaling pathway. This review summarizes the current state of the literature on the physical response of the cell nucleus to osmotic stress and presents a model of nuclear deformation under altering osmolarity to provide further insights into the mechanisms by which extracellular physical signals can modulate cellular gene expression and function.
Osmotic Properties of the Cell
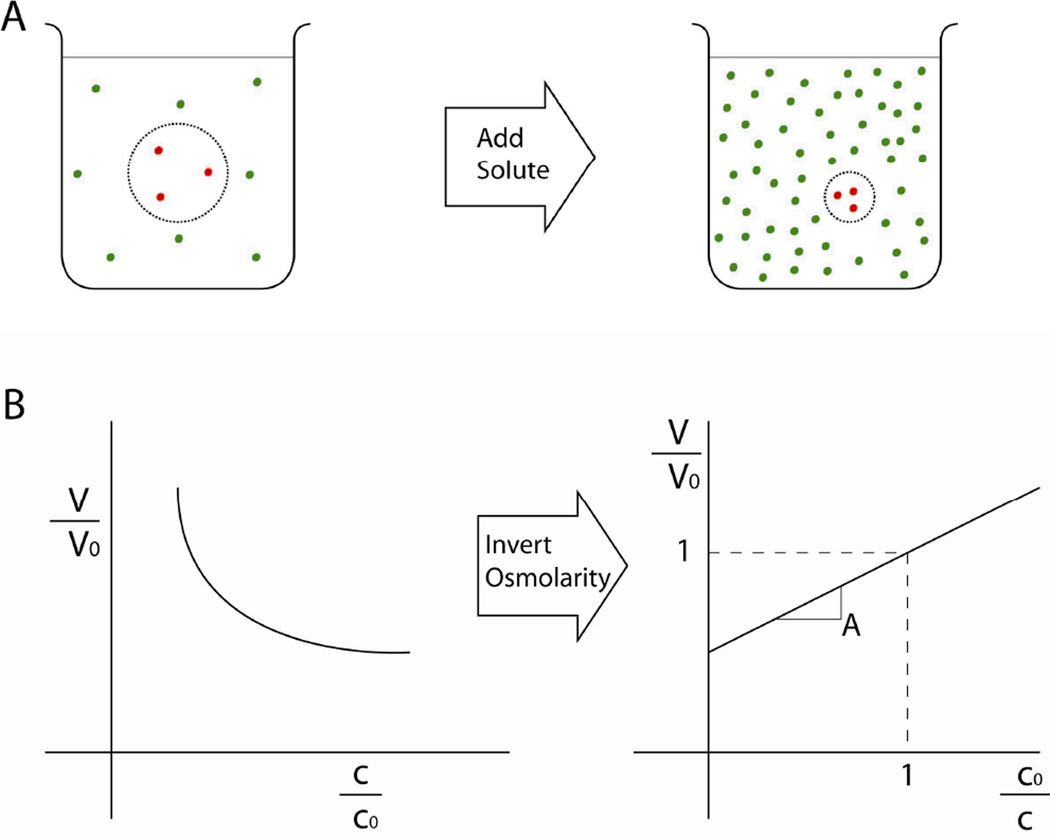
The physical response of the cell to osmotic stress has been characterized extensively [Lucke and McCutcheon, 1932]. With a few exceptions, cells behave as perfect osmometers when their active volume regulation is suppressed. A “perfect” osmometer is a theoretical model consisting of a body of solution enclosed by a semi-permeable membrane that allows free passage of water while blocking passage of solute molecules (Fig. 1). If the solute concentration in the bath surrounding the osmometer changes, water flows across the membrane until the concentration inside the osmometer matches the new environmental concentration. Hence, the volume of an osmometer is inversely proportional to the external osmolality, termed the Boyle-van’t Hoff relationship. At room temperature, cells exhibit this passive response [Guilak et al., 2002]. At body temperature, cells typically display some capacity to regulate their volume under osmotic load by pumping ions across the cell membrane so that water is osmotically obliged to cross the membrane in the same direction [Hendil and Hoffmann, 1974]. On a longer timescale, cells may also eject or produce neutral solute molecules such as taurine so that cell volume can be regulated independently of ion concentration [Hallows and Knauf, 1993]. More recently, theoretical models of cells have been proposed that may explain volume regulation from a pure physical standpoint [Albro et al., 2007].
Figure 1.
The relationship between extracellular osmolality and cell volume. (A) Schematic of a cell suspended in a solution. The dashed line represents the semi-permeable cell membrane, which allows passage of water but not solute molecules. As more solute is added to the extracellular bath, water is drawn out of the cell until the concentration inside the cell is equal to the extracellular concentration. Note that there is no mixing between the intracellular and extracellular solute species because solute molecules cannot cross the membrane. (B) The resulting relationship between cell volume and extracellular concentration. Cell volume, V, decreases as the extracellular osmolality, c, increases, creating a logarithmic relationship (both values are normalized to the iso-osmotic condition). Inverting the osmolality creates a linear plot, highlighting the inverse proportionality between cell volume and extracellular osmolality. This is the canonical presentation of the data and is known as a Ponder’s plot.
