Abstract
Background
Maximal inspiratory pressure (MIP) is an important and non-invasive index of diaphragm strength and an independent predictor of all-cause mortality. The ability of adults over a wide age range and multiple ethnicities to perform MIP tests has previously not been evaluated.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited white, African-American, Hispanic and Chinese-American participants ages 45–84 years and free of clinical cardiovascular disease in six US cities. MIP was measured using standard techniques among 3849 MESA participants. The MIP quality goal was 5 maneuvers, with the two largest values matching within 10 cmH2O. Correlates of MIP quality and values were assessed in logistic and linear regression models.
Results
The 3849 MESA-Lung participants with MIP measures were 51% female, 35% white, 26% African-American, 23% Hispanic, and 16% Chinese-American. Mean MIP±SD was 73±26 cmH2O for women and 97±29 cmH2O for men. The quality goal was achieved by 83% of the cohort and was associated with female gender, older age, race/ethnicity, study site, low FEV1/FVC ratio, and wheeze with dyspnea. The multivariate correlates of MIP were male gender, younger age, higher BMI, shorter height, higher FVC, higher systolic blood pressure (in women) and health status (in men). There were no clinically important race/ethnic differences in MIP values.
Conclusion
Race-specific reference equations for MIP are unnecessary in the United States. More than 80% of adults can be successfully coached for 5 maneuvers with repeatability within 10 cmH2O.
Keywords: diaphragm strength, respiratory muscle strength, maximal inspiratory pressure, quality control, pulmonary function testing
INTRODUCTION
Maximal inspiratory pressure (MIP) is a measure of the strength of inspiratory muscles, primarily the diaphragm, and allows for the assessment of ventilatory failure, restrictive lung disease and respiratory muscle strength. The test is quick and non-invasive, but it is highly dependent on participant effort and coaching. The range of normal values is broad and low values should be interpreted relative to the lower limit of normal values for age and gender [1].
Respiratory muscle weakness is an independent predictor of all-cause mortality, and MIP has previously been shown to be associated with incident cardiovascular events including myocardial infarction, cardiovascular death and possibly stroke [2]. These associations were independent, in large part, of other measures of pulmonary function such as the forced vital capacity (FVC).
Previous studies have reported the correlates of MIP in older whites and African-Americans [3–6]. However, these studies could not adequately evaluate potential race/ethnic differences in MIP due to limited minority recruitment [3–5] or recruitment of minorities at only one study site [6]. This type of confounding can be substantial, since MIP is highly dependent on coaching by individual technologists.
We evaluated correlates of MIP in a large multiethnic study which was specifically designed to avoid site-by-race confounding [7], and examined potential differences in MIP across these four race/ethnic groups in the whole cohort and in a subset of healthy participants. We also described correlates of achieving a MIP quality goal and provided reference equations from the healthy subset of the cohort.
METHODS
Study Sample
MESA is a multicenter prospective cohort study to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in individuals without clinical cardiovascular disease [7]. In 2000–2002, MESA recruited 6,814 men and women ages 45–84 years old from six U.S. communities. (See Table 1 for the list.) MESA participants are non-Hispanic white, African-American, Hispanic, or Asian (of Chinese origin). Multiple race/ethnic groups were recruited at all six sites. Exclusion criteria included clinical cardiovascular disease (physician diagnosis of heart attack, stroke, transient ischemic attack, heart failure, or angina), current atrial fibrillation, any cardiovascular procedure, pregnancy, active cancer treatment, weight >300 lbs, serious medical condition which precluded long term participation, nursing home residence, cognitive inability, inability to speak English, Spanish, Cantonese, or Mandarin, plan to leave the community within five years, and chest CT within the past year. The protocols were approved by the Institutional Review Boards of all collaborating institutions and the National Heart Lung Blood Institute.
Table 1.
