Abstract
INTRODUCTION
The association of murine asthma with adiposity may be mediated by adiponectin, an anti-inflammatory adipokine with reduced serum concentrations in the obese. We studied whether serum adiponectin concentration was associated with human asthma and explained the association between adiposity and asthma, particularly in women and in pre-menopausal women.
METHODS
A cross-sectional analysis of 2,890 eligible subjects at year 15 of the Coronary Artery Risk Development in Young Adults (CARDIA) cohort and its YALTA ancillary study, and had either current asthma or never asthma at that evaluation, was performed. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2. Multivariable logistic regression analysis was performed with dependent variable current asthma status.
RESULTS
Women, but not men, with current asthma had lower mean unadjusted serum adiponectin concentration than those with never asthma (p < 0.001; p for sex interaction < 0.001). Similarly, current asthma was related to obese status only in women (OR 3.31, 95% CI 2.00, 5.46, p for sex interaction 0.004); this association was little affected by adjusting for serum adiponectin. Prevalence of current asthma in pre-menopausal women was reduced in the highest vs. lowest tertile of serum adiponectin concentration (OR 0.46, 95% CI 0.26, 0.84, p 0.03), after adjusting for BMI. However, the interaction between serum adiponectin concentration and BMI category on current asthma status was not significant in pre-menopausal women or women overall.
DISCUSSION
High serum adiponectin concentration may protect against current asthma in pre-menopausal women, but does not explain the association between asthma and adiposity.
Keywords: Asthma, Adiposity, Body mass index, Waist circumference, Adiponectin
INTRODUCTION
The incidence of obesity is dramatically increasing in the United States and worldwide in both children and adults (1). Recently, it has been shown that adiposity is a risk factor for asthma, particularly in women (2–6). Mechanistic explanations that have been advanced to explain this association include mechanical effects (such as lack of tidal stretch leading to latching of airway smooth muscle), immunological effects of adiposity, as well as genetic, hormonal (particularly the effect of female sex hormones), and environmental effects (the latter including change in dietary factors and increased exposure to indoor allergens from increased sedentary habits) (7).
It has been recently demonstrated that adiposity is associated with chronic low-grade systemic inflammation (8). This inflammatory state is related to adipokines – proteins mainly produced by adipocytes – which may be pro-inflammatory, such as leptin, or anti-inflammatory, such as adiponectin. Our recent cross-sectional analysis has suggested that high serum concentrations of leptin may be an independent risk factor for asthma in pre-menopausal women (9). Similarly, it has been hypothesized that decreased serum concentrations of adiponectin observed in obese humans may also contribute to the propensity towards asthma in this population (10).
The study objective was to determine whether serum adiponectin concentration was associated with asthma and explained the association between adiposity and asthma, particularly in women and in pre-menopausal women. This was evaluated using the prospective Coronary Artery Risk Development in Young Adults (CARDIA) cohort and its Young Adult Longitudinal Trends in Antioxidants (YALTA) ancillary study. A better understanding of the role of adiponectin in asthma may suggest newer ways of treating asthma in the expanding obese population.
METHODS
Study design
This is a cross-sectional analysis of the CARDIA and YALTA datasets. This longitudinal epidemiologic study focusing on development of cardiovascular disease risk is funded by the National Heart, Lung, and Blood Institute (NHLBI). During 1985 and 1986, CARDIA recruited 5,115 black and white men and women, aged 18 to 30 years from four clinical centers in Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA, with follow-up examinations completed 2, 5, 7, 10, and 15 years later. At the year 15 examination, 3,672 persons were reexamined, constituting nearly 74% of survivors.
Inclusion and exclusion criteria
All participants with current or never asthma at the year 15 examination were included. Former asthma, pregnancy, not fasting for 8 hours, possible renal disease (men with serum creatinine > 1.8 mg/dL and women with serum creatinine > 1.5 mg/dL) were exclusion criteria. Additionally, those with missing data for independent variables and covariates were excluded from the analysis.