Structure and Osmotic Properties of the Nucleus
The nucleus has a number of physical characteristics that distinguish it from the cell, and thus affect its volumetric response to osmotic stress. Most notably, the nucleus follows the Boyle van’t Hoff relationship under hyper-osmotic conditions, but shows a nonlinear relationship between volume and inverse osmolality in the hypo-osmotic range [Finan et al., 2008]. The nucleus is surrounded by the nuclear envelope, which consists of two lipid bilayers known as the inner and outer nuclear membranes. A lumen separates the two membranes but they connect at nuclear pore complexes that penetrate the nuclear envelope and the outer nuclear membrane is continuous with the endoplasmic reticulum. Nuclear pore complexes form channels 9 nm wide in the nuclear envelope [Paine et al., 1975] and control transport of large macromolecules between the nucleus and the cytoplasm. As a result, the nuclear envelope functions as a molecular sieve [Peters, 1984], allowing free passage of ions and small solutes but inhibiting or blocking diffusion of large solute molecules. Cell shape [Feldherr and Akin, 1993] and calcium signaling (itself a consequence of osmotic stress [Erickson et al., 2001]) influence the size limit on passive diffusion across the nuclear envelope. The nuclear lamina is a layer of intermediate filament-type proteins that supports the nuclear envelope. It is composed of two families of lamin proteins: A-type and B-type. While both are localized primarily to the lamina, A-type lamins also exist at lower density throughout the nucleoplasm [Hozak et al., 1995].
Chromatin binds directly and via other proteins [Worman et al., 1988] to the lamins in the nuclear envelope. Chromosomes occupy distinct territories in the interphase nucleus [Cremer et al., 1993] and these territories are separated and penetrated by a chromatin free network of channels that terminate at nuclear pores [Schermelleh et al., 2008] called the inter-chromatin domain [Albiez et al., 2006]. The nucleus also contains numerous macro-molecular aggregates such as speckles and the largest sub-nuclear structure, the nucleolus. These structures function in the transcription and modification of RNA. Sub-nuclear bodies are confined to the interchromatin domain because they are too large to enter regions of dense chromatin [Cremer and Cremer, 2001].
Physical Mechanisms of Osmotic Signaling
Osmotic and mechanical stresses can regulate gene expression via biochemical pathways involving physical connections between the cell and extracellular matrix. It is now widely accepted that mechanical stresses also act on the genome through a biophysical pathway. Mechanical loads are transferred from extracellular matrix molecules via integrins to the actin cytoskeleton and intermediate filament network, which is in turn connected to the nuclear lamina. The nuclear lamina binds chromatin directly and via small proteins such as emerin [Dahl et al., 2008]. Therefore, there is a physical connection from the extracellular matrix to the genome along which mechanical stress can be transmitted [Maniotis et al., 1997]. Similarly, osmotic stress can also act on the genome via a direct, biophysical pathway.
At interphase, DNA is combined with histone proteins to form chromatin, which is folded and packaged hierarchically to fit inside the nucleus. The fundamental organizational unit of chromatin is the nucleosome. Four dimers of proteins called histones combine to from a core around which DNA wraps about 1.7 times to form a nucleosome. It is possible to extend the chromatin molecule under experimental conditions so that the nucleosomes can be clearly seen in a ‘beads on a string’ conformation in electromicrographs taken at very low salt. If the concentration of monovalent salt is increased to 5 mM, this conformation collapses into a zigzag arrangement of nucleosomes which collapses further as ion concentration is increased until the chromatin reaches a limiting level of conformation with the form of a continuous fiber 25 nm in diameter with no individual nucleosomes visible at about 60 mM NaCl [Thoma et al., 1979]. One consequence of this is that this chromatin condensation is not expected to change at this length scale in response to fluctuations in monovalent ion concentration under physiologic conditions because the physiologic concentration of monovalent ions is equivalent to 150 mM NaCl, far above the saturation threshold for this transition. A qualitatively similar transition is seen in response to increasing concentrations of divalent salt but at much lower concentrations, with fiber condensation beginning at 0.2 mM MgCl2 and saturating at 1 mM MgCl2. In situ, this 25 nm fiber is further folded in irregular patterns to generate either heterochromatin, which is densely packed or euchromatin, which is more diffuse. Heterochromatin is gene poor and biased towards transcriptional silence while euchromatin is rich in genes and biased towards transcriptional activation. There is a spatial distribution of chromatin density, with heterochromatin attached directly to the interior face of the nuclear lamina and euchromatin more common near the center of the nucleus.
