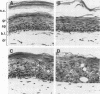
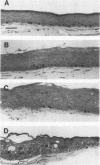
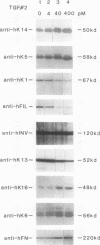
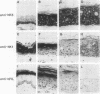
Abstract
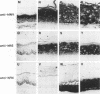
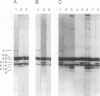
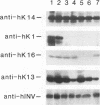
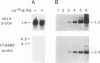
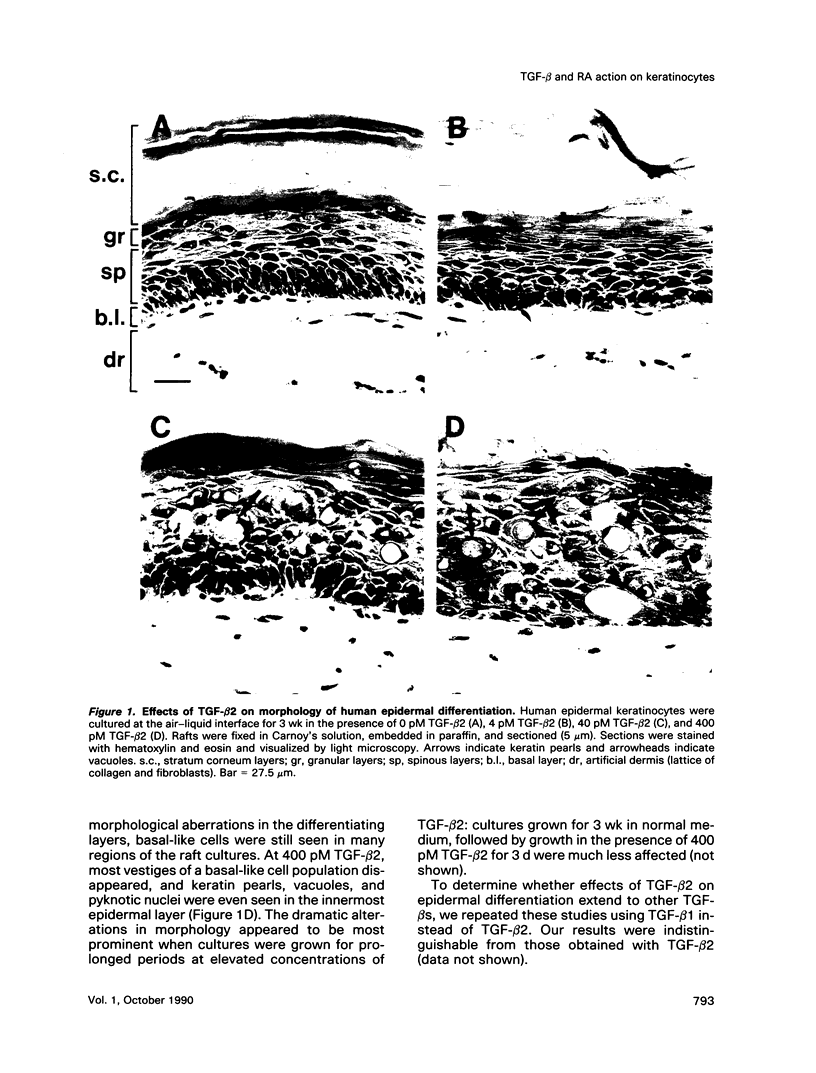
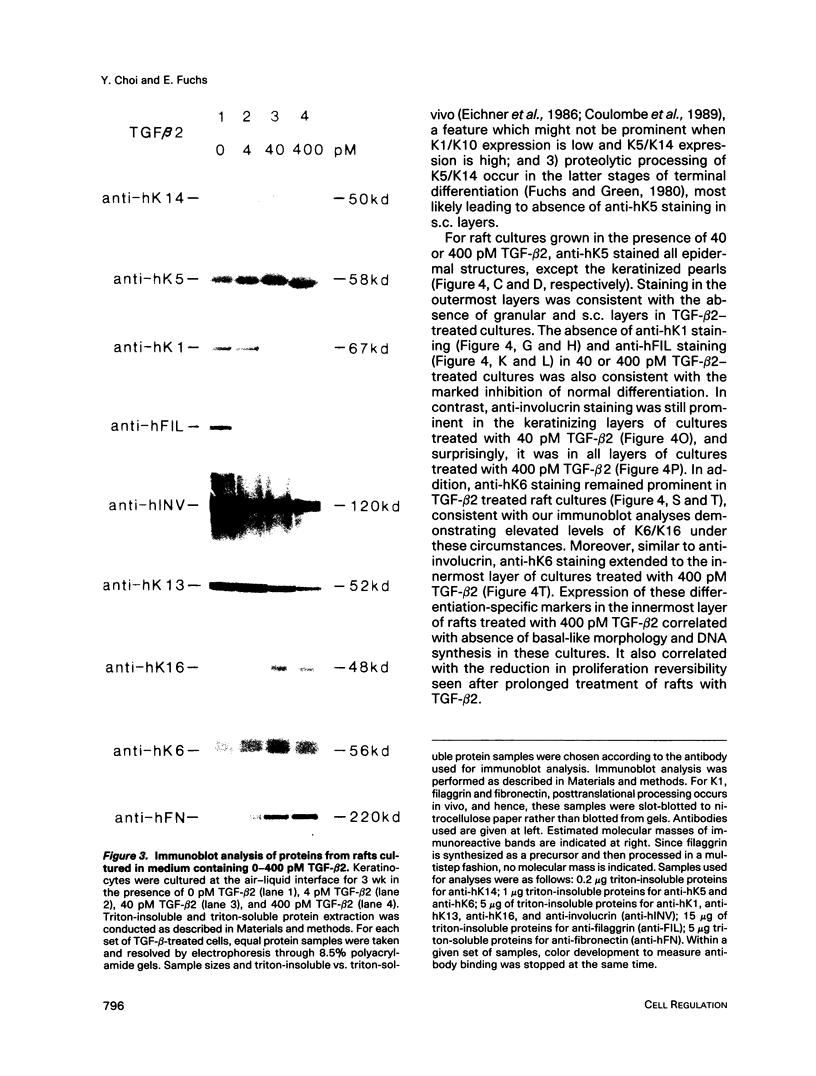
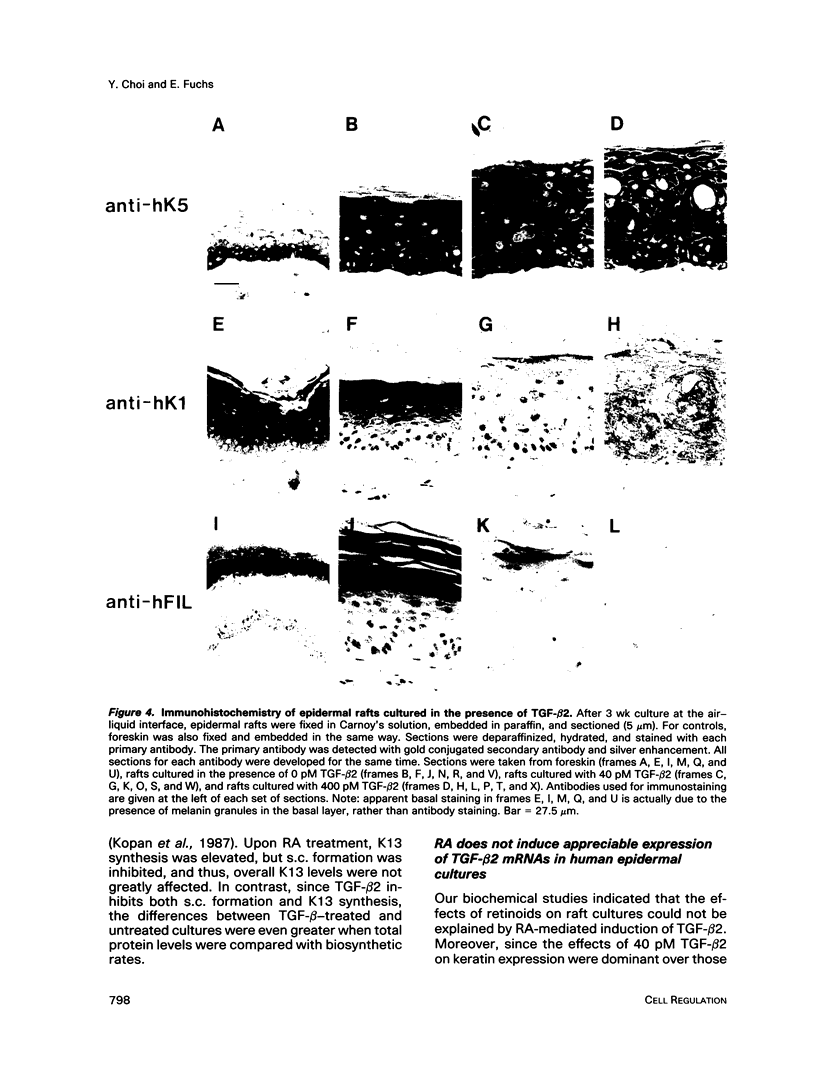
In the epidermis of skin, a fine balance exists between proliferating progenitor cells and terminally differentiating cells. We examined the effects of TGF-beta s and retinoic acid (RA) on controlling this balance in normal and malignant human epidermal keratinocytes cultured under conditions where most morphological and biochemical features of epidermis in vivo are retained. Our results revealed marked and pleiotropic effects of both TGF-beta and RA on keratinocytes. In contrast to retinoids, TGF-beta s acted on mitotically active basal cells to retard cell proliferation. Although withdrawal from the cell cycle is a necessary prerequisite for commitment to terminal differentiation, TGF-beta s inhibited normal keratinization in suprabasal cells and promoted the type of differentiation commonly associated with wound-healing and epidermal hyperproliferation. The actions of TGF-beta s and RA on normal keratinization were synergistic, whereas those on abnormal differentiation associated with hyperproliferation were antagonistic. These observations underscore the notion that environmental changes can act separately on proliferating and differentiating cells within the population. Under the conditions used here, the action of TGF-beta s on human keratinocytes was dominant over RA, and TGF-beta s did not seem to be induced as a consequence of RA treatment. This finding is consistent with the fact that RA accelerated, rather than inhibited, proliferation in raft cultures. Collectively, our data suggest that the effects of both factors on epidermal growth and differentiation are multifaceted and the extent to which their action is coupled in keratinocytes may vary under different conditions and/or in different species.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature. 1989 Jul 27;340(6231):307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J., Fee F., Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988 Jan 28;331(6154):363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- Albers K. M., Greif F., Setzer R. W., Taichman L. B. Cell-cycle withdrawal in cultured keratinocytes. Differentiation. 1987;34(3):236–240. doi: 10.1111/j.1432-0436.1987.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Asselineau D., Bernard B. A., Bailly C., Darmon M., Pruniéras M. Human epidermis reconstructed by culture: is it "normal"? J Invest Dermatol. 1986 Feb;86(2):181–186. doi: 10.1111/1523-1747.ep12284237. [DOI] [PubMed] [Google Scholar]