Characteristics of the 3849 participants who completed maximal inspiratory pressure (MIP) measures in the MESA-Lung Study.
| Study site, % | |
| Winston-Salem, NC | 14.5 |
| New York, NY | 18.6 |
| Baltimore, MD | 12.1 |
| Minneapolis/St. Paul, MN | 14.1 |
| Chicago, IL | 18.7 |
| Los Angeles, CA | 21.9 |
| Height, mean ± SD, cm | 165.9 ± 10.0 |
| BMI, kg/m2, % | |
| <18.5 | 3.2 |
| 18.5–25 | 28.9 |
| 25–30 | 37.2 |
| 30–35 | 19.8 |
| >35 | 10.8 |
| Cigarette smoking status, % | |
| Never | 50.9 |
| Past | 39.4 |
| Current | 9.7 |
| Physician-diagnosed asthma | 11.5% |
| FVC, mean ± SD, L | 3.19 ± 0.96 |
| FEV1, mean ± SD, L | 2.38 ± 0.73 |
| FVC % pred, mean ± SD, % | 95.3 ± 16.9 |
| FEV1, % pred, mean ± SD, % | 93.7 ± 18.4 |
| FEV1/FVC, mean ± SD | 0.75 ± 0.09 |
| Health Status, % | |
| Better than average | 60.3 |
| Average | 35.1 |
| Worse than average | 4.6 |
Abbreviations: SD = standard deviation; BMI = body mass index
The MESA-Lung Study enrolled 3965 participants in 2004–2006 of 4483 eligible participants who were sampled randomly among MESA participants who consented to genetic analyses, underwent baseline measures of endothelial function, and attended MESA Exam 3 or 4 (99%, 89%, and 88% of the MESA cohort, respectively). Chinese-Americans were over-sampled, such that the final cohort was 35% white, 26% African-American, 23% Hispanic, and 16% Chinese-American.
Instruments and Training
The same mechanical pressure gauge and testing techniques used by the Cardiovascular Health Study was used for this study [3]. The methods were consistent with American Thoracic Society guidelines [8]. The accuracy of each pressure gauge was checked using a mercury manometer at 50 cmH2O and verified to be within 5%. A manual of procedures was written and distributed to the six field centers. At the beginning of the study, technologists from the six field centers were centrally trained and certified for spirometry and MIP testing.
MIP Test Methods
Each participant was asked to perform five MIP maneuvers, with a goal of matching the highest two within 10 cmH2O. MIP was recorded to the nearest 5 cmH2O using a differential pressure gauge fitted with a disposable cardboard mouthpiece. The patient was seated for the test. The technologists first demonstrated the correct maneuver. The participant was instructed to exhale slowly and completely, seal lips firmly around the new mouthpiece, and then “pull in hard, like you are trying to suck up a thick milkshake.” The technologist noted the largest negative pressure sustained for at least one second on the pressure gauge. The participant was allowed to rest for about one minute and then repeat the maneuver 5 times. The pressure gauge has minor tick marks at 5 cmH2O increments, so results were rounded to the nearest 5 cmH2O.
We repeated MIP measurements on a 5% random quality control sample of participants to confirm reproducibility of the measurements in this study.
Covariate Information
Age, self-identified race/ethnicity, subjective health status and smoking status were assessed by questionnaires. Pack years was calculated as the number of years smoked times the average number of cigarettes per day divided by 20. Height and weight were measured by trained technicians using a stadiometer and a balancing scale, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Wheeze with dyspnea was defined as a positive response to the item, “In the last 12 months, have you had an attack of wheezing or whistling in the chest that has made you feel short of breath?” Participants were asked what language they speak at home. If the technician who performed the MIP exam was fluent in that language then they were classified as language concordant.
Spirometry was measured following and meeting ATS standards [9], as previously described [10]. Predicted values were calculated using Hankinson reference equations for whites, African-Americans and Hispanics [11], which we have previously validated for use in this cohort [12]. We used a correction factor of 0.88 for Chinese-Americans (using reference equations for FEV1 and FVC for whites), which we found to be superior to other approaches for this cohort [12].
Statistical Analyses
We defined MIP difference as the difference between the highest and second highest MIP values. The MIP quality goal was defined as completion of exactly five MIP maneuvers with the MIP difference less than 10 cmH2O (adequate repeatability). Using a logistic regression model with generalized estimating equations, we examined the relationship between MIP test success and age, gender, ethnicity, education, income, study site, height, BMI, smoking status, pack years of smoking, asthma, FVC, forced expiratory volume in one second (FEV1), FEV1/FVC ratio, wheeze with dyspnea, subjective health status, technician language concordance and systolic blood pressure. Age and gender adjusted univariate models were fit first, followed by a multivariate model which included the terms that were significant at the P<0.10 level in the univariate models and family income. Family income was included in the model to better control for potential confounding of race/ethnicity by socioeconomic status. Generalized estimating equations were used to account for within-site correlation at the level of the technician [13]. There were 35 technologists who administered one or more MIP tests.
