Abstract
Obesity is one of the primary risk factors for osteoarthritis. Increased adiposity is associated not only with alterations in joint loading, but also with increased systemic and joint concentrations of adipose tissue-derived cytokines, or “adipokines,” that promote a state of chronic, low-grade inflammation that may act in concert with other cytokines in the joint to increase joint degeneration. However, the direct effect of adipokines, such as leptin, visfatin, and interleukin-6 (IL-6), on joint tissues, such as articular cartilage and meniscus, are not fully understood. In this study, we examined the hypothesis that these adipokines act synergistically with interleukin-1 (IL-1) to increase catabolism and the production of pro-inflammatory mediators in cartilage and meniscus. Explants of porcine cartilage and meniscus were treated with physiologically relevant concentrations of leptin, IL-6, or visfatin, alone or in combination with IL-1. Visfatin and IL-1 promoted the catabolic degradation of both cartilage and meniscus, as evidenced by increased metalloproteinase activity, nitric oxide production, and proteoglycan release. However, leptin or IL-6 at physiologic concentrations had no effect on the breakdown of these tissues. These findings suggest that the effects of obesity-induced osteoarthritis may not be through a direct effect of leptin or IL-6 on cartilaginous tissues, but support a potential role for increased visfatin levels in this regard. These data provide an important first step in understanding the role of adipokines in regulating cartilage and meniscus metabolism; however, these adipokines may have different effects in the context of the whole joint and must be evaluated further.
Keywords: IL-1, Leptin, IL-6, Visfatin, Matrix Metalloproteinases, Adipokines
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis and is characterized by an imbalance in the degradation and synthesis of articular cartilage, as well as remodeling of the subchondral bone [1, 2]. The primary risk factors for OA are age, joint injury, and obesity [3]. Obesity is the primary avoidable risk factor for the development of OA, not only in weight-bearing joints, such as the knee and hip, but also in non-weight bearing joints in the hand [4, 5], suggesting that systemic metabolic factors, as well as biomechanical loading, may be contributing to obesity-induced OA. Furthermore, obesity has been strongly associated with increased incidence of tears in the medial meniscus [6]. Such joint injuries result in alterations in the biomechanical and inflammatory environments in the joint, ultimately progressing to OA [7–12].
In particular, recent studies have revealed the critical role played by adipose tissue, in metabolic homeostasis, by secreting a number of cytokines, termed “adipokines,” that act through autocrine, paracrine, and endocrine signaling [13]. Obesity is associated with a chronic, low-grade, systemic, pro-inflammatory state, attributed to the actions of adipokines, such as leptin, resistin, adiponectin, and visfatin. The serum levels of the adipokines positively correlate with changes in body mass index (BMI) [13]. Additionally, plasma levels of leptin, adiponectin, and visfatin are elevated in patients with rheumatoid arthritis (RA) but there is no change in resistin concentrations, as compared to non-arthritic controls [14]. Leptin is a hormone with a well-established role in mediating food intake and energy expenditure. Leptin also seems to play a role in joint homeostasis, as it has been related to cartilage volume loss with obesity in females [15]. Synovial fluid leptin concentrations have also been correlated with the severity of knee OA [16]. Healthy chondrocytes generally do not produce leptin, but the expression of both leptin [17] and the signaling leptin receptor Ob-Rb are increased in advanced OA cartilage [18]. Mice fed a high-fat diet show increased OA severity that is highly correlated with serum leptin levels, whereas mice deficient in leptin or leptin receptor exhibit morbid obesity but no signs of OA, suggesting that leptin itself may play a role in joint degeneration [19, 20]. However, the direct effects of leptin on cartilage metabolism are not fully understood and appear to depend on various factors, such as concentration and the presence of serum. Similarly, the increased concentrations of leptin (and other adipokines) in the synovial fluid may influence the physiology of other joint tissues such as the meniscus; however, no studies thus far have examined the effects of leptin on meniscus tissue.
In addition to mediators that are produced by the adipocytes, adipose tissue macrophages are a major source of the pro-inflammatory cytokines interleukin-1 (IL-1) and IL-6 [13], which have been implicated as important mediators of cartilage degeneration [21, 22] through their anti-anabolic and pro-catabolic activities in cartilage and meniscus. IL-1β upregulation in RA and OA patients [23, 24] induces many degradative processes in the joint [21, 22, 25, 26]. IL-1 enhances the production of the inflammatory mediators prostaglandin E2 (PGE2) and nitric oxide (NO), upregulates matrix metalloproteinases (MMPs), enhances proteoglycan release, and alters collagen synthesis in both cartilage [25, 27–29] and meniscus [30, 31]. In addition, the inflammatory cytokine IL-1β causes increased IL-6 production by chondrocytes [32, 33]. High serum levels of IL-6 and high BMI are predictors of radiographic knee OA [34], suggesting an important role for IL-6 in the development of OA. IL-6 binds to the IL-6 receptor (IL-6R), which is either membrane bound or soluble (sIL-6R), and this complex then binds to the cell surface glycoprotein 130 (gp130), which signals intracellularly [35]. Chondrocytes express the gp130 receptor on the cell surface [36], and thus can signal upon binding by IL-6 and the sIL-6R from the synovial fluid. Unlike chondrocytes and synoviocytes [37], the infrapatellar fat pad [38] and inflammatory cells secrete sIL-6R [37]. As sIL-6R and IL-6 are both present in the synovial fluid, their catabolic effects may contribute to degenerative changes, and potential failure, of the meniscus. However, no studies to date have analyzed the effects of IL-6 signaling in the presence or absence of IL-1 on cartilage MMP activity and on the catabolism of meniscal tissue.
Although there appears to be significant cross-regulation of pro-inflammatory cytokines and adipokines, the interactive effects of adipokines and IL-1 on joint tissues are complex and not fully understood [39]. For example, leptin increases the production of IL-6 in human OA cartilage explants [40]. Additionally, IL-6 and IL-1 regulate leptin mRNA expression and IL-1β stimulates the short term release of leptin [41]. Furthermore, IL-6 may have either pro- or anti-inflammatory effects, depending on interactions with other cytokines, such as IL-1 [21]. Some studies have shown IL-6 and sIL-6R decrease IL-1 mediated catabolism [42], while other studies have observed synergism, resulting in increased matrix degradation [36, 43]. IL-6 and sIL-6 receptor signaling upregulates visfatin in RA synovial fibroblasts [44].
Visfatin, also known as pre-B cell colony-enhancing factor (PBEF), was originally identified as a secreted growth factor for lymphocytes but has a variety of functions [45]. Visfatin is involved in the synthesis of nicotinamide adenine dinucleotide (NAD), an essential cofactor in cell metabolism [45], can act as an insulin mimetic by binding to the insulin receptor, and has anti-apoptotic actions [14]. Additionally, in RA patients visfatin serum concentrations are associated with radiographic joint damage [46] and serum and synovial fluid levels correlate with inflammation and disease activity [47]. The infrapatellar fat pad [48], human OA chondrocytes [49], and RA synovial fibroblasts [47] produce visfatin, and synthesis of this cytokine is increased by IL-1β [49]. Thus far, no studies have evaluated the effects of visfatin or the synergism of visfatin with IL-1 on cartilage or meniscus MMP activity, proteoglycan breakdown, or NO production.
The goal of this study was to assess the effects of these adipokines in the presence or absence of the cytokine IL-1 on cartilage and meniscus catabolism. We hypothesized that physiologic concentrations of adipokines would enhance the catabolism of cartilage and meniscus and would synergize with IL-1 to further promote the pro-inflammatory breakdown of these tissues. We used porcine cartilage and meniscus explants to examine the effects of leptin, IL-6, and visfatin either with or without IL-1. Explants were cultured for 72 hours and the media was assessed for MMP activity, sulfated glycosaminoglycan (S-GAG) release, and NO production.