- Asselineau D., Bernard B. A., Bailly C., Darmon M. Retinoic acid improves epidermal morphogenesis. Dev Biol. 1989 Jun;133(2):322–335. doi: 10.1016/0012-1606(89)90037-7. [DOI] [PubMed] [Google Scholar]
- Asselineau D., Bernhard B., Bailly C., Darmon M. Epidermal morphogenesis and induction of the 67 kD keratin polypeptide by culture of human keratinocytes at the liquid-air interface. Exp Cell Res. 1985 Aug;159(2):536–539. doi: 10.1016/s0014-4827(85)80027-6. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Morgan J. R., Mulligan R. C., Green H. Restoration of growth potential in paraclones of human keratinocytes by a viral oncogene. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4102–4106. doi: 10.1073/pnas.86.11.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom C. C., Wolfshohl J. R., Coffey R. J., Jr, Madisen L., Webb N. R., Purchio A. R., Derynck R., Moses H. L. Complex regulation of transforming growth factor beta 1, beta 2, and beta 3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors beta 1 and beta 2. Mol Cell Biol. 1989 Dec;9(12):5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Bascom C. C., Sipes N. J., Graves-Deal R., Weissman B. E., Moses H. L. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988 Aug;8(8):3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Kopan R., Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J Cell Biol. 1989 Nov;109(5):2295–2312. doi: 10.1083/jcb.109.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Steinert P. M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978 Dec 14;276(5689):729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Dover R., Potten C. S. Heterogeneity and cell cycle analyses from time-lapse studies of human keratinocytes in vitro. J Cell Sci. 1988 Mar;89(Pt 3):359–364. doi: 10.1242/jcs.89.3.359. [DOI] [PubMed] [Google Scholar]
- Eichner R., Sun T. T., Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986 May;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman B. A., Harper R. A. Primary cell culture for biochemical studies of human keratinocytes. A method for production of very large numbers of cells without the necessity of subculturing techniques. Br J Dermatol. 1975 Mar;92(3):305–309. doi: 10.1111/j.1365-2133.1975.tb03080.x. [DOI] [PubMed] [Google Scholar]