We defined the maximum MIP (max MIP) for each participant as the largest MIP from each participant's test session for participants who achieved the quality goal. Using a linear regression model, we examined the relationship between max MIP and age, height, BMI, race/ethnicity, study site, smoking status, FVC, systolic blood pressure, wheezes with dyspnea, and self-reported subjective health status. We developed gender-specific models using generalized estimating equations to account for the correlation within technician [13]. The standard errors are based on the empirical covariance estimates.
Since the overall sample included smokers and some patients with lung disease, we performed secondary analyses of race/ethic differences restricted to “healthy” participants, as previously defined [3]. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) and R version 2.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The characteristics of the 3,849 participants in the MESA-Lung Study who were asked to perform MIP measures are shown in Table 1. Mean age was 66 years, 51% were women; race/ethnicity was 35% white, 26% African-American, 23% Hispanic, and 16% Chinese-America. Half had smoked cigarettes and 60% reported their health as “better than average.” Mean MIP was 85 cmH2O (SD 30).
Achievement of Quality Goal
Eighty-three percent of those asked to perform MIP testing successfully achieved the quality goal (Table 1). Of the remaining 17%, two participants were unable to complete the required five tests and the remainder had a difference of the highest two measures of MIP of greater than 10 cmH2O. Overall, the median ± interquartile range of the difference between the highest and second highest MIP values was 5 ± 5 cmH2O for men and 5 ± 5 cmH2O for women. Among those who successfully achieved the quality goal, the median ± interquartile range of the difference between the highest and second highest MIP values was 5 ± 5 cmH20 for men and 0 ± 5 cmH20 for women.
Table 2 shows the age-sex-adjusted and independent correlates of achieving the quality goal for the MIP test of five MIP maneuvers with the highest and second highest MIP values within 10cmH2O. In age-sex-adjusted analyses, older age, female gender, race/ethnicity, study site, low FEV1 /FVC ratio, and worse health status were associated with successful achievement of the quality goal. In multivariate analyses, female gender, race/ethnicity, study site, low FEV1/FVC ratio, and wheeze with dyspnea were independently associated with achievement of the quality goal. (Older age was of borderline significance.)
Table 2.
Odds ratios for achieving the MIP test quality goal
| OR | 95% CI | P | |
|---|---|---|---|
|
| |||
| Age, +10 years | 1.15 | 1.07 – 1.23 | .0001 |
|
| |||
| Female gender | 1.32 | 1.02 – 1.70 | .003 |
|
| |||
| Race/ethnicity | .009 | ||
| Non-Hispanic White | 1.00 | ref | |
| African-American | 0.79 | 0.65 – 0.96 | |
| Chinese-American | 1.16 | 0.79 – 1.69 | |
| Hispanic | 0.85 | 0.66 – 1.10 | |
|
| |||
| Study site | .0003 | ||
| Winston-Salem | 0.60 | 0.34 – 1.05 | |
| New York | 0.46 | 0.32 – 0.64 | |
| Baltimore | 0.71 | 0.44 – 1.15 | |
| Minneapolis | 0.46 | 0.29 – 0.73 | |
| Chicago | 0.54 | 0.38 – 0.77 | |
| Los Angeles | 1.00 | ref | |
|
| |||
| FEV1/FVC | 0.28 | 0.10 – 0.79 | .02 |
|
| |||
| Health Status | .04 | ||
| Better than average | 0.81 | 0.68 – 0.97 | |
| Average | 1.00 | ref | |
| Worse than average | 1.08 | 0.69 – 1.71 | |
Abbreviations: OR = odds ratio, CI = confidence interval, ref = reference group
These variables remained significant predictors in a multivariate model which included age, gender, race, study site, income, FEV1/FVC, wheeze, and health status.