MATERIALS AND METHODS
Explant Culture
Femoral condyles and medial menisci were isolated from 2–3 year old skeletally mature female pig knees obtained from a local abattoir. Explants were harvested from the femoral surface of the medial meniscus, using a 5 mm biopsy punch (Miltex, Inc, York, PA) oriented perpendicular to the meniscal surface. Full thickness cartilage explants (5 mm diameter) were harvested from the opposing medial femoral condyle in the region that articulates with the meniscus. Samples were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA), containing 1000 U/mL penicillin/streptomycin (Invitrogen) for 1 hour at 37°C. This medium was removed, and explants were cultured in DMEM containing 10% heat inactivated FBS (HyClone, Logan, UT), 0.1 mM non-essential amino acids (Invitrogen), 10 mM HEPES buffer solution (Invitrogen), 100 U/mL penicillin/streptomycin, and 37.5 µg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO) for 3 days to allow the tissue to equilibrate at 37°C and 5% CO2. Explants were then cultured for 3 days with the following adipokines at concentrations of 1 ng/mL, 10 ng/mL, or 100 ng/mL: recombinant porcine leptin (RayBiotech, Inc., Norcross, GA); recombinant porcine IL-6 (R & D Systems, Minneapolis, MN); or recombinant human visfatin (RayBiotech, Inc.). These concentrations were chosen to cover the range of concentrations that have previously been measured in the synovial fluid of patients with arthritis or joint injury (Table 1). In experiments testing the effects of IL-6, 100 ng/mL recombinant human IL-6Rα (R & D Systems) was also included. In order to test the synergism between inflammation and adipokines, explants were also cultured with 1 ng/mL recombinant porcine IL-1β (R & D Systems). Media was collected on day 3 and measured for MMP activity, S-GAG release, and NO production.
Table 1.
Physiologic Concentrations of Adipokines Measured in Synovial Fluid from Patients with Arthritis and Joint Injury
| Concentrations in Synovial Fluid | |
|---|---|
| Leptin | 1 – 20 ng/mL in OAa |
| IL-6 | 0.01 – 20 ng/mL in OAb 22 ng/mL day 1 after ACL injury; decreases to 1 – 2 ng/mL days 7 – 21c Levels are 2× higher with ACL injury + meniscal tear than ACL injury aloned |
| IL-6R | 10 – 50 ng/mL in OAe 25 – 140 ng/mL in RAe |
| Visfatin | 10 – 50 ng/mL in OAf 100 – 125 ng/mL in RAf |
MMP Activity Assay
In order to activate latent MMPs, a stock solution of 10mM p-aminophenylmercuric acetate (APMA; Sigma, St. Louis, MO) was prepared in 0.1 M NaOH. Media samples (90µl) were incubated with 10µl of 2.5 mM APMA pH 7.0 – 7.5 in assay buffer (200 mM NaCl, 50 mM Tris, 5 mM CaCl2, 10 µM ZnSO4, 0.01% Brij 35, pH 7.5) or 10µl assay buffer alone for 5 hours at 37°C. Total specific MMP activity was then measured using the quenched fluorogenic substrate Dab-Gly-Pro-Leu-Gly-Met-Arg-Gly-Lys-Flu (New England Peptide; Gardner, MA) as described previously [50–53].
S-GAG Release
Total S-GAG release was measured using the 1,9-dimethylmethylene blue (DMB) assay [54]. For this assay, standards ranging from 0 – 100 µg/mL bovine trachea chondroitin-4-sulfate type A (Sigma, St. Louis, MO) were prepared in control media. Standards and samples (20µl) were added to a 96 well plate in duplicate and 125 µl/well of DMB reagent was also added to each well. Absorbance was read at 540 nm within 5 min of DMB addition and the total µg of S-GAG released from each sample was corrected for the wet weight of the tissue.
NO Production
Total NO production was assessed by measuring the concentration of total nitrate and nitrite, as detailed previously [55, 56]. All media samples and standards (0 – 320 µM NaNO3 in culture media) were filtered through Micron Ultracel YM-10 10,000 molecular weight cut-off filters (Millipore, Bedford, MA) and diluted 1:2 in dH2O. Nitrate was then converted to nitrite, using nitrate reductase (Roche Diagnostics; Mannheim, Germany), and total nitrite concentrations were determined using the Greiss reagent. Absorbance was read at 540 nm, and total µmol NO were normalized to the wet weight of the tissue.
Statistical Analyses
Statistical analyses were performed using Statistica 7.0 (StatSoft Inc., Tulsa, OK). A one-way ANOVA and the Fisher LSD post hoc test were performed to determine significant differences (α=0.05) between treatment groups.
RESULTS
Effects of Leptin and IL-1 on Cartilage Explants
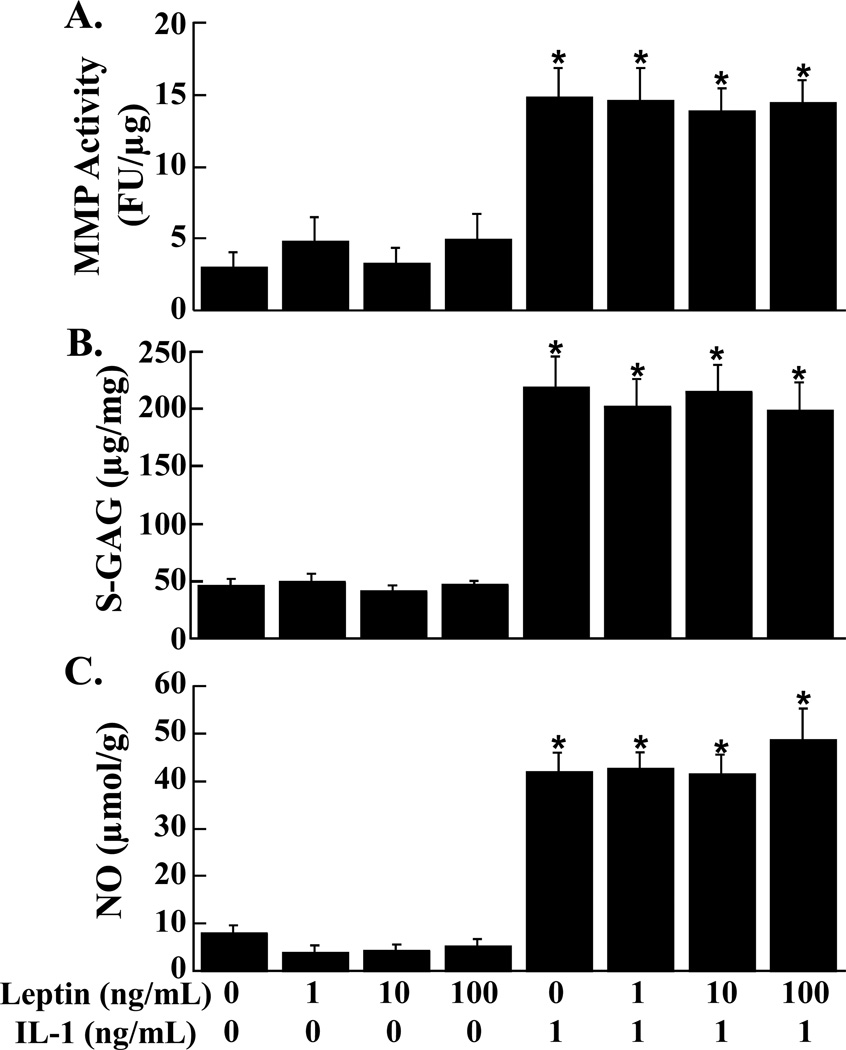
Total MMP activity (Figure 1A), S-GAG release (Figure 1B), and NO release (Figure 1C) into the media of cartilage explants was not altered by increasing concentrations of leptin. In both the presence and absence of leptin, IL-1 treatment resulted in an approximately 4-fold increase in total MMP activity, S-GAG release, and NO release (p < 0.0001) in cartilage explants.
FIG. 1.