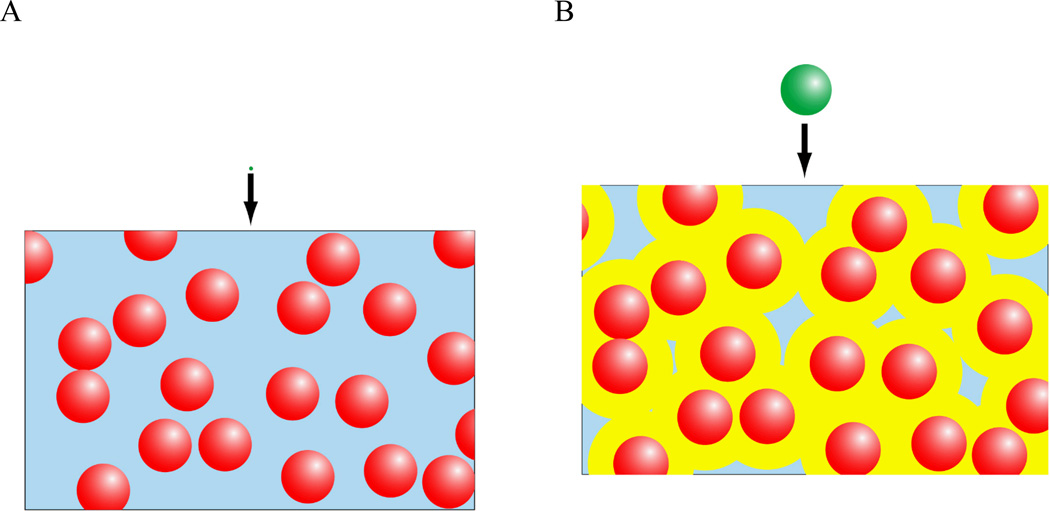
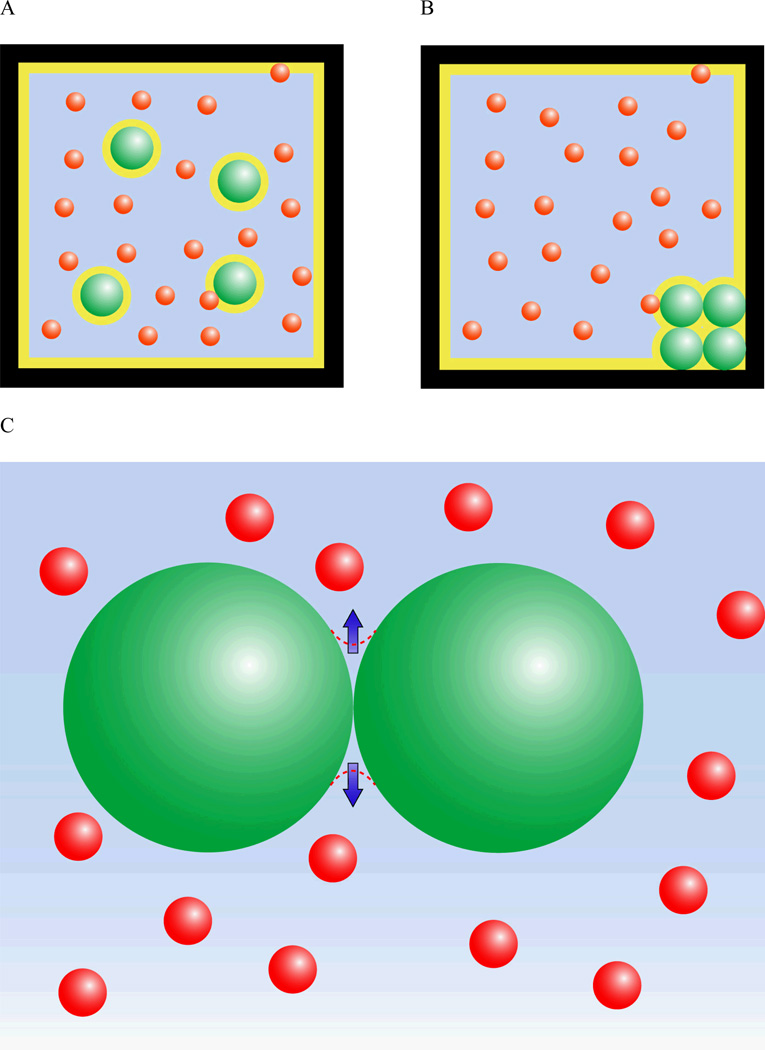
The coupling between the mechanical and osmotic properties of the nucleus thus provide several mechanisms by which extracellular, and subsequently intracellular, changes in osmolarity can influence the structure of the nuclear components. One potential mechanism by which extracellular osmolarity may influence the nucleus is through alterations in intracellular macromolecular concentrations, which can influence nuclear size and chromatin condensation. Macromolecule concentration has a powerful influence on the nucleus due to a phenomenon known as the excluded volume effect. Macromolecules by definition have finite radius. This means that the center of the molecule is excluded not only from space occupied by another molecule but also from a region one radius deep surrounding that other molecule. This region is the excluded volume. Excluded volume effects can greatly accelerate reaction kinetics because they raise the effective concentration of a macromolecule by reducing the volume through which it diffuses (Fig. 2). For example, the effective concentration for hemoglobin under physiological levels of crowding (approximately 300 mg of solute per 1 ml of water) is 80 times the actual concentration [Minton, 2001]. As two molecules approach one another, their excluded volumes overlap, creating an attractive force that can be understood in entropic or osmotic terms. From an entropic perspective, overlap of excluded volumes reduces the total excluded volume in the system (Fig. 3A). This increases the volume available to other solute molecules, allowing them to occupy a greater number of position states and become more disordered. This gain in entropy outweighs the loss of entropy due to ordering of the aggregate. The attractive force between molecules in a crowded solution can equivalently be modeled as an osmotic pressure. There is an inaccessible region around the contact between two spherical molecules that can be thought of as an osmometer (Fig. 3B). The concentration is zero in this region because solute molecules are too large to enter it so osmotic pressure tends to draw water out of it to equilibrate it with the rest of the solution. This pressure creates an attractive force at the site of contact. This concept of osmotic pressure due to steric exclusion can be extended to the more general geometry of a porous gel permeated by a solution of macromolecules. The concentration of macromolecules is lower in the pore fluid of the gel than in the solution outside the gel as larger macromolecules are excluded from the smallest pores and channels in the gel. This difference in concentration is quantified by the partition coefficient, which is the ratio between the concentration in the solution and the concentration in the pore fluid of the gel. This difference leads to an osmotic gradient that draws water out of the gel. One important conclusion from this uneven partition model of osmotic pressurization is that the pressure on the gel increases with increasing concentration of the macromolecule solution [Albro et al., 2007].