In order to further evaluate differences in quality by site, we examined the intra-class correlation coefficient (ICC) in the 5% quality control sample in whom MIP was repeated. The ICC is the ratio of the between subject variance of MIP to the total variance. ICCs did not vary significantly by site in the quality control sample. The ICCs were 84%, 89%, 86%, 88%, 89%, and 86% at Wake Forest, Columbia, Johns Hopkins, Minnesota, Northwestern, and Los Angeles, respectively. An ICC greater than 80% demonstrates excellent reproducibility. For comparison, the ICCs for FEV1 and FVC were both 99%.
Correlates of MIP
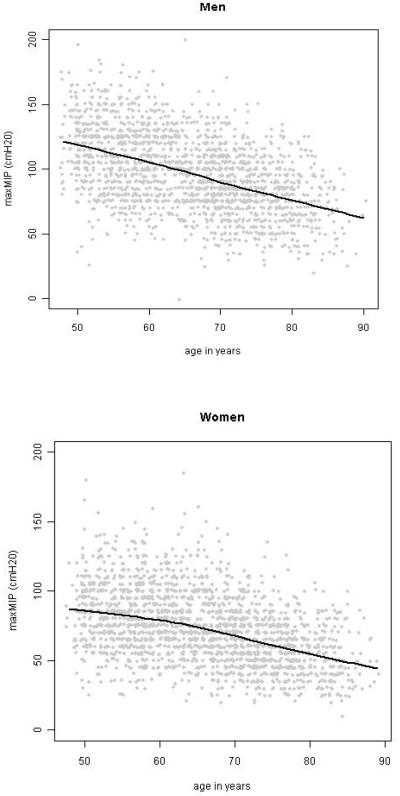
The distributions of MIP for men and women were both unimodal and approximately symmetric. Strong, linear associations were observed between MIP and age, BMI, and FVC in both genders, with the exception that the relationship between MIP and BMI flattened at a BMI of about 30 kg/m2 in men. The relationship between age and MIP is shown in Figure 1.
Figure 1.
The association of MIP with age, in adult men and women from the MESA-Lung study. Solid lines are lowess smooths.
The mean ± standard deviation MIP was 73 ± 26 cmH2O for women who achieved the quality goal. The age-sex-adjusted and multivariate correlates of MIP among women are shown in Table 3. The independent correlates of MIP among women from the multivariate model were younger age, ethnicity, study site, shorter height, higher BMI, higher FVC and higher systolic blood pressure. Higher MIP was correlated with higher height in age-adjusted analyses but with lower height in multivariate analyses. Race/ethnicity was significant in age-adjusted and multivariate analyses among women; however, the statistical significance was due to an observed difference principally among Chinese-Americans in age-adjusted analyses and small differences among African-Americans and Hispanics in multivariate analyses.
Table 3.
Predictors of MIP in Women (age adjusted)
| Coeff | 95% CI | p-value | ||
|---|---|---|---|---|
| Age (yrs) | −1.05 | −1.23 | −0.86 | <.0001 |
|
| ||||
| White | ref | <.0001 | ||
| African-American | 0.6 | −3.1 | 4.4 | |
| Chinese | −11.0 | −14.9 | −7.2 | |
| Hispanic | −4.1 | −8.1 | −0.1 | |
|
| ||||
| Wake Forest | 9.8 | 0.5 | 19.1 | 0.01 |
| Columbia | 2.7 | 2.6 | 16.5 | |
| Johns Hopkins | 7.7 | −0.3 | 5.7 | |
| Minnesota | 3.2 | −0.5 | 15.9 | |
| Northwestern | 3.2 | −2.8 | 9.2 | |
| UCLA | Ref | |||
|
| ||||
| Height (cm) | 0.4 | 0.3 | 0.6 | <.0001 |
|
| ||||
| BMI < 18.5 | −2.6 | −10.7 | 5.6 | <.0001 |
| BMI 18.5 – 25 | ref | |||
| BMI 25 – 30 | 3.8 | 1.0 | 6.7 | |
| BMI 30 – 35 | 7.6 | 3.3 | 11.9 | |
| BMI > 35 | 10.7 | 6.6 | 14.9 | |
|
| ||||
| FVC (liters) | 8.5 | 6.0 | 11.0 | <.0001 |
|
| ||||
| Systolic BP (mmHg) | 0.1 | 0.01 | 0.1 | 0.03 |
Abbreviations: Coeff = average difference in MIP per unit change in the variable CI = confidence interval, BMI = body mass index, ref = reference group.