Effects of leptin and IL-1 on cartilage explants. MMP activity (A), S-GAG release (B), and NO production (C) were measured in the media of cartilage explants that were exposed to 0 – 100 ng/mL leptin in the presence or absence of 1 ng/mL IL-1 (n = 12 per treatment group). (A) The MMP activity is expressed as fluorescence units (FU)/µg wet weight + standard error. (B) The S-GAG release is expressed as µg S-GAG/mg wet weight + standard error. (C) The NO release is expressed as µmol/g wet weight + standard error. *: p < 0.0001 compared to control, 1 ng/mL leptin, 10 ng/mL leptin, and 100 ng/mL leptin.
Effects of Leptin and IL-1 on Meniscal Explants
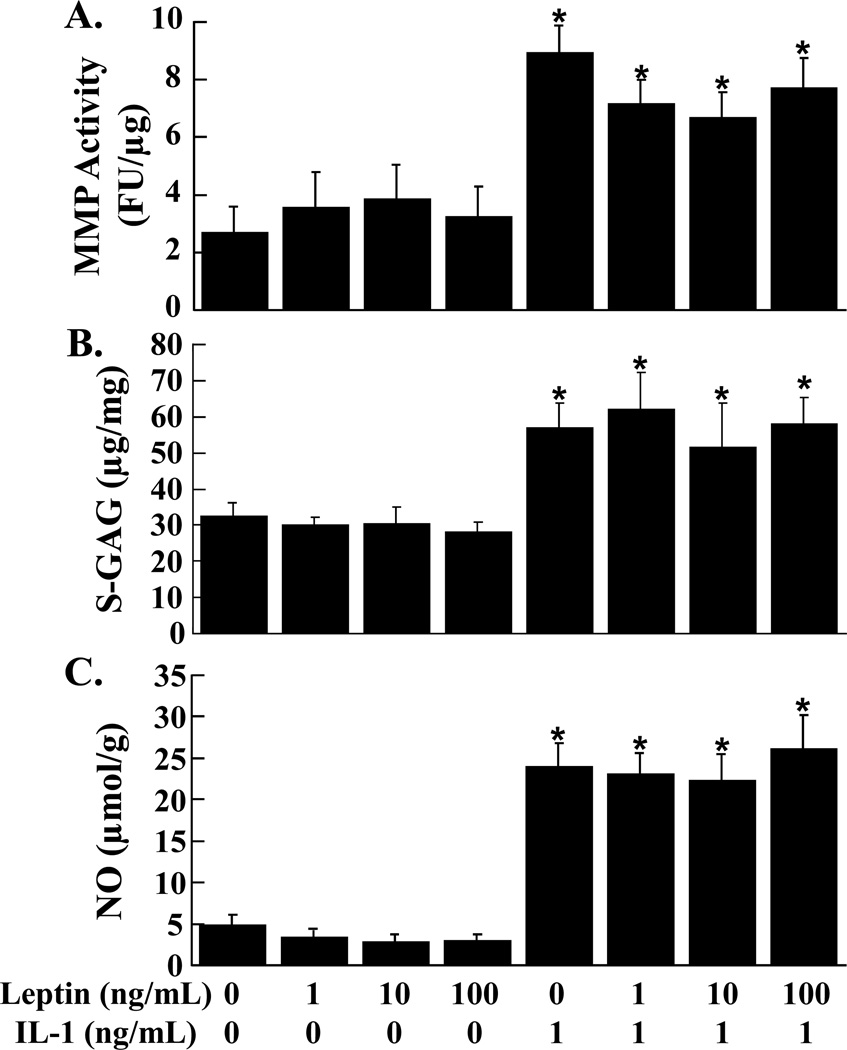
The addition of leptin to meniscal explants did not change the total MMP activity (Figure 2A), S-GAG release (Figure 2B), and NO release (Figure 2C) in the media. However, IL-1 treatment of meniscal explants increased MMP activity and S-GAG release 2-fold and NO release (p < 0.05) nearly 7-fold independent of leptin.
FIG. 2.
Effects of leptin and IL-1 on meniscal explants. MMP activity (A), S-GAG release (B), and NO production (C) were measured in the media of meniscal explants that were exposed to 0 – 100 ng/mL leptin in the presence or absence of 1 ng/mL IL-1 (n = 12 per treatment group). (A) The MMP activity is expressed as fluorescence units (FU)/µg wet weight + standard error. (B) The S-GAG release is expressed as µg S-GAG/mg wet weight + standard error. (C) The NO release is expressed as µmol/g wet weight + standard error. *: p < 0.05 compared to control, 1 ng/mL leptin, 10 ng/mL leptin, and 100 ng/mL leptin.
Effects of IL-6, sIL-6R, and IL-1 on Cartilage Explants
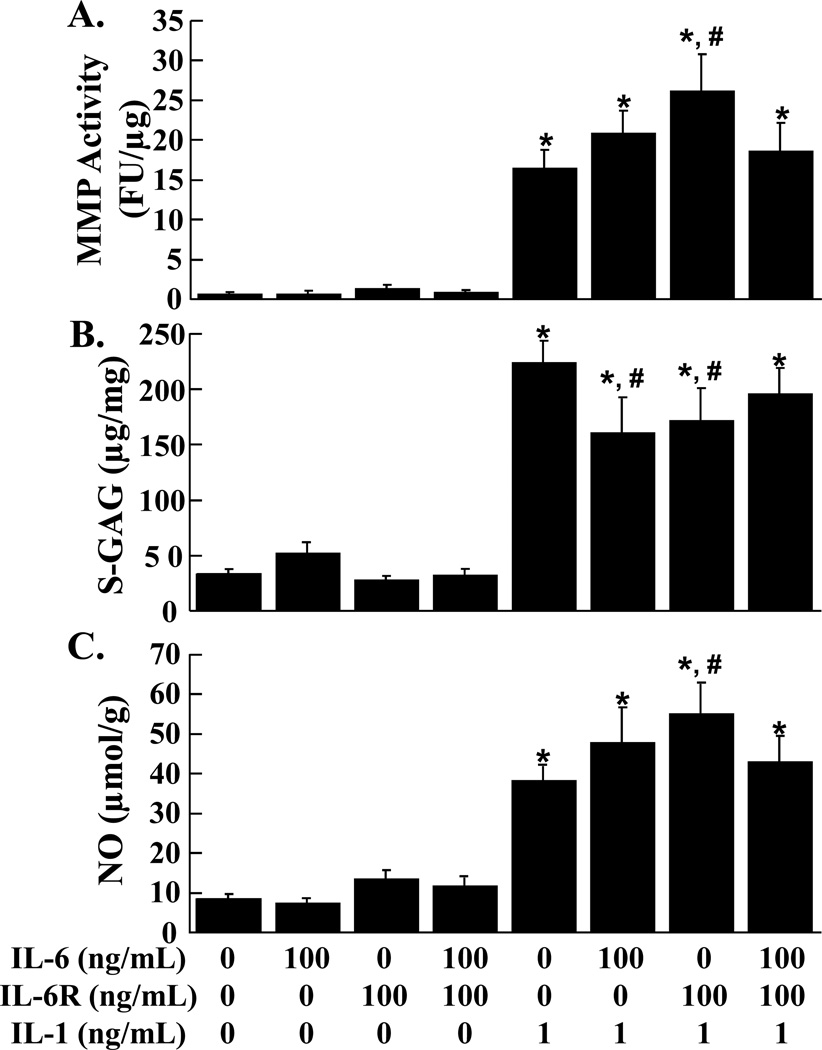
Total MMP activity, S-GAG release, and NO release from cartilage explants were not altered by IL-6 at any concentration tested (1 ng/mL, 10 ng/mL, and 100 ng/mL), as compared to control explants (data not shown). IL-6, sIL-6R, and the combination of IL-6 and sIL-6R failed to alter the MMP activity (Figure 3A), S-GAG release (Figure 3B), and NO release (Figure 3C) in the media of cartilage explants. The addition of IL-1 independent of IL-6, sIL-6R, or both, upregulated MMP activity, S-GAG release, and NO release (p < 0.001). The addition of the sIL-6R in combination with IL-1 caused a further increase in MMP activity and NO release, as compared to IL-1 alone (p < 0.05). On the other hand, both IL-6 and sIL-6R in the presence of IL-1 decreased S-GAG release, as compared to IL-1 alone (p < 0.05), but the combination of IL-6, sIL-6R, and IL-1 did not change S-GAG release, as compared to IL-1 treated cartilage.