Independent and Dependent variables
Independent variables measured at year 15 evaluation, included standard categories of body mass index (BMI < 18.5 kg/m2 or underweight; BMI 18.5–24.9 kg/m2 or normal weight; BMI 25–29.9 kg/m2 or overweight; and BMI ≥ 30 kg/m2 or obese), quartiles of waist circumference (which may correlate better with visceral adiposity than global adiposity), as well as three categories (tertiles) of serum adiponectin concentration.
The dependent variable measured at the same time was current asthma status, defined as 1) a subject report of taking a medication for asthma at year 15 evaluation or 2) a self-reported doctor or nurse asthma diagnosis along with asthma symptoms in the year previous to the year 15 evaluation. Those who denied both taking an asthma medication and a provider-given diagnosis of asthma at all evaluations (years 0, 2, 5, 7, 10, and 15) were classified as never asthma.
Covariates
The following variables at year 15 were evaluated as covariates - diabetes mellitus, insulin resistance, self-reported smoking status, race, age, and physical activity level. The above listed covariates were selected since they have been shown to affect either asthma status or serum adiponectin concentration (11, 12). Further details about the covariates are available in the online data supplement.
Study Measurements
At the year 15 evaluation, self-reported information was obtained from study subjects using standardized questionnaires. Certified technicians measured height (within 0.5 cm), weight (within 0.09 kg) and waist circumference (within 0.5 cm) using standardized techniques. Body mass index (BMI) was defined as weight/height2 in kg/m2. Blood samples were collected after at least 8 hours of fasting. Adiponectin was measured as part of the YALTA ancillary study in serum by radioimmunoassay at Linco Research, Inc. using a polyclonal antibody raised in a rabbit and purified recombinant adiponectin standards with an effective range of 0.2 to 40 mg/L (13). This assay measures total adiponectin and not the various molecular weight forms of adiponectin. Further details about the study measurements are available in the online data supplement.
Statistical Analysis
We performed descriptive analyses (to calculate frequency distributions), univariate analyses (such as chi-square and t tests for categorical and continuous variables respectively), and multivariable logistic regression analyses using asthma status (current vs. never) as the categorical dependent variable. Consistent with our a priori hypothesis, we examined subgroups defined by sex and menopausal status and performed formal tests for interaction. Issues of confounding were resolved by including the above-described covariates to the models. A two-sided p-value of < 0.05 was considered statistically significant. All statistical analysis was done using the Statistical Analysis Software (SAS) package version 9.1 (Cary, NC). This study was approved by the University of New Mexico Human Research Review Committee, Albuquerque, NM.
RESULTS
Among the 3, 672 participants in the CARDIA year 15 evaluation, 424 met the exclusion criteria, including 404 participants with former asthma. An additional 358 participants were excluded either because of missing serum adiponectin values (n = 317) or missing covariates (n = 41). Among the 2,890 eligible participants whose data was analyzed, 246 had current asthma, and 2,644 had never asthma. This population included 1,603 women (55%). Of the 1,554 women for whom these data were available, 1,391 (90%) were pre-menopausal. As shown in Table 1, participants with current asthma had significantly higher mean values for body mass index (BMI), waist circumference, and insulin resistance, lower mean values of FEV1/FVC ratio (obtained 5 years prior to the year 15 evaluation), and were more likely to be women and diabetic, when compared to those with never asthma (p values for all comparisons < 0.05).
Table 1.