These variables remained significant predictors in a multivariate model which included age, race, study site, height, BMI, smoking status, FVC, and systolic blood pressure.
Among men who achieved the quality goal, mean ± standard deviation MIP was 97 ± 29 cmH2O, which was significantly higher than the mean MIP among women (P<.0001). The age-sex-adjusted and multivariate correlates of MIP among men are shown in Table 4. The independent correlates of MIP among men from the multivariate model were younger age, study site, shorter height, higher BMI, and higher FVC. Similar to among women, higher MIP was correlated with taller height in age-adjusted analyses and shorter height in multivariate analyses among men. No statistically or clinically significant differences in MIP were observed by race/ethnicity in age-adjusted or multivariate models.
Table 4.
Predictors of MIP in Men (age adjusted)
| Coeff | 95% CI | p-value | ||
|---|---|---|---|---|
| Age (yrs) | −1.44 | −1.63 | −1.26 | <.0001 |
|
| ||||
| White | ref | ns | ||
| African-American | −3.6 | −7.7 | 0.6 | |
| Chinese | −5.8 | −11.3 | −0.2 | |
| Hispanic | 0.1 | −4.13 | 4.4 | |
|
| ||||
| Wake Forest | 2.5 | −4.0 | 9.0 | <.0001 |
| Columbia | 4.3 | −0.2 | 8.7 | |
| Johns Hopkins | −3.0 | −6.3 | 0.2 | |
| Minnesota | 8.0 | 3.8 | 12.1 | |
| Northwestern | 1.9 | −5.6 | 9.4 | |
| UCLA | Ref | |||
|
| ||||
| Height (cm) | 0.19 | 0.06 | 0.32 | 0.005 |
|
| ||||
| BMI < 18.5 | −7.6 | −15.5 | 0.4 | <.0001 |
| BMI 18.5 – 25 | ref | |||
| BMI 25 – 30 | 9.3 | 5.9 | 12.7 | |
| BMI 30 – 35 | 8.0 | 3.4 | 12.6 | |
| BMI > 35 | 3.3 | −2.1 | 8.8 | |
|
| ||||
| FVC (liters) | 8.1 | 6.09 | 10.0 | <.0001 |
|
| ||||
| Health >norm | 3.6 | 0.9 | 6.2 | <.0001 |
| health average | ref | |||
| health worse | −8.8 | −16.1 | −1.5 | |
Abbreviations: Coeff = average difference in MIP per unit change in the variable CI = confidence interval, BMI = body mass index, ref = reference group; ns = not significant. These variables remained significant predictors in a multivariate model which included age, race, study site, height, BMI, FVC, and health status.
MIP Reference Equations
We repeated analyses of MIP and race/ethnicity among non-smoking participants in good health. We excluded those who failed to achieve the quality goal, current smokers, those with any respiratory illness, and those with FEV1 < 65% of predicted, as suggested by a previous study [4]. 872 men and 883 women remained in the healthy subgroup. The lower limits of the normal range for men and women from our study were very similar to the LLNs from previous large studies of adults (see Figure 2). No significant interactions with ethnicity were found in the multivariate models. The gender-specific reference equations from our study are in Table 5.
Figure 2.