Figure 2.
The effects of molecular size on macromolecular crowding and apparent solute concentration. (A) In a solution of macromolecules, an infinitesimal test molecule diffuses through a volume equal to the total water volume (i.e., the blue region). (B) A molecule of finite size cannot approach closer than its radius to molecules of the background species (red) so it is excluded from the yellow region. For high levels of crowding, this dramatically reduces the water volume available to the test molecule, increasing the effective concentration.
Figure 3.
The effects of molecular aggregation on excluded volume and osmotic pressure. The diagram depicts large molecules (green) in solution with another smaller but not infinitesimal species (red) contained in a finite volume (the black box). The area in yellow is the excluded volume. The scenario on the left (A) is less favored entropically over the scenario on the right (B) because molecular crowding minimizes the excluded volume and maximizes the number of position states available to the red molecules. (C) A region shaped like a concave lens around the contact between the two spherical, green molecules is inaccessible to the smaller red molecules in solution. The concentration in this region is zero, while the concentration in the solution is finite so there is osmotic pressure drawing water out of this region. This pressure thus creates an attractive force between the two green molecules.
The nucleoplasm differs from the cytoplasm in that it is organized into macromolecular aggregates rather than membrane-bound vesicles. These aggregates are sustained by macromolecule-dependent excluded volume effects and dissolve in dilute buffer [Hancock, 2004]. Osmotically-induced changes in cell volume change the concentration of macromolecules and influence aggregation. For example, the nucleolus shrinks under hypo-osmotic stress [Delpire et al., 1985]. Excluded volume effects influence aggregation of long coiled molecules and chromatin condenses when the concentration of macromolecules increases [Richter et al., 2007]. Under hyper-osmotic stress, the intracellular concentration of macromolecules increases and chromatin condenses within the nucleus, enlarging the inter-chromatin domain [Albiez et al., 2006]. The geometry of the inter-chromatin domain is important because gene transcription occurs at the edge of the inter-chromatin domain and mRNA processing occurs at sub-nuclear bodies within the inter-chromatin domain. Altered chromatin compaction throughout the nucleus accumulates to change the volume of the nucleus. The uneven partition model of osmotic pressurization can replicate experimental observations of this process [Finan et al., 2008]. This model states that the nucleoplasm is compressed by an osmotic pressure that is proportional to intracellular macromolecule concentration and therefore, inversely proportional to cell volume. However, the uneven partition model of osmotic pressurization cannot describe all the observed nonlinear behaviors of the nucleus unless the action of the nuclear lamina is incorporated [Finan et al., 2008].