Distribution of selected characteristics according to asthma status (all variables assessed at year 15, except where noted)
| Characteristics | Current Asthma (n = 246) | Never Asthma (n = 2,644) | p value |
|---|---|---|---|
| Mean Age (in years) | 40.1 ± 3.6 | 40.2 ± 3.6 | 0.61 |
| Women | 168/246 (68%) | 1,435/2,644 (54%) | <0.001 |
| Pre-menopausal women | 132/163 (81%) | 1,259/1,391 (91%) | <0.001 |
| White race | 119/246 (48%) | 1,443/2,644 (55%) | 0.06 |
| Current Smokers | 53/246 (22%) | 555/2,644 (21%) | 0.84 |
| Mean FEV1/FVC ratio 5 years prior | 0.79 ± 0.18 (n = 210) | 0.83 ± 0.11 (n = 2,308) | 0.01 |
| Geometric Mean Physical Activity | 186.4; 95% C.I. 51.6, 672.8 | 221.5; 95% C.I. 61.5, | 0.04 |
| Score (Exercise units) | 797.7 | ||
| History of Diabetes Mellitus | 21/246 (9%) | 141/2,644 (5%) | 0.04 |
| Geometric Mean Insulin Resistance | 2.9; 95% C.I. 1.6, 5.3 | 2.5; 95% C.I. 1.3, 4.8 | 0.002 |
| Use of Cholesterol Lowering | 9/246 (4%) | 60/2,643 (2%) | 0.17 |
| Medication | |||
| Mean Body Mass Index (kg/m2) | 31.6 ± 8.3 | 28.4 ± 6.5 | <0.001 |
| Men | 28.3 ± 5.3 (n = 78) | 28.2 ± 5.3 (n = 1,209) | 0.81 |
| Women | 33.2 ± 9.0 (n = 168) | 28.6 ±7.4 (n = 1,435) | <0.001 |
| Pre-menopausal women | 32.4 ± 9.2 (n = 132) | 28.3 ± 7.2 (n = 1,259) | <0.001 |
| Mean Waist Circumference (cm.) | 93.8 ± 16.1 | 88.9 ± 14.8 | <0.001 |
| Men | 93.6 ± 12.7 (n = 78) | 93. 8 ± 13.1 (n = 1,209) | 0.91 |
| Women | 93.8 ± 17.4 (n = 168) | 84.8 ±14.9 (n = 1,435) | <0.001 |
| Pre-menopausal women | 92.3 ± 18.0 (n = 132) | 84.2 ± 14.7 (n = 1,259) | <0.001 |
| Geometric Mean Serum Adiponectin (mg/L) | 8.9; 95% C.I. 4.9, 16.1 | 9.0; 95% C.I. 4. 8, 16.8 | 0.77 |
| Men | 8.0; 95% C.I. 4.5, 14.3 (n = 78) | 7.0; 95% C.I. 3.8, 12.9 (n = 1,209) | 0.06 |
| Women | 9.3; 95% C.I. 5.1, 16.9 (n = 168) | 11.1; 95% C.I. 6.3, 19.3 (n = 1,435) | <0.001 |
| Pre-menopausal women | 9.8; 95% C.I. 5.7, 16.8 (n = 132) | 11.3; 95% C.I. 6.5, 19.7 (n = 1,259) | 0.005 |
Note 1: For untransformed variables, the data are expressed as mean ± S.D (n); for geometric means, the data are expressed as mean; 95% confidence intervals (n).
Note 2: The total n value for various comparisons is not uniform because of missing data. The n value for a specific cell is listed in parenthesis when it differed from the n value at the top of the column.
As has been shown previously, serum adiponectin concentration correlated better with waist circumference than with BMI (Spearman correlation coefficient of − 0.42 vs. − 0.31 respectively) (11). Women had higher mean serum adiponectin concentrations than men (geometric means 10.9 mg/L vs. 7.1 mg/L; p <0.001). Among women, pre-menopausal women had higher mean serum adiponectin concentration than post-menopausal women (geometric mean 11.2 mg/L vs. 8.9 mg/L; p < 0.001). Although there was no difference in mean serum adiponectin concentrations between the current asthma and never asthma groups in men and women combined, women with current asthma had lower mean serum adiponectin concentrations compared to women with never asthma (geometric means 9.3 vs. 11.1 mg/L; p < 0.001, Table 2). This association between current asthma and low serum adiponectin concentration was not seen in men (p for interaction between sex and serum adiponectin in estimation of current asthma < 0.001).
Table 2.