FIG. 3.
Effects of IL-6, sIL-6R, and IL-1 on cartilage explants. MMP activity (A), S-GAG release (B), and NO production (C) were measured in the media of cartilage explants that were exposed to control media, 100 ng/mL IL-6, and 100 ng/mL sIL-6R in the presence or absence of 1 ng/mL IL-1 (n = 6 per treatment group). (A) The MMP activity is expressed as fluorescence units (FU)/µg wet weight + standard error. (B) The S-GAG release is expressed as µg S-GAG/mg wet weight + standard error. (C) The NO release is expressed as µmol/g wet weight + standard error. *: p < 0.001 compared to control, 100 ng/mL IL-6, 100 ng/mL sIL-6R, 100 ng/mL IL-6 + sIL-6R. #: p < 0.05 compared to IL-1 treated explants.
Effects of IL-6, sIL-6R, and IL-1 on Meniscal Explants
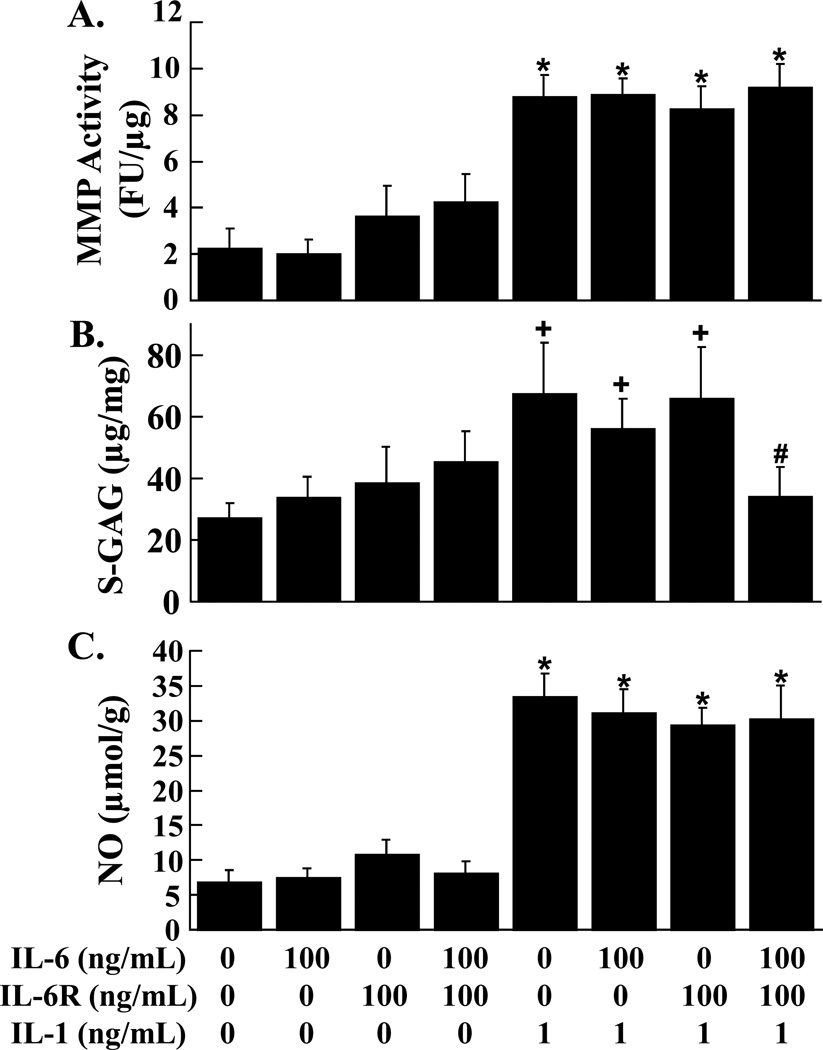
Total MMP activity, S-GAG release, and NO release from meniscal explants were not affected by IL-6 at any concentration (1 ng/mL, 10 ng/mL, and 100 ng/mL), as compared to control explants (data not shown). MMP activity (Figure 4A), S-GAG release (Figure 4B), and NO release (Figure 4C) were not altered by IL-6, sIL-6R, and the combination of IL-6 and sIL-6R. The addition of IL-1 independent of IL-6, sIL-6R, or both, increased MMP activity and NO release (p < 0.01) in meniscal explants. Treatment of meniscal explants with IL-1 alone, IL-6 and IL-1, or sIL-6R and IL-1 resulted in the upregulation of S-GAG release, as compared to control explants (p < 0.05). However, the combination of IL-6, sIL-6R, and IL-1 suppressed the IL-1 mediated release of S-GAGs into the meniscal media (p < 0.05).
FIG. 4.
Effects of IL-6, sIL-6R, and IL-1 on meniscal explants. MMP activity (A), S-GAG release (B), and NO production (C) were measured in the media of meniscal explants that were exposed to control media, 100 ng/mL IL-6, and 100 ng/mL sIL-6R in the presence or absence of 1 ng/mL IL-1 (n ≥ 9 per treatment group). (A) The MMP activity is expressed as fluorescence units (FU)/µg wet weight + standard error. (B) The S-GAG release is expressed as µg S-GAG/mg wet weight + standard error. (C) The NO release is expressed as µmol/g wet weight + standard error. *: p < 0.01 compared to control, 100 ng/mL IL-6, 100 ng/mL sIL-6R, 100 ng/mL IL-6 + sIL-6R. #: p < 0.05 compared to IL-1 treated explants. +: p < 0.05 compared to control.
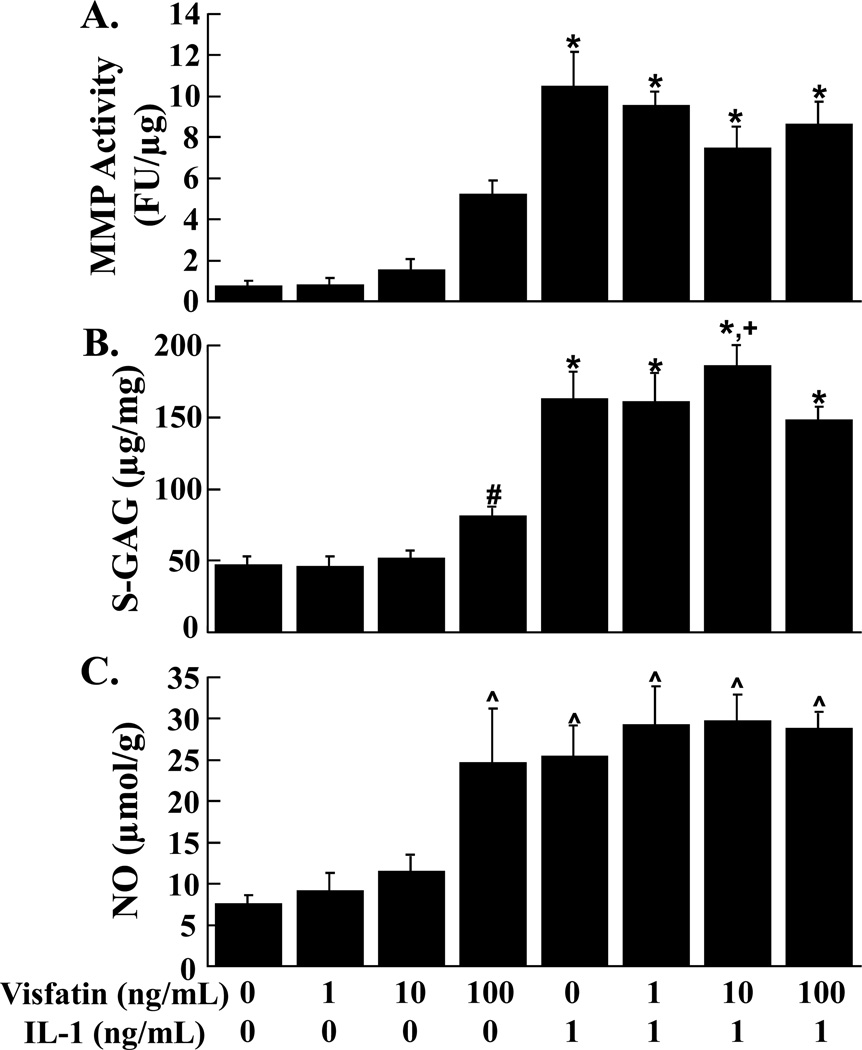
Effects of Visfatin and IL-1 on Cartilage Explants
MMP activity by cartilage explants was unchanged by the addition of increasing concentrations of visfatin (Figure 5A). Independent of visfatin, IL-1 upregulated MMP activity in the media of cartilage explants (p < 0.01). The 100 ng/mL concentration of visfatin increased S-GAG release into the media, as compared to control (Figure 5B, p < 0.05). In both the presence and absence of visfatin, IL-1 promoted the release of S-GAG from cartilage explants (p < 0.01). Additionally, IL-1 and 10 ng/mL visfatin synergized to enhance S-GAG release, as compared to IL-1 and 100 ng/mL visfatin (p < 0.05). NO release by cartilage explants was also enhanced by 100 ng/mL visfatin (Figure 5C, p < 0.01). IL-1 treatment of cartilage explants increased NO release (p < 0.01), however there was no synergism between IL-1 and 100 ng/mL visfatin.