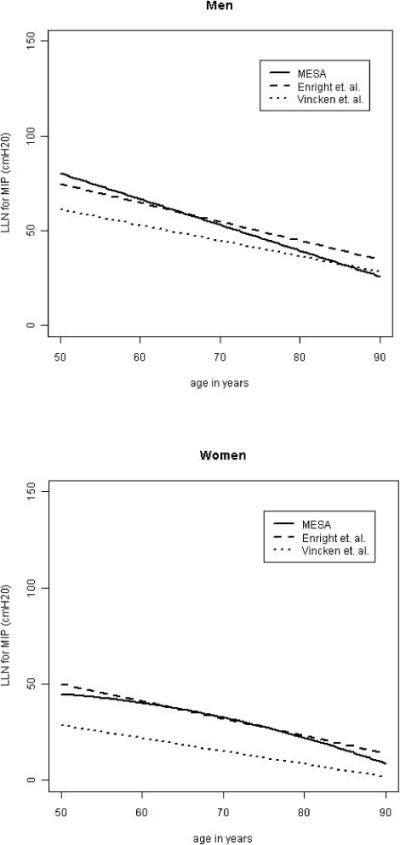
Comparison of the MIP lower limit of the normal range (LLN) from other studies (Vincken [14] and Enright [4]) stratified by gender, using average height (172cm for men, 158cm for women) and weight (177lbs for men, 150 lbs for women).
Table 5.
Gender-specific MIP reference equations
| coefficient | age | age2 | wt | wt2 | age*wt | ht | ht2 | LLN | R2 |
|---|---|---|---|---|---|---|---|---|---|
| men = +9.8 | −0.31 | 0 | 1.47 | −.0026 | −.0059 | 0 | 0 | 40 | 0.27 |
| women = −388 | 1.77 | −.0014 | 0.41 | 0 | −.0041 | 4.69 | −.014 | 36 | 0.21 |
Footnotes: MIP in cmH2O; weight in pounds; height in centimeters
To obtain the lower limit of the normal range (LLN) for men, subtract 40 from the predicted MIP (subtract 36 for women).
DISCUSSION
Male gender, younger age, obesity, higher FVC, and shorter height were strongly and independently associated with higher values of MIP in relatively healthy adults in four race/ethnic groups. In contrast, differences in MIP by race/ethnicity were of small magnitude and inconsistent statistical significance in the overall cohort, and were non-significant among the subset of non-smoking participants in good health. These results suggest that race/ethnic-specific reference equations for MIP are not warranted.
This is the first study of which we are aware to evaluate correlates of MIP in a multiethnic sample and is unique in avoiding site-by-race confounding by enrolling multiple ethnicities at all sites. The main correlates of higher values of MIP observed across the four race/ethnic groups in the present study, including male gender, younger age, obesity and higher FVC, are consistent with prior studies of MIP in whites and African-Americans [3–6]. We observed linear relationships between advancing age and lower MIP, similar to results from the Atherosclerosis Risk in Communities (ARIC) study, the largest study to date [6]. The relationship of height to MIP was positive in age-adjusted analyses in both the current study and ARIC. After multivariate adjustment for BMI and other correlates, height became non-significant among women in ARIC and inverse in the current study. Similar to ARIC, we observed a non-linear relationship of BMI to MIP among men, which was positive in the normal-to-over-weight range and was flat or slightly inverse in obesity. Among women, higher BMI was associated with higher MIP values among normal weight, overweight and obese women in both studies.
In contrast to other measures of lung function [12], values of MIP did not vary appreciably by race/ethnic group. There was no statistically significant difference in MIP by race/ethnicity among men in models controlling for multiple potential confounders. Mean MIP was higher among African-American women (+3.6 cmH20) and lower among Hispanic women (−5.2 cmH20) when compared to white women; however, these differences were small relative to the differences between men and women (23 cmH20), between technologists (up to 10 cmH20), or with advancing age (− 10 cmH20 per 10 years), and thus of little clinical significance. Restriction to non-smoking participants in good health, as has been previously used for the derivation of reference equations [4], yielded no significant differences by race/ethnicity. Neither of three prior studies that included whites and African-Americans observed a difference in MIP by race/ethnicity, although two were potentially limited by small numbers of African-Americans [3,5] and the other by site-by-race confounding [6].
We did not observe a significant relationship of current smoking and lower MIP, which has been noted in some [3, 5] but not other [14,15] prior studies. The negative association likely would have been significant had MESA enrolled more current smokers.
The best index of quality is the repeatability of the MIP tests completed, as measured by the difference between the highest and second highest value for test sessions with at least two non-zero MIP measurements. However, meeting the quality goal does not necessarily mean that the subject's effort was maximal [16]. Differences in the MIP quality measure by site, for example, did not correspond to differences in maximal MIP by site. Furthermore, ICCs in the 5% quality control replicate sample also did not vary by site.