Association between risk for current asthma and adiposity with and without adjustment for serum adiponectin concentration
| Models for adiposity measure | Women | Men | |||
|---|---|---|---|---|---|
| n with current asthma/Total n | Odds Ratio (95% C.I.) | n with current asthma/Total n | Odds Ratio (95% C.I.) | ||
| BMI | Under & Normal weight | 31/585 | 1 | 18/356 | 1 |
| Over weight | 37/420 | 1.68 (1.01, 2.79) | 39/559 | 1.54 (0.84, 2.80) | |
| Obese | 100/598 | 3.31 (2.00, 5.46) | 21/372 | 1.33 (0.61, 2.89) | |
| p-value for Wald χ2 | <0.001 | 0.37 | |||
| BMI adjusted for Adiponectin | Under & Normal weight | 31/585 | 1 | 18/356 | 1 |
| Over weight | 37/420 | 1.63 (0.98, 2.74) | 39/559 | 1.64 (0.89, 3.02) | |
| Obese | 100/598 | 3.19 (1.91, 5.32) | 21/372 | 1.45 (0.66, 3.17) | |
| p-value for Wald χ2 | <0.001 | 0.28 | |||
Note 1: Covariates adjusted in each logistic regression model include age, race, smoking status, ln(physical activity), history of diabetes, and ln(insulin resistance). In addition, when indicated, group-specific tertiles of serum adiponectin concentration were also adjusted.
Note 2: The interaction between sex and BMI on current asthma was significant (p value 0.004 in both models).
Note 3: Adiponectin tertile cut points are 1.2–9, 9–14.4, and 14.4–94.8 mg/L for women and 1–6, 6–9.6, and 9.6–64 mg/L for men.
Note 4: Similar results were obtained when the underweight and normal weight categories were analyzed separately with the latter serving as the referent category.
Note 5: BMI categories are defined as follows - Underweight - BMI < 18.5 kg/m2; Normal weight - BMI 18.5–24.9 kg/m2; Overweight - BMI 25–29.9 kg/m2; Obese - BMI ≥ 30 kg/m2.
Further, since this population included only 42 underweight participants (1%) and since initial evaluation showed a linear and not a bowl-shaped relationship between BMI categories and asthma risk, underweight and normal weight subjects were combined to form the referent category for subsequent analyses in Tables 2 and 4. As shown in Table 2, current asthma was associated with overweight and obese status for women with odds ratio of 1.68 (95% C.I. 1.01, 2.79) and of 3.31 (95% C.I. 2.00, 5.46) respectively. The formal test for interaction between sex and BMI category in estimation of current asthma was significant (p =0.004). Similarly, current asthma was associated with the third and fourth quartiles of waist circumference for women with odds ratio of 2.58 (95% C.I. 1.52, 4.38) and of 3.09 (95% C.I. 1.77, 5.37) respectively (expanded Table 2, online data supplement). The formal test for interaction between sex and waist circumference category in estimation of current asthma was similarly significant (p = 0.02). However, the association between the risk for current asthma and adiposity (BMI or waist circumference) was not affected by adjusting for the serum adiponectin concentration in women. Similar results were obtained when the analysis was limited to pre-menopausal women alone (expanded Table 2, online data supplement).
Table 4.
Stratified analysis of the association between current asthma and Body Mass Index (BMI) categories in pre-menopausal women, according to the various tertiles of serum adiponectin concentration
| Tertiles of adiponectin | BMI Category | Pre-menopausal women | |
|---|---|---|---|
| n with current asthma/total n | n with current asthma/total n | ||
| Tertile 1 | Under & Normal weight | 5/88 | 1 |
| Over weight | 10/130 | 1.82 (0.58, 5.72) | |
| Obese | 47/277 | 5.77 (1.96, 17.01) | |
| p-value for Wald χ2 | 0.001 | ||
| Tertile 2 | Under & Normal weight | 15/186 | 1 |
| Over weight | 14/145 | 1.21 (0.56, 2.62) | |
| Obese | 22/156 | 1.93 (0.87, 4.24) | |
| p-value for Wald χ2 | 0.24 | ||
| Tertile 3 | Under & Normal weight | 8/259 | 1 |
| Over weight | 8/98 | 2.30 (0.80, 6.63) | |
| Obese | 3/52 | 1.11 (0.24, 5.14) | |
| p-value for Wald χ2 | 0.26 | ||
Note1: All models were adjusted for age, race, smoking status, ln(physical activity), history of diabetes, and ln(insulin resistance).
Note 2: The interactions between BMI categories and serum adiponectin on current asthma status were not significant (p = 0.45). Similar results were obtained when BMI and ln(adiponectin) were used as continuous variables (p = 0.75).