FIG. 5.
Effects of visfatin and IL-1 on cartilage explants. MMP activity (A), S-GAG release (B), and NO production (C) were measured in the media of cartilage explants that were exposed to 0 – 100 ng/mL visfatin in the presence or absence of 1 ng/mL IL-1 (n ≥ 9 per treatment group). (A) The MMP activity is expressed as fluorescence units (FU)/µg wet weight + standard error. (B) The S-GAG release is expressed as µg S-GAG/mg wet weight + standard error. (C) The NO release is expressed as µmol/g wet weight + standard error. *: p < 0.01 compared to control, 1 ng/mL visfatin, 10 ng/mL visfatin, and 100 ng/mL visfatin. #: p < 0.05 compared to control and 1 ng/mL visfatin. +: p < 0.05 compared to IL-1 + 100 ng/mL visfatin. ^: p < 0.01 compared to control, 1 ng/mL visfatin, and 10 ng/mL visfatin.
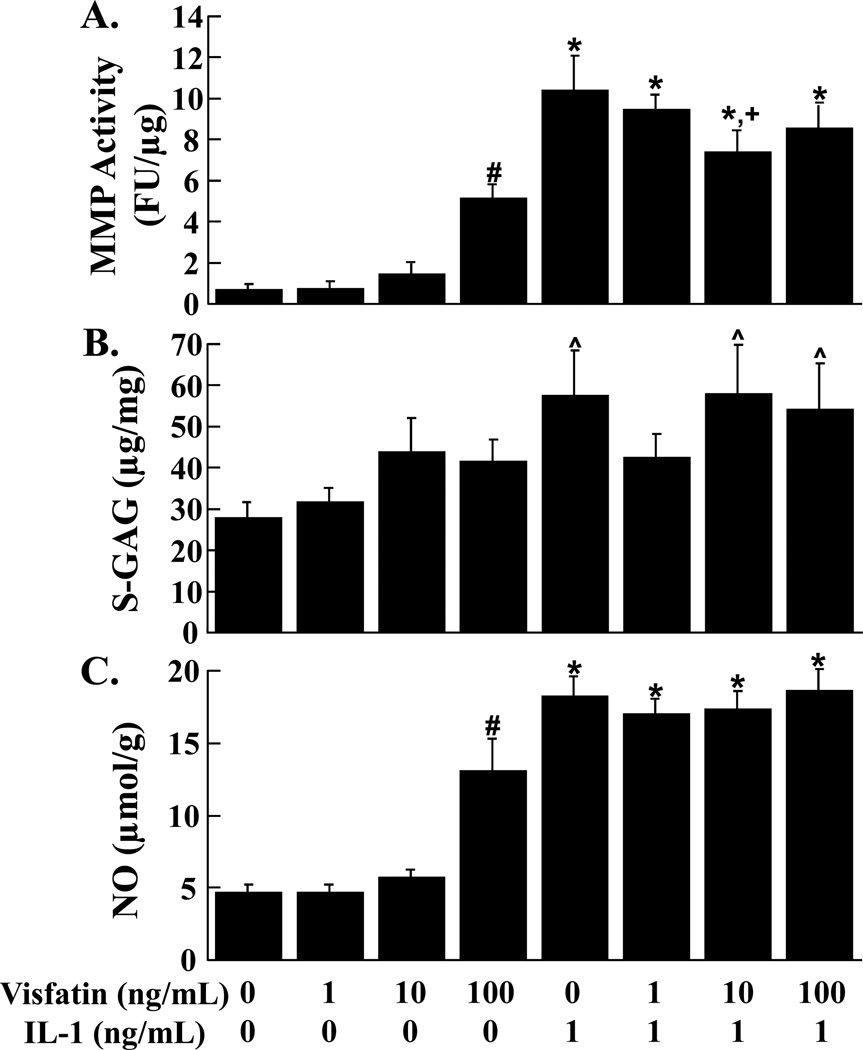
Effects of Visfatin and IL-1 on Meniscal Explants
MMP activity (Figure 6A, p < 0.05) and NO release (Figure 6C, p < 0.05) from meniscal explants were enhanced by addition of 100 ng/mL visfatin. Treatment of meniscal explants with IL-1 resulted in upregulation of MMP activity and NO release (p < 0.05). IL-1 mediated MMP activity was suppressed by 10 ng/mL visfatin (p < 0.05). Visfatin did not alter S-GAG release from meniscal explants (Figure 6B). S-GAG release was upregulated by IL-1 alone, IL-1 and 10 ng/mL visfatin, and IL-1 and 100 ng/mL visfatin, as compared to control (p < 0.05).
FIG. 6.
Effects of visfatin and IL-1 on meniscal explants. MMP activity (A), S-GAG release (B), and NO production (C) were measured in the media of meniscal explants that were exposed to 0 – 100 ng/mL visfatin in the presence or absence of 1 ng/mL IL-1 (n ≥ 9 per treatment group). (A) The MMP activity is expressed as fluorescence units (FU)/µg wet weight + standard error. (B) The S-GAG release is expressed as µg S-GAG/mg wet weight + standard error. (C) The NO release is expressed as µmol/g wet weight + standard error. *: p < 0.05 compared to control, 1 ng/mL visfatin, 10 ng/mL visfatin, and 100 ng/mL visfatin. #: p < 0.05 compared to all other treatment groups. +: p < 0.05 compared to IL-1 treated explants. ^: p < 0.05 compared to control and 1 ng/mL visfatin.
DISCUSSION
Our results demonstrated that visfatin and IL-1 promoted the catabolism of cartilage and meniscus, whereas physiologic concentrations of leptin or IL-6 did not alter MMP activity, S-GAG release, or NO production in either cartilage or meniscus explants. Consistent with previous studies, IL-1 upregulated MMP activity, S-GAG release, and NO production in cartilage and meniscus. A novel result of the present study was that physiologic concentrations of visfatin increased cartilage S-GAG release and NO production and enhanced MMP activity and NO release from the meniscus. Visfatin synergized with IL-1 to upregulate cartilage S-GAG release but suppressed the IL-1 mediated MMP activity in meniscus. Furthermore in cartilage, sIL-6R synergized with IL-1 to enhance MMP activity and NO production, but suppressed the IL-1 mediated S-GAG release. IL-6 alone also suppressed the IL-1 mediated cartilage S-GAG release and IL-6 and sIL-6R decreased the IL-1 effects on meniscus S-GAG release. Our findings support the hypothesis that specific adipokines (i.e., visfatin) can induce catabolism of cartilage and meniscus, exhibiting synergistic catabolic effects with IL-1. However, the findings that leptin or IL-6, alone or in combination with IL-1, do not have direct acute effects on the catabolism of these tissues suggests that cartilage and meniscus tissue degradation associated with obesity is not from a direct effect of these adipokines.