About 83% of our participants met the quality goal of a 10 cmH2O match, a success rate similar to that of Cardiovascular Health Study participants [3]. MIP measurements depend greatly on patient effort, thus the technologist must be an enthusiastic coach. Many patients (both children and adults) exhibit a rather large learning effect, so that their best values during a test session are often obtained after several maneuvers, even up to fifteen [17, 18]. However, most MIP reference studies were done using 5 maneuvers, so most clinical laboratories follow the same procedure [8]. However, up to 3 additional maneuvers should be done if the last value was the highest or if the second highest value is not at least 90% of the highest value.
Achieving the quality goal was associated with female gender, older age, race/ethnicity, study site, low FEV1/FVC ratio, and wheeze with dyspnea. The quality of maximal inspiratory pressure (MIP) has been previously been found to be related to both participant and technician factors [19, 20]. Our results for gender, age and airway obstruction match prior studies. Although African-American participants achieved the quality goal less frequently than did white participants, mean MIP values for African-Americans were slightly higher than for whites, as noted above.
The impact of the technician can be large. We therefore used generalized estimating equations in our multivariate modeling to account for the potential technician clustering effect and adjusted all analyses by study site. There were 35 technicians who administered at least one MIP test. Study site remained a significant correlate of both achievement of the quality goal and mean MIP in multivariate models, as has been noted in prior studies [3, 6].
Limitations of our study include possible residual site-by-ethnicity confounding. For example, the majority of the Chinese-Americans were recruited from the Chicago site. Since mean MIP varies by technologist skills, the slightly lower mean MIP values for Chinese-Americans (when compared to other ethnic groups) could have been due to slightly less effort provided by the technologists in Chicago. The body habitus and nutrition of various ethnic groups within mainland China vary markedly, and we did not ascertain the location of birth and childhood within China of our study participants. Application of our study results is limited by the age range of our participants (45–84), which did not include children or young adults. Therefore, it is possible that MIP values for healthy African-American children differ substantially from healthy Japanese-American children.
In conclusion, male gender, younger age, and shorter height, obesity and higher FVC were strongly and independently associated with higher values of MIP in this large multiethnic cohort study. Unlike other measures of pulmonary function, no large or consistently significant differences in MIP were observed between whites, African-Americans, Hispanics and Chinese-Americans. This finding suggests that race-specific reference equations for MIP are unnecessary and that previously published reference values for MIP in healthy white adults [3, 14] are appropriate for patients in these four race/ethnic groups.
ACKNOWLEDGEMENTS
This research was supported by contracts N01-HC-95159 through N01-HC-95165, N01-HC95169 and grant R01-HL077612 from the National Heart, Lung, and Blood Institute. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The authors wish to thank participants in the MESA-Lung study at Wake Forest University, Columbia University, Johns Hopkins University, University of California-Los Angeles, University of Minnesota, and Northwestern University. We also wish to thank the other MESA-Lung investigators, Jeffrey Carr, Robert Detrano, John Hankinson, Eric Hoffman, Steve Kawut, Richard Kronmal, Kiang Liu, Naresh Punjabi, Eyal Shahar, Lewis J. Smith, and Russell Tracy, and the study technicians: Brenda Brewer, Terry Tembreull, Cathy Nunn, Corliss Cook, Diane Hightower, Cecilia Castro, Vijakumar Nayudupalli, Olga Gonzales, Flor Camarena, Sergio Teruya, Carol Christman, Rosie Zelke, Lance Ambrose, Sonia Watkins, Gail Murton, Jackie Munoz, Gina Paciotti, Elizabeth Justiniano, Esther Ruiz, Kara Edwards, Chabela (Ana Diaz), Cheryl Westbrook, Jihong Wu, Grace Ho, Tiffany Houston, Virginia Vignolles, Ivy Wong, Karen Mancera-Cuevas, Valerie Bruce, Jiesi Lin, Ying Mou, Kathy Ngo, Yanli Wang, Hamid Eskandari, Shakiba Amini-Mobaraki, Mauricio Becerra, Carlos Martinez, and Kapila Marambage.
Funding
NIH/NHLBI: N01-HC95159-HC95165, N01-HC95169, R01-HL077612 None of the authors has any financial interest in companies who make devices to measure respiratory pressures.