Note 3: Adiponectin tertile cut points used in the above analysis were 1.2–9, 9–15, and 15–94.8 mg/L mg/L.
Note 4: Similar results were obtained when the underweight and normal weight categories were analyzed separately with the latter serving as the referent category.
Note 5: BMI categories are defined as follows - Underweight - BMI < 18.5 kg/m2; Normal weight - BMI 18.5–24.9 kg/m2; Overweight - BMI 25–29.9 kg/m2; Obese - BMI ≥ 30 kg/m2.
Since the associations between adiposity and asthma (Table 2) and between serum adiponectin and asthma (Table 1) were seen only in women and since most women in this study were pre-menopausal (90%), further analyses in Tables 3 and 4 were focused on pre-menopausal women. Analyses related to all women and men are presented in the expanded Tables 3 and 4 in the online data supplement. Uniform adiponectin cut points generated from the pre-menopausal group were used for these analyses.
Table 3.
Association between current asthma status and serum adiponectin concentration in pre-menopausal women, with and without adjusting for adiposity
| Adiponectin models | Pre-menopausal women | ||
|---|---|---|---|
| n with current asthma/total n | Odds Ratio (95% C.I.) | ||
| Adiponectin | 1st Tertile | 62/495 | 1 |
| 2nd Tertile | 51/487 | 0.87 (0.57, 1.33) | |
| 3rd Tertile | 19/409 | 0.38 (0.21, 0.69) | |
| p-value for Wald χ2 | 0.004 | ||
| Adiponectin adjusted for Body Mass Index | 1st Tertile | 62/495 | 1 |
| 2nd Tertile | 51/487 | 0.93 (0.61, 1.42) | |
| 3rd Tertile | 19/409 | 0.46 (0.26, 0.84) | |
| p-value for Wald χ2 | 0.03 | ||
Note 1: Covariates adjusted in each logistic regression model include age, race, smoking status, ln(physical activity), history of diabetes, and ln(insulin resistance).
Note 2: Adiponectin tertile cut points used in the above analysis were derived from pre-menopausal women (1.2–9, 9–15, and 15–94.8 mg/L mg/L).
Note 3: Further, when the third tertile of adiponectin was further split into two halves, the more extreme half had the lowest odds ratio. Additionally, similar trends were obtained when ln (adiponectin) was used as a continuous (instead of categorical) independent variable in the above analysis.
Table 3 shows that high serum adiponectin concentration was associated with reduced odds of current asthma in pre-menopausal women. After adjusting for BMI and other covariates, the highest tertile of serum adiponectin concentration in pre-menopausal women was associated with reduced odds of current asthma, with an O.R. of 0.46 (95% C.I. 0.26, 0.84) compared to the lowest tertile (p = 0.03). Similar results were obtained after adjusting for waist circumference in the analysis instead of BMI (expanded Table 3 in online data supplement).
The interaction between menopausal status and serum adiponectin category on current asthma status in women was not significant (the range of p values in the unadjusted pair wise comparisons was 0.32–0.92, expanded Table 3). However, this analysis was limited by the small number of participants in the post-menopausal group (n = 163).
A stratified analysis suggested that the odds ratio of the association between current asthma and obese status may be the highest in those with the lowest tertile of serum adiponectin concentration, both in pre-menopausal women and in women overall (Table 4 below and Expanded Table 4 in data supplement), although the formal test of interaction between BMI and adiponectin in estimation of current asthma was not statistically significant in either group (the range of p values in the unadjusted pair wise comparisons was 0.45–0.99). Similar results were obtained after adjusting for waist circumference in the analysis instead of BMI (data not shown).
DISCUSSION
This study demonstrates three possible and important results. First, lower serum adiponectin concentration is associated with current asthma only in women in an unadjusted analysis. Second, the serum adiponectin concentration does not appear to explain the adiposity-asthma association in women. Third, high serum adiponectin concentration may be associated with reduced odds of asthma in pre-menopausal women, an effect that may be independent of adiposity. In addition, we confirm results from other studies that showed that asthma is associated with adiposity only in women.