The Roles of the Nucleoplasm and Lamina
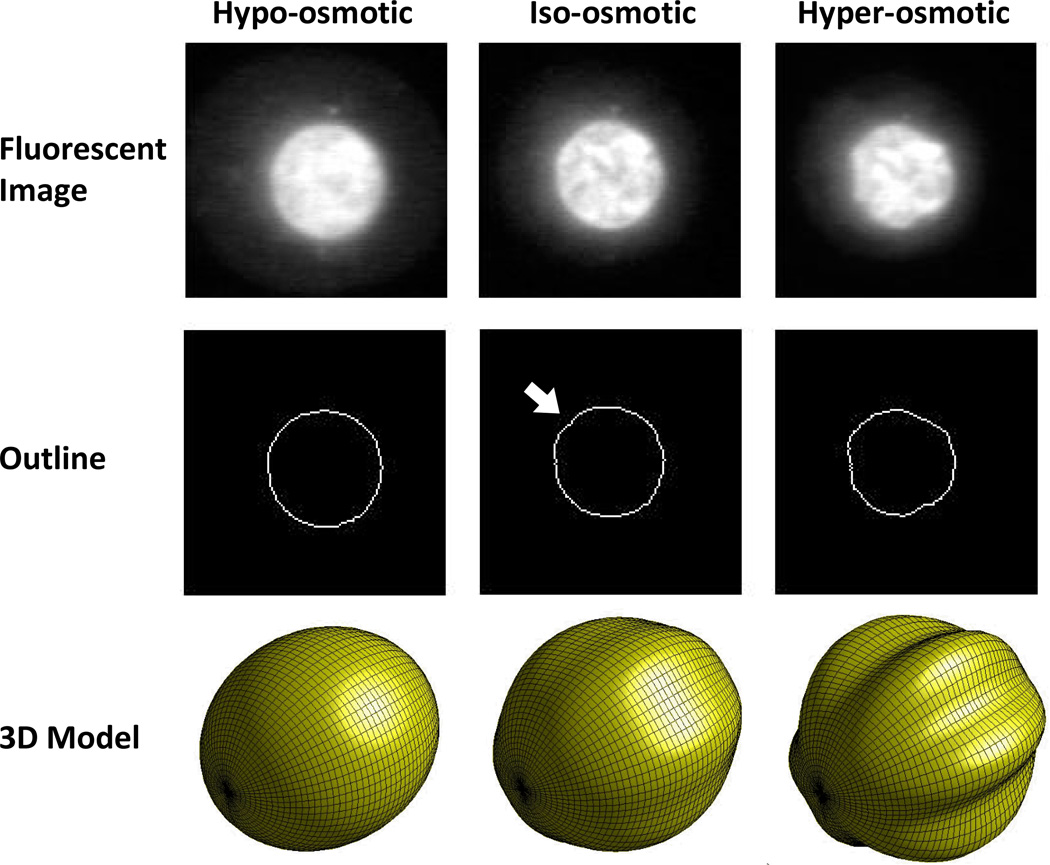
The nucleoplasm and the nuclear lamina have distinct mechanical properties that likely contribute to the overall response of the nucleus to osmotic stress. Nanoparticle rheology studies of the nucleoplasm have modeled it as a viscoelastic fluid [de Vries et al., 2007]. Micropipette aspiration experiments on Xenopus Oocyte nuclei designed to examine the difference between the lamina and the nucleoplasm report that both structures exhibit power law rheology, suggesting that they are close to the transition between solid and fluid, with the lamina being more elastic and the nucleoplasm being more viscous [Dahl et al., 2005]. Studies treating the whole nucleus as a single structure report a viscoelastic solid response [Guilak et al., 2000] and the nuclear lamina has been modeled as a two dimensional elastic solid [Rowat et al., 2005]. The nuclear lamina has also been observed to buckle under shear load [Rowat et al., 2006]. This characteristic is significant because it indicates resistance to shear loads, a property exclusive to solid materials. Taken together, these data are consistent with a model of the nucleus as a soft core (the nucleoplasm) encased in a stiffer shell (the lamina). In such a system, expansion or contraction of the core induces tension or compression, respectively, in the shell, and compressive stress above a certain threshold causes the shell to buckle. In this model, osmotic pressurization of the nucleoplasm is high enough at the in situ condition to initiate buckling of the lamina. If the extracellular osmolality rises, the cell shrinks and the osmotic pressurization of the nucleoplasm increases. In this case, the nucleus would contract, and the lamina would compress into a more convoluted shape. If the extracellular osmolality decreases, the cell enlarges and the pressurization of the nucleoplasm falls. This causes the nucleoplasm to expand, stretching the lamina into a smooth shape. The tension in the lamina inhibits any further expansion of the nucleus (Fig 4). Observed trends in the evolution of nucleus size and shape under osmotic load in articular chondrocytes conform to this model [Ateshian et al., 2006; Finan et al., 2008], although osmotic pressurization of a core/shell structure cannot explain all the possible permutations of nuclear morphology. Nonetheless, this model provides a useful representation of nuclear morphology under certain experimental conditions.
Figure 4.
Changes in nuclear morphology with osmotic stress. The central column depicts the iso-osmotic or equilibrium condition (380 mOsm). The osmotic pressurization of the nucleus is barely sufficient to induce mild buckling of the nuclear lamina, as evidenced by the mild undulation in the outline of the nucleus highlighted by the white arrow. The bottom panel in the central column shows a 3D view of a mildly post-buckled spheroid. The column on the left depicts the hypo-osmotic condition (180 mOsm). The undulation has disappeared and the outline of the nucleus is now smooth. The column on the right depicts the hyper-osmotic condition (580 mOsm). Now there are pronounced undulations along the entire circumference of the nuclear outline. The bottom panel in this column shows a 3D view of a spheroidal geometry with pronounced longitudinal buckling. These reconstructions are hypothesized to be representative of the nuclear outlines depicted in the middle row.