Abbreviation List
- BMI
Body mass index
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- ICC
Intra-class correlation coefficient
- MESA
Multi-Ethnic Study of Atherosclerosis
- MIP
Maximal inspiratory pressure
REFERENCES
- 1.Moxham J. Respiratory Muscles. In: Hughes JMB, Pride NB, editors. Lung Function Tests: Physiological Principles and Clinical Applications. WB Saunders; London: 1999. Chapter 4. [Google Scholar]
- 2.van der Palen J, Rea TD, Manolio TA, Lumley T, Newman AB, Tracy RP, et al. Respiratory muscle strength and the risk of incident cardiovascular events. Thorax. 2004;59(12):1063–1067. doi: 10.1136/thx.2004.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enright PL, Kronmal RA, Manolino TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. Correlates and reference values. Am J Respir Crit Care Med. 1994;149(2):430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 4.Enright PL, Adams AB, Boyle PJ, Sherrill DL. Spirometry and maximal respiratory pressure references from healthy Minnesota 65–85 year old women and men. Chest. 1995;108(3):663–669. doi: 10.1378/chest.108.3.663. [DOI] [PubMed] [Google Scholar]
- 5.Harik-Khan RI, Wise RA, Fozard JL. Determinants of maximal inspiratory pressure: the Baltimore Longitudinal Study of Aging. Am J Respir Crit Care Med. 1998;158(5):1459–1464. doi: 10.1164/ajrccm.158.5.9712006. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter MA, Tockman MS, Hutchinson RG, Davis CE, Heiss G. Demographic and anthropometric correlates of maximal inspiratory pressure: the Atherosclerosis Risk in Communities Study. Am J Respir Crit Care Med. 1999;159(2):415–422. doi: 10.1164/ajrccm.159.2.9708076. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Green M, Road J, Sieck GC, Similowski T. ATS+ERS Statement on Respiratory Muscle Testing: 2. Tests of respiratory muscle strength. Am J Respir Crit Care Med. 2002;166(4):527–547. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardization of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Stukovsky K, Hankinson J, Jiang R, Johnson WC, Kawut S, Smith L, Barr RG. Participant and technician predictors of spirometry quality factors in the Multi-Ethnic Study of Atherosclerosis (abstract) Eur Respir Soc. 2006;451s [Google Scholar]
- 11.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of spirometry reference values in a multiethnic population. The MESA-Lung Study [abstract] Amer Thorac Soc. 2007;A605 doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 14.Vincken W, Ghezzo H. Cosio MG. Maximal static respiratory pressure in adults: normal values and their relationship to determinant of respiratory function. Bull. Eur. Physiopathol. Respir. 1987;23(5):435–39. [PubMed] [Google Scholar]
- 15.Karvonen J, Saarelainen S, Nieminen MM. Measurement of respiratory muscle forces based on maximal inspiratory and expiratory pressures. Respiration. 1994;61(1):28–31. doi: 10.1159/000196299. [DOI] [PubMed] [Google Scholar]
- 16.Aldrich TK, Spiro P. Maximal inspiratory pressure: does reproducibility indicate full effort? Thorax. 1995;50(1):40–43. doi: 10.1136/thx.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson JL, Covey MK, Vitalo CA, Alex CG, Patel M, Kim MJ. Maximal inspiratory pressure, Learning effect and test-retest reliability in patients with chronic obstructive pulmonary disease. Chest. 1993;104(2):448–453. doi: 10.1378/chest.104.2.448. [DOI] [PubMed] [Google Scholar]
- 18.Wen AS, Woo MS, Keens TG. How many maneuvers are required to measure maximal respiratory pressure accurately? Chest. 1997;111(3):802–807. doi: 10.1378/chest.111.3.802. [DOI] [PubMed] [Google Scholar]
- 19.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 20.Hughes PD, Polkey MI, Harrus ML, Coats AJ, Moxham J, Green M. Diaphragm strength in chronic heart failure. Am J Respir Crit Care Med. 1999;160(2):529–534. doi: 10.1164/ajrccm.160.2.9810081. [DOI] [PubMed] [Google Scholar]