In 2005, more than 21 million people in the United States were estimated to be affected by asthma, amounting to 7.6% of the total population (14). Between 1980 and 1996, the prevalence of and morbidity trends related to asthma increased in the United States (15). Paralleling the rise in prevalence of asthma has been a rise in the prevalence of obesity in the United States (1). In 1999–2002, 65.1% of adults in the United States were either overweight or obese and 30.4% were obese (16). The somewhat contemporaneous course of the two epidemics has sparked interest in adiposity as a risk factor for asthma. Some (but not all) studies have also shown that adiposity is a stronger risk factor for asthma in women than in men (2–6).
Adipose tissue produces and releases a variety of pro-and anti-inflammatory factors (17). Some of these substances may play a role in the development of a variety of inflammatory conditions such as cardiovascular disease, type II diabetes, and possibly asthma, although their pathogenic role is far from proven. One of the anti-inflammatory adipokines is adiponectin. Although central (visceral) adipocytes are the most important source of adiponectin (11), serum adiponectin concentration does not increase with obesity as serum leptin concentration does. On the contrary, there is a tendency for reduced serum adiponectin concentration among obese subjects (18).
Obesity is associated with high rates of necrosis of adipocytes, exposing naked lipid droplets to the interstitium (19). Macrophages gather around the necrosing adipocytes and become functionally activated, forming syncytia (19). Macrophages in the adipose tissue produce tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) which in turn may directly inhibit the local production of adiponectin in a paracrine fashion (20). This may explain the reduced systemic adiponectin concentration seen in obesity.
The anti-inflammatory properties of adiponectin include inhibition of pro-inflammatory cytokines such as TNF-α and IL-6 (21, 22) and endothelial adhesion molecules such as ICAM-1, VCAM-1 and p-selectin (23) as well as induction of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist (22, 24, 25). Therefore, adiponectin may modulate the activation, proliferation, cytotoxicity, and cytokine production by inflammatory cells as well as affect interactions of T-cells with other T-cells, target cells, and B-cells. Since ICAM-1 is the receptor for most rhinoviruses (the most important cause of asthma exacerbations) (26) and p-selectin-mediated adhesion of leukocytes to the vascular endothelium is a key early event in the initiation of inflammatory response, it is conjectured that the effects of adiponectin on endothelial adhesion molecules may play a role in asthma causation. Additionally, adiponectin inhibits proliferation and migration of cultured vascular smooth muscle cells induced by mitogens (27–29) and may have similar effects on murine airway smooth muscle (Figure 1) (10). Given that adiponectin receptors are expressed in cultured human airway smooth cells (30), it has been hypothesized that a decline in serum adiponectin concentration in the obese may contribute to the increased airway smooth muscle mass seen with remodeling in chronic asthma (31).
Figure 1.
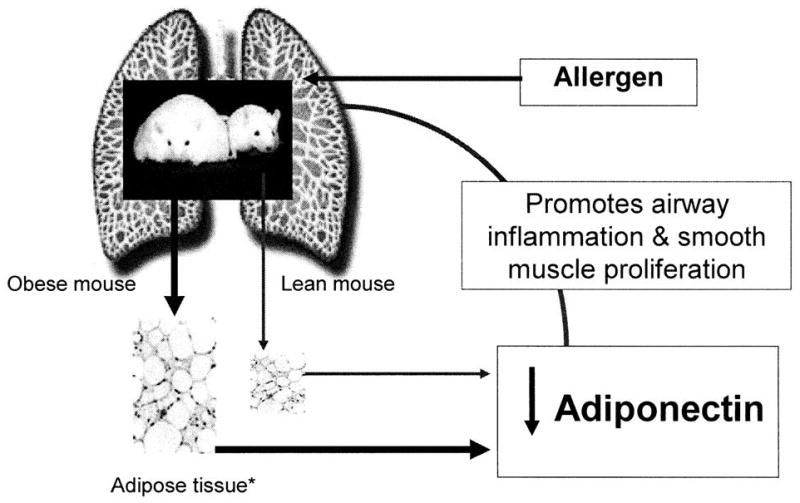
A schematic representation of the suggested role of adiponectin in murine asthma, based on work by Shore et al. (10, 30, 31).