The current study did not include two other major adipokines, adiponectin and resistin, which future studies may wish to address. Adiponectin synovial fluid concentrations are decreased with increasing severity of OA, suggesting a possible protective role of adiponectin against OA [57]. In addition, adiponectin is negatively associated with knee OA scores in a mouse model of diet induced obesity [19]. Therefore, it is unlikely that adiponectin exerts catabolic effects on cartilage and meniscus that may contribute to obesity induced OA. On the other hand, while resistin levels have been shown to be unchanged in plasma samples from RA patients as compared to non-arthritic patients [14], they are elevated in synovial fluid from RA patients [58–60]. RA serum and synovial fluid resistin concentrations are significantly higher than in OA patients. Additionally, resistin levels in OA patients are significantly lower in the synovial fluid than in paired serum samples [59]. These data suggest a possible role for resistin in the systemic inflammatory disease of RA but a less likely role for resistin in joint tissue degradation.
Previous studies have shown varying effects of leptin or IL-6 on chondrocytes. Many of the differences between the various studies may be attributable to the inherent differences in the response of chondrocytes that have been enzymatically isolated versus chondrocytes that are cultured in the context of the cartilage extracellular matrix, as well as the arthritic state of the tissue. In addition, some experiments have included serum in the culture media, whereas other experiments have been performed in serum-free conditions. Furthermore, the inherent differences between species used in different studies may also explain some of the conflicting results in the literature. In our studies, we utilized non-arthritic porcine cartilage and meniscus explants to better maintain the chondrocytes and meniscal cells in the native environment of the extracellular matrix.
In this study, we observed no effect of leptin on cartilage and meniscus catabolism, as measured by MMP activity or S-GAG release. Porcine chondrocytes express the Ob-Rb leptin receptor [61], and therefore should be able to activate signaling cascades in response to leptin treatment. In previous studies, leptin has been shown to decrease chondrocyte proliferation, increase the release of IL-1β, and increase MMP-9 and MMP-13 protein expression after 5 – 7 days in serum free culture of freshly-isolated chondrocytes [18]. In contrast, in the present study our results showed no increase in MMP activity after 72 hours, which may be due to the presence of serum, the use of cartilage explants, lower leptin concentrations, and/or the shorter time in culture. Cartilage from OA patients produces increased MMP-1 and MMP-3 levels that correlate with synovial fluid leptin concentrations [62], suggesting a catabolic role for leptin in OA tissues. Similar to our results, primary human chondrocytes cultured in serum-free conditions with leptin alone did not modulate NO; however IL-1 and 12,800 ng/mL leptin (as compared to a maximum of 100 ng/mL in the present study) synergized to increase NO release [63]. Furthermore, intra-articular injection of 2 mg/mL leptin (approximately 100,000 times the physiological levels) into the rat knee increases MMP expression and production and decreases proteoglycan staining [64]. These observed effects of leptin likely reflect the tremendously high concentrations tested, as compared to the present study. Human OA cartilage shows elevated expression of the leptin receptor Ob-Rb [18], therefore it is not surprising that studies using human OA cartilage explants have shown that 100 ng/mL leptin increases NO release [40], whereas we did not observe a change in non-arthritic explants. In addition, consistent with our lack of catabolic response in the presence of leptin, Simopoulou and coworkers have shown that 100 ng/mL leptin decreases the expression of the leptin Ob-Rb receptor in normal cartilage [18]. The supra-physiologic concentration of 10,000 ng/mL leptin was required to increase inducible nitric oxide synthase (iNOS) mRNA and NO release and synergize with IL-1β to further upregulate these pro-inflammatory factors [40]. A recent study by Pallu and coworkers shows a positive association between chondrocyte responsiveness and BMI in primary OA chondrocytes treated with 500 ng/mL leptin but a negative association when treated with 100 ng/mL leptin [65].
There is little or no previous data on the effects of leptin on meniscus, and our results suggest that, similar to cartilage, physiologic concentrations of leptin do not have direct catabolic effects on meniscus. Leptin signaling may be necessary for the development of OA in obese individuals, since leptin knockout mice and leptin receptor deficient mice do not develop OA, despite extreme obesity [20]. However, the role of leptin in OA may not involve direct effects on cartilage or meniscus catabolism at physiologic concentrations. Nonetheless, the significant association of OA with leptin or IL-6 [17, 66–68], suggests that the effects of these adipokines on joint degeneration may be regulated through their effects on other tissues, such as bone [69].
IL-6, sIL-6R, and the combination of IL-6 and sIL-6R did not alter cartilage or meniscus breakdown in this study. Consistent with our results, other studies have demonstrated that IL-6 alone does not alter collagenase production [70] and the combination of IL-6 and sIL-6R has no effect on S-GAG release [43, 71]. In addition, IL-6 and sIL-6R have been shown to increase TIMP-1 expression [42] and production and increase MMP-3, resulting in no change in the ratio of MMP-3/TIMP-1 [32] and thus likely no net change in MMP activity. In serum-starved isolated bovine chondrocytes, IL-6 and IL-6R have been shown to increase the mRNA expression of MMP-1, MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 [72]; however, synthesis and activity of these enzymes was not assessed. The total MMP activity in these experiments was a measure of the activity of both the pro-MMPs and active MMPs secreted into the media. On average, the active MMPs accounted for 10 – 20% of the total MMP activity (data not shown), therefore the majority of the MMPs were in the pro-enzyme form. Thus, longer time in culture and/or increased expression of MMP activators [73], such as plasmin and membrane type MMPs (MT-MMPs), likely would contribute to an increase in the active fraction of MMPs.
The combination of sIL-6R and IL-1 resulted in an additional increase in MMP activity and NO release, as compared to IL-1 alone in cartilage explants. IL-1 may be inducing an increase in endogenous IL-6, which in the presence of the sIL-6R is able to promote these changes, similar to previous observations [36]. However, upon addition of exogenous IL-6 this increase was no longer observed. The mechanism for this phenomenon is not known, but may be due to exogenous IL-6 failing to activate these signaling cascades and downregulating the endogenous IL-6, as has been shown previously in an ischemia/reperfusion injury model where exogenous IL-6 administration decreased serum concentrations of IL-6 [74]. Additionally, previous studies have also shown that IL-6 may have anti-inflammatory effects and promotes the production of IL-1 receptor antagonist (IL-1ra) [75, 76]. On the other hand, we found that the combination of IL-6 and sIL-6R in the presence of IL-1 decreased S-GAG release, compared to IL-1 treated cartilage, suggesting that the breakdown of the S-GAGs in this case is mediated by proteolytic enzymes other than MMPs. In serum-free bovine cartilage explants, IL-6 and sIL-6R in combination with IL-1 synergizes to increase GAG release that is mediated by aggrecanase-2 and not MMPs [43], suggesting that other degradative enzymes could be mediating S-GAG release.
In meniscal explants, the combination of IL-6, sIL-6R, and IL-1 suppressed the IL-1 mediated release of S-GAGs into the media, suggesting that the effects of IL-6 on cartilage and meniscus are slightly different. While the effects of IL-6 on the meniscus have not been previously reported, these findings may reflect the relatively low concentration of proteoglycans in native meniscus, as compared to articular cartilage. In IL-6 knockout mice, old males but not females develop more advanced OA [77], highlighting the importance of sex on the activity of IL-6. Other studies have shown a species-specificity to IL-6 effects, since IL-6 and sIL-6R treatment did not change the biosynthesis of proteoglycans and collagens in bovine cartilage but decreased synthesis in human cartilage [71]. Cross-species homologies for IL-6 are only approximately 50% [36] so there may be low species cross-reactivity of human sIL-6R and porcine IL-6. IL-6 and sIL-6R and IL-1β treatment of human OA chondrocytes in alginate synergized to decrease MMP-3 after day 3 in culture [32], suggesting that the time course of these events may be critical. Consistent with our results, IL-6 has been shown to be an unnecessary cofactor in IL-1 induced proteoglycan synthesis suppression and NO release [78]. However, tocilizumab, a humanized anti-IL-6R monoclonal antibody has proven to be an effective treatment to reduce the severity of RA [35] and serum IL-6 levels are associated with radiographic knee OA and cartilage loss over three years [66], suggesting that in vivo IL-6 signaling is important during arthritis disease progression.