- Fleckman P., Dale B. A., Holbrook K. A. Profilaggrin, a high-molecular-weight precursor of filaggrin in human epidermis and cultured keratinocytes. J Invest Dermatol. 1985 Dec;85(6):507–512. doi: 10.1111/1523-1747.ep12277306. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Galvin S., Loomis C., Manabe M., Dhouailly D., Sun T. T. The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 1989;4:277–300. [PubMed] [Google Scholar]
- Glick A. B., Flanders K. C., Danielpour D., Yuspa S. H., Sporn M. B. Retinoic acid induces transforming growth factor-beta 2 in cultured keratinocytes and mouse epidermis. Cell Regul. 1989 Nov;1(1):87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Schwartz F., Fuchs E. Differences in keratin synthesis between normal epithelial cells and squamous cell carcinomas are mediated by vitamin A. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4280–4284. doi: 10.1073/pnas.81.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Fuchs E. The use of retinoic acid to probe the relation between hyperproliferation-associated keratins and cell proliferation in normal and malignant epidermal cells. J Cell Biol. 1989 Jul;109(1):295–307. doi: 10.1083/jcb.109.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. H., MacCallum D. K., Jepsen A. Fine structure of subcultivated stratified squamous epithelium grown on collagen rafts. Exp Cell Res. 1980 Jan;125(1):153–165. doi: 10.1016/0014-4827(80)90199-8. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Pelton R. W., Hogan B. L. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989 Nov;3(11):1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Webb N. R., Rose T. M., Marquardt H., Ikeda T., Twardzik D., Seyedin S., Purchio A. F. Transforming growth factor-beta 2: cDNA cloning and sequence analysis. DNA. 1988 Jan-Feb;7(1):1–8. doi: 10.1089/dna.1988.7.1. [DOI] [PubMed] [Google Scholar]
- Mansbridge J. N., Hanawalt P. C. Role of transforming growth factor beta in the maturation of human epidermal keratinocytes. J Invest Dermatol. 1988 Mar;90(3):336–341. doi: 10.1111/1523-1747.ep12456286. [DOI] [PubMed] [Google Scholar]
- Mansbridge J. N., Knapp A. M. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987 Sep;89(3):253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Nelson W. G., Sun T. T. The 50- and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol. 1983 Jul;97(1):244–251. doi: 10.1083/jcb.97.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Yamaai T., Koyama E., Nohno T., Fujimoto W., Arata J., Taniguchi S. Expression of retinoic acid receptor genes in keratinizing front of skin. FEBS Lett. 1989 Dec 18;259(1):86–90. doi: 10.1016/0014-5793(89)81501-7. [DOI] [PubMed] [Google Scholar]