* Adipocytes and stromal macrophages are both hypertrophic and hyperplastic in obese mice, as compared to lean mice.
Murine models have also shown that subcutaneous injection of adiponectin in ovalbumin-sensitized mice prevents the subsequent development of increased airway responsiveness on exposure to ovalbumin (10, 31). Taken together, the known anti-inflammatory effects of adiponectin in both mice and humans, and the prevention of both allergen-induced airway responsiveness and airway smooth muscle proliferation in murine models has led to the hypothesis that the decreased serum concentrations of adiponectin in obese humans may contribute to the propensity towards asthma in this population (30).
This study suggests that higher serum adiponectin concentration may be a protective factor for current asthma in women, particularly those who are pre-menopausal. The observed association was independent of adiposity. These results are consistent with a previous cross-sectional study from a different population that suggested that high serum concentrations of leptin, another adipokine, are an independent risk factor for asthma in pre-menopausal women and that this effect may also be independent of adiposity (9). These results suggest that there may be interplay of female sex hormones, selected adipokines, and asthma, although this remains to be proven.
Despite the strong biological plausibility cited above, this study fails to convincingly demonstrate that serum adiponectin concentration modifies the association between adiposity and asthma. There are three possible explanations. First, human asthma and murine asthma have different pathophysiologic pathways (32) and therefore, adiponectin may not play the same pathogenic role in the association between adiposity and human asthma as it does in the murine asthma model. Second, human cross sectional studies may not be able to show clear associations, because of the presence of multiple confounding factors and the lack of knowledge of temporal sequencing of the variables. For instance, it may be necessary to replicate the mouse intervention study design in human subjects in order to better understand the role of adiponectin on the human airway. This may be possible by studying either the effect of factors that affect serum adiponectin (such as exercise, weight reduction, and peroxisome proliferator-activated receptor (PPAR) γ agonists) on asthma outcome measures or the effect of specific allergen inhalation on serum adiponectin concentration in subjects with asthma. Third and most important, it is likely that the association between adiposity and a complex disease trait such as asthma is mediated not by one adipokine but a balance of several pro-inflammatory and anti-inflammatory adipokines, and adiponectin is only a part of the larger puzzle. On the same line of reasoning, it is possible that other mechanistic pathways such as the mechanical effect of adiposity, and genetic, hormonal, and environmental pathways are more important than the immunologic effects of adiposity, in the asthma-adiposity association in humans.
The strengths of the study include its relatively large sample size and its clinical translational character, based on the recently published data on the role of adiponectin in asthma in the mouse model by Shore et al. (10, 31). The study however, has some limitations. Asthma diagnosis was self-reported, and this may result in misclassification bias, particularly among the obese population (33). Further, this is an observational cross sectional study which can only allow for hypothesis generation but does not prove causality or the direction of causation. The number of post-menopausal subjects in this relatively young cohort is small (about 10%), making it difficult to compare with pre-menopausal women. Additionally, the observed lack of association among men may be due to relatively smaller number of asthmatics among men, compared to women, rather than a true lack of association. Further, in order to confirm definitively that adiponectin has a different effect on asthma status in various groups, a statistically significant interaction is required. This exploratory analysis lacks the power to detect a statistically significant interaction. Finally, adiponectin is present in serum in hexameric low molecular weight and multimeric high molecular weight forms, which were not separately measured in this study. Debate exists as to whether the various forms have the same in vivo activity (34).
In summary, high serum adiponectin concentration may exert a protective effect against asthma in pre-menopausal women and this effect may be independent of adiposity. However, the adiposity-asthma association seen among women subjects in the CARDIA study can not be explained by serum adiponectin concentration. Future studies will need to expand on these observations to improve our understanding of the association between asthma and adiposity. A better understanding of the role of adiponectin in asthma may suggest newer ways of treating asthma in the obese population.
Supplementary Material
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the Coronary Artery Risk Development in Young Adults Study Investigators.
FUNDING:
Dr. Sood is supported by the University of New Mexico Clinical Translational Science Center Scholar Award and DHHS/NIH/NCRR/GCRC Grant # 5M01 RR00997; Dr. Beckett is supported by ES01247. Dr. Steffes has several grants/contracts from NIDDK, NHLBI and NICHD.