Recent advances in theory and instrumentation have brought quantitative application of this model to the nucleus within reach. The stiffer the substrate beneath a thin buckled shell, the smaller and more numerous the ripples in the buckled layer will be. There are now simple, powerful mechanical theories that relate the post-buckled shape of a thin layer on a soft substrate to the relative stiffness of the layer and the substrate [Cerda and Mahadevan, 2003]. A theory for the evolution of the post-buckled shape was recently presented that describes how multiple, evenly distributed undulations can collapse into a single, large invagination under certain conditions [Pocivavsek et al., 2008]. This expands the range of geometric features that can be modeled as buckling phenomena. The specific case of a core/shell structure was recently modeled [Yin et al., 2008]. Such models, in combination with precise measurements of nuclear geometry, may facilitate separate measurements of the properties of the nuclear lamina and the nucleoplasm without the need for harsh treatments to physically separate the two structures. Such precise geometric measurements are currently frustrated by the limited resolution of confocal microscopy. However, techniques have recently emerged that overcome the traditional resolution limitations of light microscopy [Schermelleh et al., 2008] and may provide new insights into such phenomena.
The Role of the Actin Cytoskeleton in Nuclear Properties
The shape and mechanics of the nucleus also depend on contractility in the actin cytoskeleton. This dependence is another mechanism by which osmotic stress may act on the nucleus, since hypo-osmotic stress can trigger disassembly of the actin cytoskeleton [Erickson et al., 2003]. Actin filaments assemble into one of two structures: bundled stress fibers or cross-linked filament networks. Actin cross-linking proteins influence the nature of the actin structure assembled. Some, such as filamin, join actin filaments at an angle to form a gel. Others, such as α-actinin, join actin filaments in a parallel orientation to form thick bundles that can exert tension when acted upon by myosin motor proteins. The physical properties of the cell environment affect the actin cytoskeleton. Adherent cells pull on their substrate with actin-myosin bundles. As the stiffness of the substrate increases, so does the tension and concomitant bundling of the actin cytoskeleton. This tension is transferred into the nuclear lamina, where it alters nuclear shape and increases nucleocytoplasmic transport [Feldherr and Akin, 1993]. It is possible nucleocytoplasmic transport increases in a similar fashion under hypo-osmotic load as a result of tension introduced to the lamina by swelling of the nucleoplasm.
Articular chondrocytes are a simple system for osmotic loading experiments because they remain rounded with minimal actin bundling in monolayer culture for up to 48 hours so the osmotic response of the nucleus is not greatly influenced by actin organization. However, this behavior is unusual and mammalian cells in monolayer culture typically spread aggressively and form highly bundled, contractile actin cytoskeletons on conventional stiff substrates such as glass or plastic. Hyper-osmotic loading of such cells causes shrinkage primarily in the direction normal to the coverslip with very little change in cross sectional area since the perimeter of the nucleus is constrained by actin attachments in this plane [Albiez et al., 2006]. The molecular make-up of attachments between the actin cytoskeleton and the nucleus have been reviewed in detail elsewhere [Worman and Gundersen, 2006]. In summary, actin and intermediate filaments associate with nesprin proteins that bridge the lumen between the inner and outer nuclear membranes and bind to SUN proteins sitting in the inner nuclear membrane. The SUN proteins bind lamins on the inside of the inner nuclear membrane which in turn bind chromatin directly and via lamin associated proteins such as emerin. There is also an interesting biochemical link between actin contractility and the nucleus. Actin contractility signals also regulate histone acetylation [Kim et al., 2005]. Histone acetylation is a chromatin modification with complex biological consequences, primarily involving gene activation. It also decondenses chromatin, leading to changes in genome architecture [Toth et al., 2004].
Conclusions
The biological significance of influence of mechanical and osmotic signals on nuclear morphology and membrane mechanics are profound. Diseases caused by lamin mutations such as Hutchinson-Gilford Progeria Syndrome lead to accelerated aging in tissues throughout the body. Cells with the mutation exhibit altered mechanical properties in the nucleus, inhibition of DNA repair and misshapen nuclei [Dahl et al., 2006]. Similar changes have been observed in the nuclei of genetically normal aged subjects [Scaffidi and Misteli, 2006], suggesting that nuclear mechanics and DNA repair are intertwined even in the absence of a mutation. Lamin deficient cells exhibit defective mechanotransduction in monolayer culture [Lammerding et al., 2004]. Most mature cells express A-type lamins but expression is low in stem cells [Constantinescu et al., 2006]. It has been suggested that this allows stem cells to infiltrate tissues more easily because it makes the largest organelle in the cell more deformable. Expression of A-type lamins is also low in neutrophils, possibly for the same reason. In this context, it is intriguing to note that A-type lamin expression is generally reduced in cancerous cells. The application of novel mechanical or osmotically based experiments, coupled with appropriate structural information and mathematical models of the cell and nucleus, could yield important new insights into the mechanics of the nuclear lamina in these cell types.