Our results show that visfatin enhances cartilage and meniscus catabolism. Cartilage MMP activity trended towards an increase with increasing concentrations of visfatin. A previous study that used supraphysiologic concentrations of visfatin in immature mouse chondrocytes reported that visfatin increased MMP-3 and MMP-13 synthesis and release [49]. Interestingly, the 10 ng/mL dose of visfatin synergized with IL-1 to increase S-GAG release from cartilage, while this same combination suppressed MMP activity in meniscus. Previous studies in chondrocytes point to the ability of visfatin to synergize with IL-1β, resulting in an increase in PGE2 production [49]. Therefore, visfatin has slightly different signaling pathways in cartilage and meniscus. Our study provides the first analysis of the effects of visfatin on meniscus. Interestingly, in a collagen-induced arthritis mouse model, visfatin expression was increased in both the serum and arthritic paw [79]. Inhibition of visfatin with APO866, a competitive inhibitor of visfatin enzymatic function, in this animal model decreased arthritis severity and also IL-1β and IL-6 production. Furthermore, there is a positive correlation between synovial fluid visfatin concentrations and cartilage matrix degradation [80], suggesting the importance of visfatin in arthritis progression.
CONCLUSIONS
In conclusion, we have shown that visfatin and IL-1 promoted the catabolic degradation of both cartilage and meniscus, as evidenced by increased MMP activity, NO production, and proteoglycan release. However, leptin or IL-6 at physiologic concentrations had no effect on the breakdown of these tissues. These findings suggest that the effects of obesity-induced OA may not be through a direct effect of leptin or IL-6 on cartilage or meniscus, but support a potential role for increased visfatin levels in this regard. These data provide an important first step in understanding the potential role of adipokines in regulating cartilage and meniscus metabolism; however, these adipokines may have different effects in the context of the whole joint and must be evaluated further.
ACKNOWLEDGMENTS
This study was supported by the Arthritis Foundation and NIH Grants AR50245, AG15768, AR48182, AR48852, and AR55434. We thank Poston Pritchett and Katherine Riera for technical assistance.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Kraus VB. Pathogenesis and Treatment of Osteoarthritis. Medical Clinics of North America. 1997;81(1):85–112. doi: 10.1016/s0025-7125(05)70506-x. [DOI] [PubMed] [Google Scholar]
- 2.Poole AR, Guilak F, Abramson SB. Etiopathogenesis of Osteoarthritis. In: Moskowitz RW, Altman R, Hochberg M, Buckwalter J, Goldberg VM, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4th Ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 27–49. [Google Scholar]
- 3.Hochberg MC. Osteoarthritis: Clinical Features and Treatment. In: Klippel JH, editor. Primer on the Rheumatic Diseases. 11 ed. Atlanta: Arthritis Foundation; 1997. pp. 218–221. [Google Scholar]
- 4.Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996 Jul;23(7):1221–1226. [PubMed] [Google Scholar]
- 5.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozkoc G, Circi E, Gonc U, Irgit K, Pourbagher A, Tandogan RN. Radial tears in the root of the posterior horn of the medial meniscus. Knee Surg Sports Traumatol Arthrosc. 2008 Sep;16(9):849–854. doi: 10.1007/s00167-008-0569-z. [DOI] [PubMed] [Google Scholar]
- 7.Wyland DJ, Guilak F, Elliott DM, Setton LA, Vail TP. Chondropathy after meniscal tear or partial meniscectomy in a canine model. J Orthop Res. 2002 Sep;20(5):996–1002. doi: 10.1016/S0736-0266(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 8.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy-the Journal of Arthroscopic and Related Surgery. 2005 Nov;21(11):1366–1369. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998 Apr;41(4):687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, Guermazi A, Grigorian M, Gale D, Felson DT. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006 Mar;54(3):795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 11.Burger C, Kabir K, Mueller M, Rangger C, Minor T, Tolba RH. Retropatellar chondromalacia associated with medial osteoarthritis after meniscus injury. One year of observations in sheep. Eur Surg Res. 2006;38(2):102–108. doi: 10.1159/000093281. [DOI] [PubMed] [Google Scholar]
- 12.Lindhorst E, Vail TP, Guilak F, Wang H, Setton LA, Vilim V, Kraus VB. Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. J Orthop Res. 2000 Mar;18(2):269–280. doi: 10.1002/jor.1100180216. [DOI] [PubMed] [Google Scholar]
- 13.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and Cellular Endocrinology. 2010 Mar 25;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006 Sep;65(9):1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding C, Parameswaran V, Cicuttini F, Burgess J, Zhai G, Quinn S, Jones G. Association between leptin, body composition, sex and knee cartilage morphology in older adults: the Tasmanian older adult cohort (TASOAC) study. Ann Rheum Dis. 2008 Sep;67(9):1256–1261. doi: 10.1136/ard.2007.082651. [DOI] [PubMed] [Google Scholar]
- 16.Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, Cho HL. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009 Dec;28(12):1431–1435. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 17.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003 Nov;48(11):3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 18.Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A. Differential expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007 Aug;15(8):872–883. doi: 10.1016/j.joca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, Cao L, Setton LA, Guilak F. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12(4):R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009 Oct;60(10):2935–2944. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–246. [PubMed] [Google Scholar]
- 22.Lotz M. Cytokines in cartilage injury and repair. Clinical Orthopaedics & Related Research. 2001;391(Suppl)(391 Suppl):S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins SJ, Humphreys M, Jayson MI. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clinical & Experimental Immunology. 1988;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- 24.Kahle P, Saal JG, Schaudt K, Zacher J, Fritz P, Pawelec G. Determination of cytokines in synovial fluids: correlation with diagnosis and histomorphological characteristics of synovial tissue. Annals of the Rheumatic Diseases. 1992;51(6):731–734. doi: 10.1136/ard.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995 Feb;43:104–108. [PubMed] [Google Scholar]
- 26.van den Berg WB, Joosten LA, van de Loo FA. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999 Nov-Dec;17(Suppl 18)(6):S105–S114. [PubMed] [Google Scholar]
- 27.Hubbard JR, Steinberg JJ, Bednar MS, Sledge CB. Effect of purified human interleukin-1 on cartilage degradation. J Orthop Res. 1988;6(2):180–187. doi: 10.1002/jor.1100060204. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 1993 Aug;19(3):545–568. [PubMed] [Google Scholar]
- 29.Stefanovic-Racic M, Mollers MO, Miller LA, Evans CH. Nitric oxide and proteoglycan turnover in rabbit articular cartilage. J Orthop Res. 1997 May;15(3):442–449. doi: 10.1002/jor.1100150318. [DOI] [PubMed] [Google Scholar]
- 30.Cao M, Stefanovic-Racic M, Georgescu HI, Miller LA, Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. J Orthop Res. 1998 Jan;16(1):104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]
- 31.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003 Jul;95(1):308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez C, Deberg MA, Burton S, Devel P, Reginster JY, Henrotin YE. Differential regulation of chondrocyte metabolism by oncostatin M and interleukin-6. Osteoarthritis Cartilage. 2004 Oct;12(10):801–810. doi: 10.1016/j.joca.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- 34.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, Spector TD. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009 Jul;60(7):2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonseca JE, Santos MJ, Canhao H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009 Jun;8(7):538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Rowan AD, Koshy PJ, Shingleton WD, Degnan BA, Heath JK, Vernallis AB, Spaull JR, Life PF, Hudson K, Cawston TE. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum. 2001 Jul;44(7):1620–1632. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 37.Desgeorges A, Gabay C, Silacci P, Novick D, Roux-Lombard P, Grau G, Dayer JM, Vischer T, Guerne PA. Concentrations and origins of soluble interleukin 6 receptor-alpha in serum and synovial fluid. J Rheumatol. 1997 Aug;24(8):1510–1516. [PubMed] [Google Scholar]