Footnotes
COMPETING INTERESTS:
None
Contributor Information
Akshay Sood, University of New Mexico Health Sciences Center School of Medicine, Albuquerque, NM, U.S.A
Xichun Cui, University of New Mexico Health Sciences Center School of Medicine, Albuquerque, NM, U.S.A
Clifford Qualls, University of New Mexico Health Sciences Center School of Medicine, Albuquerque, NM, U.S.A
William S. Beckett, University of Rochester School of Medicine and Dentistry, Rochester, NY, U.S.A
Myron D. Gross, University of Minnesota, Minneapolis, MN, U.S.A
Michael W. Steffes, University of Minnesota Minneapolis, MN, U.S.A
Lewis J. Smith, The Feinberg School of Medicine, Northwestern University, Chicago, IL, U.S.A.
David R. Jacobs, Jr., University of Minnesota, Minneapolis, MN, U.S.A. (also affiliated with the University of Oslo, Oslo, Norway).
References
- 1.Kuczmarski RJ, Carroll MD, Flegal KM, Troiano RP. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5(6):542–8. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 2.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164(11):2045–50. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 3.Celedon JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, Fang Z, et al. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1835–40. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163(6):1344–9. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa-Munoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4–11 year old children in the UK. Thorax. 2001;56(2):133–7. doi: 10.1136/thorax.56.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult- onset asthma in women. Arch Intern Med. 1999;159(21):2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 7.Sood A. Does obesity weigh heavily on the health of the human airway? J Allergy Clin Immunol. 2005 May;115(5):921–4. doi: 10.1016/j.jaci.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003 Nov 21;278(47):46654–60. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 9.Sood A, Camargo CA, Jr, Ford ES. Association between leptin and asthma in adults. Thorax. 2006;61(4):300–5. doi: 10.1136/thx.2004.031468. Epub 2006 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006 Aug;118(2):389–95. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, et al. Serum adiponectin in young adults--interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol. 2004 Aug;14(7):492–8. doi: 10.1016/j.annepidem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Platts-Mills TA, Carter MC, Heymann PW. Specific and nonspecific obstructive lung disease in childhood: causes of changes in the prevalence of asthma. Environ Health Perspect. 2000 Aug;108(Suppl 4):725–31. doi: 10.1289/ehp.00108s4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003 Apr;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. National Health Interview Survey, 2001–2005. 2005. [Google Scholar]
- 15.From the Centers for Disease Control and Prevention. Self-reported asthma prevalence among adults--United States, 2000. JAMA. 2001;286(13):1571–2. [PubMed] [Google Scholar]
- 16.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004 Jun 16;291(23):2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 17.Fantuzzi G. Adipose tissue, adipokines and inflammation. J Allergy Clin Immunol. 2005:04–1164. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999 Apr 2;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 19.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005 Nov;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003 Sep;285(3):E527–33. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 21.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004 Jul;40(1):177–84. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 22.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004 Apr 9;316(3):924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 23.Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Effects of adipocyte-derived cytokines on endothelial functions: implication of vascular disease. J Surg Res. 2005 Jun 1;126(1):121–9. doi: 10.1016/j.jss.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004 May 4;109(17):2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 25.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004 Oct 15;323(2):630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 26.Stanciu LA, Djukanovic R. The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J. 1998 Apr;11(4):949–57. doi: 10.1183/09031936.98.11040949. [DOI] [PubMed] [Google Scholar]
- 27.Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes. 2002 Jul;51(7):2325–8. doi: 10.2337/diabetes.51.7.2325. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002 Nov 26;106(22):2767–70. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999 Dec 21–28;100(25):2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 30.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006 Apr;110(1):83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Shore SA. Obesity and asthma: cause for concern. Curr Opin Pharmacol. 2006 Mar 7; doi: 10.1016/j.coph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel S, Holgate ST. The mouse trap: It still yields few answers in asthma. Am J Respir Crit Care Med. 2006 Dec 1;174(11):1173–6. doi: 10.1164/rccm.2609002. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 33.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56(1):4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004 Mar 5;94(4):e27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