- Pittelkow M. R., Lindquist P. B., Abraham R. T., Graves-Deal R., Derynck R., Coffey R. J., Jr Induction of transforming growth factor-alpha expression in human keratinocytes by phorbol esters. J Biol Chem. 1989 Mar 25;264(9):5164–5171. [PubMed] [Google Scholar]
- Pittelkow M. R., Wille J. J., Jr, Scott R. E. Two functionally distinct classes of growth arrest states in human prokeratinocytes that regulate clonogenic potential. J Invest Dermatol. 1986 Apr;86(4):410–417. doi: 10.1111/1523-1747.ep12285684. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rieger M., Franke W. W. Identification of an orthologous mammalian cytokeratin gene. High degree of intron sequence conservation during evolution of human cytokeratin 10. J Mol Biol. 1988 Dec 20;204(4):841–856. doi: 10.1016/0022-2836(88)90045-9. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., O'Connell T. M., Bennett G., Burton L. E., Stiles C. D., Rheinwald J. G. Environment-dependent growth inhibition of human epidermal keratinocytes by recombinant human transforming growth factor-beta. J Cell Physiol. 1989 Jun;139(3):455–462. doi: 10.1002/jcp.1041390302. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., RayChaudhury A., Shows T. B., Le Beau M. M., Fuchs E. A group of type I keratin genes on human chromosome 17: characterization and expression. Mol Cell Biol. 1988 Feb;8(2):722–736. doi: 10.1128/mcb.8.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. D., Pittelkow M. R., Wille J. J., Jr, Scott R. E., Moses H. L. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986 Apr;46(4 Pt 2):2068–2071. [PubMed] [Google Scholar]
- Stellmach V. M., Fuchs E. Exploring the mechanisms underlying cell type-specific and retinoid-mediated expression of keratins. New Biol. 1989 Dec;1(3):305–317. [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner A. L., Eichman M. J., Fuchs E. The sequence of a type II keratin gene expressed in human skin: conservation of structure among all intermediate filament genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4683–4687. doi: 10.1073/pnas.82.14.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Rosenberg M., Ross S., Tyner A., Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M., Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982 Feb 4;295(5848):434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Mattey D. L., Garrod D. R. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol. 1984 Dec;99(6):2211–2215. doi: 10.1083/jcb.99.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Harris C. C. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Franke W. W., Achtstätter T., Haasnoot W. H., Ponec M., Warnaar S. O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986 Jan;162(1):97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]