The nuclear interior has a complex, heterogeneous architecture and that architecture determines biological function. One controversial paradigm holds that this architecture arises from an elaborate pattern of binding domains on an intricate molecular scaffold called the nuclear matrix [Pederson, 2000]. However, a new paradigm has emerged recently stating that nuclear architecture arises stochastically from self-organization of molecular aggregates [Kaiser et al., 2008]. The consequences of this for transduction of physical signals are profound. Aggregation of molecules is highly sensitive to macromolecule concentration so macromolecule concentration is the key physical parameter in this new paradigm. Osmotic stress changes cell volume, directly altering macromolecule concentration. This means that osmotic stress is an essential component in the emerging picture of how extracellular physical signals act on the genome. Osmotic pressurization of a gel due to uneven partition provides a physical model for osmotically induced changes in nuclear size. If the lamina is represented separately as a shell encasing the gel, the model also describes osmotically-induced changes in shape via buckling phenomena. Quantitative application of this model to osmotic loading experiments offers the prospect of novel insights into the mechanics of the nucleus in general and the lamina in particular. Such insights could yield increased understanding of DNA repair, migration, differentiation, mechanotransduction and tumorigenesis. Despite this great promise, there is relatively little published work in the field so opportunities for fresh discoveries are plentiful.
Acknowledgments
Supported by NIH grants AR50245, AG15768, AR48182, and AR48852.
References
- Albiez H, Cremer M, Tiberi C, Vecchio L, Schermelleh L, Dittrich S, Kupper K, Joffe B, Thormeyer T, von Hase J, Yang S, Rohr K, Leonhardt H, Solovei I, Cremer C, Fakan S, Cremer T. Chromatin domains and the interchromatin compartment form structurally defined and functionally interacting nuclear networks. Chromosome Res. 2006;14:707–733. doi: 10.1007/s10577-006-1086-x. [DOI] [PubMed] [Google Scholar]
- Albro MB, Chahine NO, Caligaris M, Wei VI, Likhitpanichkul M, Ng KW, Hung CT, Ateshian GA. Osmotic loading of spherical gels: a biomimetic study of hindered transport in the cell protoplasm. J Biomech Eng. 2007;129:503–510. doi: 10.1115/1.2746371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieff AI, Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976;10:104–116. doi: 10.1038/ki.1976.82. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Likhitpanichicul M, Hung CT. A mixture theory analysis for passive transport in osmotic loading of cells. Journal of Biomechanics. 2006;39:464–475. doi: 10.1016/j.jbiomech.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Cerda E, Mahadevan L. Geometry and physics of wrinkling. Phys Rev Lett. 2003;90:074302. doi: 10.1103/PhysRevLett.90.074302. [DOI] [PubMed] [Google Scholar]
- Chalut K, Chen S, Finan J, Giacomelli M, Guilak F, Leong K, Wax A. Label-free, high-throughput measurements of dynamic changes in cell nuclei using angle-resolved low coherence interferometry. Biophys J. 2008 doi: 10.1529/biophysj.107.124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- Cremer T, Kurz A, Zirbel R, Dietzel S, Rinke B, Schrock E, Speicher MR, Mathieu U, Jauch A, Emmerich P, Scherthan H, Ried T, Cremer C, Lichter P. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol. 1993;58:777–792. doi: 10.1101/sqb.1993.058.01.085. [DOI] [PubMed] [Google Scholar]
- Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J. 2005;89:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries AH, Krenn BE, van Driel R, Subramaniam V, Kanger JS. Direct observation of nanomechanical properties of chromatin in living cells. Nano Lett. 2007;7:1424–1427. doi: 10.1021/nl070603+. [DOI] [PubMed] [Google Scholar]
- Delpire E, Duchene C, Goessens G, Gilles R. Effects of osmotic shocks on the ultrastructure of different tissues and cell types. Exp Cell Res. 1985;160:106–116. doi: 10.1016/0014-4827(85)90240-x. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34:1527–1535. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis Cartilage. 2003;11:187–197. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- Fedan JS, Yuan LX, Chang VC, Viola JO, Cutler D, Pettit LL. Osmotic regulation of airway reactivity by epithelium. J Pharmacol Exp Ther. 1999;289:901–910. [PubMed] [Google Scholar]
- Feldherr CM, Akin D. Regulation of nuclear transport in proliferating and quiescent cells. Exp Cell Res. 1993;205:179–186. doi: 10.1006/excr.1993.1073. [DOI] [PubMed] [Google Scholar]
- Finan JD, Chalut KJ, Wax A, Guilak F. Nonlinear Osmotic Properties of the Cell Nucleus. Ann Biomed Eng. 2008 doi: 10.1007/s10439-008-9618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst J, Gschwentner M, Ritter M, Botta G, Jakab M, Mayer M, Garavaglia L, Bazzini C, Rodighiero S, Meyer G, Eichmuller S, Woll E, Paulmichl M. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch. 2002;444:1–25. doi: 10.1007/s00424-002-0805-1. [DOI] [PubMed] [Google Scholar]
- Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529–1541. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82:720–727. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000;269:781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Knauf PA. Principles of Cell Volume Regulation. In: Strange K, editor. Cellular and Molecular Physiology of Cell Volume Regulation. Boa Raton, FL: CRC Press; 1993. [Google Scholar]
- Hancock R. A role for macromolecular crowding effects in the assembly and function of compartments in the nucleus. J Struct Biol. 2004;146:281–290. doi: 10.1016/j.jsb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Hendil KB, Hoffmann EK. Cell-Volume Regulation in Ehrlich Ascites Tumor-Cells. Journal of Cellular Physiology. 1974;84:115–125. doi: 10.1002/jcp.1040840113. [DOI] [PubMed] [Google Scholar]
- Hozak P, Sasseville AM, Raymond Y, Cook PR. Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. J Cell Sci. 1995;108(Pt 2):635–644. doi: 10.1242/jcs.108.2.635. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol. 2008;67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- Kaiser TE, Intine RV, Dundr M. De Novo Formation of a Subnuclear Body. Science. 2008 doi: 10.1126/science.1165216. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Mori T, Sato S, Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg Focus. 2007;22:E5. doi: 10.3171/foc.2007.22.5.6. [DOI] [PubMed] [Google Scholar]
- Kim YB, Yu J, Lee SY, Lee MS, Ko SG, Ye SK, Jong HS, Kim TY, Bang YJ, Lee JW. Cell adhesion status-dependent histone acetylation is regulated through intracellular contractility-related signaling activities. J Biol Chem. 2005;280:28357–28364. doi: 10.1074/jbc.M412608200. [DOI] [PubMed] [Google Scholar]
- Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke B, McCutcheon M. The living cell as an osmotic system and its permeability to water. Physiological Reviews. 1932;12:0068–0139. [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Azen SP. Mechanism of NaCl reabsorption by hamster thin ascending limbs of Henle's loop. Am J Physiol. 1975;228:71–79. doi: 10.1152/ajplegacy.1975.228.1.71. [DOI] [PubMed] [Google Scholar]
- Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Woo SK, Chen CP, Dahl SC, Handler JS, Kwon HM. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am J Physiol. 1998;274:F753–F761. doi: 10.1152/ajprenal.1998.274.4.F753. [DOI] [PubMed] [Google Scholar]
- Oswald ES, Chao PG, Bulinski JC, Ateshian GA, Hung CT. Chondrocyte nuclear response to osmotic loading. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3659–3661. doi: 10.1109/IEMBS.2006.259394. [DOI] [PubMed] [Google Scholar]
- Owsianik G, D'Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature. 1975;254:109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Palmoski M, Perricone E, Brandt KD. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979;22:508–517. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- Pederson T. Half a century of "the nuclear matrix". Mol Biol Cell. 2000;11:799–805. doi: 10.1091/mbc.11.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Nucleocytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. Embo Journal. 1984;3:1831–1836. doi: 10.1002/j.1460-2075.1984.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek L, Dellsy R, Kern A, Johnson S, Lin B, Lee KY, Cerda E. Stress and fold localization in thin elastic membranes. Science. 2008;320:912–916. doi: 10.1126/science.1154069. [DOI] [PubMed] [Google Scholar]
- Richter K, Nessling M, Lichter P. Experimental evidence for the influence of molecular crowding on nuclear architecture. J Cell Sci. 2007;120:1673–1680. doi: 10.1242/jcs.03440. [DOI] [PubMed] [Google Scholar]
- Rowat AC, Foster LJ, Nielsen MM, Weiss M, Ipsen JH. Characterization of the elastic properties of the nuclear envelope. Journal of the Royal Society Interface. 2005;2:63–69. doi: 10.1098/rsif.2004.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowat AC, Lammerding J, Ipsen JH. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys J. 2006;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. [Google Scholar]
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, Leonhardt H, Sedat JW. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320:1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F, Koller T, Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth KF, Knoch TA, Wachsmuth M, Frank-Stohr M, Stohr M, Bacher CP, Muller G, Rippe K. Trichostatin A-induced histone acetylation causes decondensation of interphase chromatin. J Cell Sci. 2004;117:4277–4287. doi: 10.1242/jcs.01293. [DOI] [PubMed] [Google Scholar]
- Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Gundersen GG. Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 2006;16:67–69. doi: 10.1016/j.tcb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Yuan J, Blobel G, Georgatos SD. A lamin B receptor in the nuclear envelope. Proc Natl Acad Sci U S A. 1988;85:8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yin J, Cao Z, Li C, Sheinman I, Chen X. Stress-driven buckling patterns in spheroidal core/shell structures. Proc Natl Acad Sci U S A. 2008;105:19132–19135. doi: 10.1073/pnas.0810443105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YY, Yao JA, Tseng GN. Role of tyrosine kinase activity in cardiac slow delayed rectifier channel modulation by cell swelling. Pflugers Arch. 1997;433:750–757. doi: 10.1007/s004240050341. [DOI] [PubMed] [Google Scholar]