- 38.Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009 Nov;60(11):3374–3377. doi: 10.1002/art.24881. [DOI] [PubMed] [Google Scholar]
- 39.Loeser RF. Systemic and local regulation of articular cartilage metabolism: where does leptin fit in the puzzle? Arthritis Rheum. 2003 Nov;48(11):3009–3012. doi: 10.1002/art.11315. [DOI] [PubMed] [Google Scholar]
- 40.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Paivarinta U, Moilanen T, Moilanen E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otero M, Lago R, Gomez R, Dieguez C, Lago F, Gomez-Reino J, Gualillo O. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 2006 Aug;45(8):944–950. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 42.Silacci P, Dayer JM, Desgeorges A, Peter R, Manueddu C, Guerne PA. Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J Biol Chem. 1998 May 29;273(22):13625–13629. doi: 10.1074/jbc.273.22.13625. [DOI] [PubMed] [Google Scholar]
- 43.Flannery CR, Little CB, Hughes CE, Curtis CL, Caterson B, Jones SA. IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Biol. 2000 Nov;19(6):549–553. doi: 10.1016/s0945-053x(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 44.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, Bryant-Greenwood G, Jones SA. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006 Jul;54(7):2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 45.Bao JP, Chen WP, Wu LD. Visfatin: a potential therapeutic target for rheumatoid arthritis. J Int Med Res. 2009 Nov-Dec;37(6):1655–1661. doi: 10.1177/147323000903700601. [DOI] [PubMed] [Google Scholar]
- 46.Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, Shintani A, Pincus T, Stein CM. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009 Jul;60(7):1906–1914. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, Gay S, Kyburz D. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007 Sep;56(9):2829–2839. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- 48.Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ, Toes RE, Ioan-Facsinay A. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011 Jan 17; doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 49.Gosset M, Berenbaum F, Salvat C, Sautet A, Pigenet A, Tahiri K, Jacques C. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum. 2008 May;58(5):1399–1409. doi: 10.1002/art.23431. [DOI] [PubMed] [Google Scholar]
- 50.Deng S-J, Bickett DM, Mitchell JL, Lambert MH, Blackburn RK, Carter HLC, III, Neugebauer J, Pahel G, Weiner MP, Moss ML. Substrate Specificity of Human Collagenase 3 Assessed Using a Phage-displayed Peptide Library. The Journal of Biological Chemistry. 2000 Oct 6;275:31422–31427. doi: 10.1074/jbc.M004538200. 2000. [DOI] [PubMed] [Google Scholar]
- 51.McNulty AL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage. 2010 Jun;18(6):830–838. doi: 10.1016/j.joca.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009 Jun;467(6):1557–1567. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of Integrative Repair of the Meniscus Following Acute Exposure to Interleukin-1 in vitro. Journal of Orthopaedic Research. 2008;26:504–512. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 55.Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol. 1999;301:49–61. doi: 10.1016/s0076-6879(99)01068-x. [DOI] [PubMed] [Google Scholar]
- 56.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002 Oct;10(10):792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 57.Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010 Nov;41(8):593–598. doi: 10.1016/j.arcmed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, Muller-Ladner U, Gay S. Adipocytokines in synovial fluid. JAMA. 2003 Oct 1;290(13):1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 59.Senolt L, Housa D, Vernerova Z, Jirasek T, Svobodova R, Veigl D, Anderlova K, Muller-Ladner U, Pavelka K, Haluzik M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis. 2007 Apr;66(4):458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005 May 1;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 61.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000 Aug 25;92(1–3):73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 62.Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp Rheumatol. 2011 Jan-Feb;29(1):57–64. [PubMed] [Google Scholar]
- 63.Otero M, Lago R, Lago F, Reino JJ, Gualillo O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res Ther. 2005;7(3):R581–R591. doi: 10.1186/ar1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bao JP, Chen WP, Feng J, Hu PF, Shi ZL, Wu LD. Leptin plays a catabolic role on articular cartilage. Mol Biol Rep. 2010 Oct;37(7):3265–3272. doi: 10.1007/s11033-009-9911-x. [DOI] [PubMed] [Google Scholar]
- 65.Pallu S, Francin PJ, Guillaume C, Gegout-Pottie P, Netter P, Mainard D, Terlain B, Presle N. Obesity affects the chondrocyte responsiveness to leptin in patients with osteoarthritis. Arthritis Res Ther. 2010;12(3):R112. doi: 10.1186/ar3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, Ding C. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010 Nov;18(11):1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Gandhi R, Takahashi M, Smith H, Rizek R, Mahomed NN. The synovial fluid adiponectin-leptin ratio predicts pain with knee osteoarthritis. Clin Rheumatol. 2010 Nov;29(11):1223–1228. doi: 10.1007/s10067-010-1429-z. [DOI] [PubMed] [Google Scholar]
- 68.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45(3–4):387–398. [PMC free article] [PubMed] [Google Scholar]
- 69.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lotz M, Guerne PA. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA) J Biol Chem. 1991 Feb 5;266(4):2017–2020. [PubMed] [Google Scholar]
- 71.Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, Plaas AH, James IE, Song XY, Lark MW, Grodzinsky AJ. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009 Oct;60(10):2985–2996. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 72.Legendre F, Bogdanowicz P, Boumediene K, Pujol JP. Role of interleukin 6 (IL-6)/IL-6R-induced signal tranducers and activators of transcription and mitogen-activated protein kinase/extracellular. J Rheumatol. 2005 Jul;32(7):1307–1316. [PubMed] [Google Scholar]
- 73.Milner JM, Elliott S-F, Cawston TE. Activation of Procollagenases is a Key Control Point in Cartilage Collagen Degradation: Interaction of Serine and Metalloproteinase Pathways. Arthritis and Rheumatism. 2001 Sep;44(9):2084–2096. doi: 10.1002/1529-0131(200109)44:9<2084::AID-ART359>3.0.CO;2-R. 2001. [DOI] [PubMed] [Google Scholar]
- 74.Kimizuka K, Nakao A, Nalesnik MA, Demetris AJ, Uchiyama T, Ruppert K, Fink MP, Stolz DB, Murase N. Exogenous IL-6 inhibits acute inflammatory responses and prevents ischemia/reperfusion injury after intestinal transplantation. Am J Transplant. 2004 Apr;4(4):482–494. doi: 10.1111/j.1600-6143.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 75.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003 Aug;285(2):E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 76.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994 Jan 1;83(1):113–118. [PubMed] [Google Scholar]
- 77.de Hooge AS, van de Loo FA, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage. 2005 Jan;13(1):66–73. doi: 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Van de Loo FA, Arntz OJ, Van den Berg WB. Effect of interleukin 1 and leukaemia inhibitory factor on chondrocyte metabolism in articular cartilage from normal and interleukin-6-deficient mice: role of nitric oxide and IL-6 in the suppression of proteoglycan synthesis. Cytokine. 1997 Jul;9(7):453–462. doi: 10.1006/cyto.1997.0188. [DOI] [PubMed] [Google Scholar]
- 79.Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, Leo O, So A, De Smedt T. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008;3(5):e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duan Y, Hao D, Li M, Wu Z, Li D, Yang X, Qiu G. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatol Int. 2011 Jan 19; doi: 10.1007/s00296-010-1731-8. [DOI] [PubMed] [Google Scholar]
- 81.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006 Jul;14(7):690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003 Feb;62(2):108–112. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003 Mar;10(1):93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 84.Higuchi H, Shirakura K, Kimura M, Terauchi M, Shinozaki T, Watanabe H, Takagishi K. Changes in biochemical parameters after anterior cruciate ligament injury. Int Orthop. 2006 Feb;30(1):43–47. doi: 10.1007/s00264-005-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Suda T, Kashiwazaki S. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996 Jan;11(1):88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]